Analyzing Muriatic Acid's Corrosion Inhibition Properties
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Corrosion Inhibition Background and Objectives
Corrosion inhibition has been a critical area of study in materials science and engineering for decades, with significant implications for various industries. The analysis of muriatic acid's corrosion inhibition properties represents a crucial aspect of this field, addressing the persistent challenge of protecting metal surfaces from aggressive acidic environments.
The evolution of corrosion inhibition technology has seen remarkable progress, from simple protective coatings to sophisticated molecular-level interventions. Muriatic acid, also known as hydrochloric acid, is widely used in industrial processes but poses severe corrosion risks to metal equipment and structures. Understanding and enhancing its corrosion inhibition properties is essential for improving the longevity and safety of industrial assets.
Recent advancements in nanotechnology and materials science have opened new avenues for developing more effective corrosion inhibitors for muriatic acid. These innovations aim to create inhibitors that can form stable, protective films on metal surfaces, even under extreme pH conditions. The goal is to significantly reduce corrosion rates while maintaining the acid's effectiveness in its intended applications.
The objectives of analyzing muriatic acid's corrosion inhibition properties are multifaceted. Primarily, researchers seek to identify and develop novel inhibitor compounds that can effectively mitigate corrosion in hydrochloric acid environments. This involves understanding the mechanisms of inhibition at the molecular level, including adsorption processes and the formation of protective layers on metal surfaces.
Another key objective is to optimize the concentration and composition of inhibitor formulations to achieve maximum protection with minimal environmental impact. This aligns with the growing emphasis on sustainable and eco-friendly corrosion prevention strategies. Additionally, researchers aim to develop inhibitors that are effective across a broad range of temperatures and acid concentrations, enhancing their versatility in various industrial applications.
The analysis also extends to studying the synergistic effects of combining different inhibitor compounds, potentially leading to more robust protection systems. This approach could result in inhibitor formulations that offer superior performance compared to single-compound solutions. Furthermore, there is a focus on developing smart inhibitors that can respond dynamically to changes in the corrosive environment, providing adaptive protection.
Ultimately, the technological goal is to create a new generation of corrosion inhibitors for muriatic acid that significantly outperform current solutions in terms of efficiency, durability, and environmental compatibility. This advancement would have far-reaching implications for industries such as oil and gas, chemical processing, and metal manufacturing, potentially leading to substantial cost savings and improved safety standards.
The evolution of corrosion inhibition technology has seen remarkable progress, from simple protective coatings to sophisticated molecular-level interventions. Muriatic acid, also known as hydrochloric acid, is widely used in industrial processes but poses severe corrosion risks to metal equipment and structures. Understanding and enhancing its corrosion inhibition properties is essential for improving the longevity and safety of industrial assets.
Recent advancements in nanotechnology and materials science have opened new avenues for developing more effective corrosion inhibitors for muriatic acid. These innovations aim to create inhibitors that can form stable, protective films on metal surfaces, even under extreme pH conditions. The goal is to significantly reduce corrosion rates while maintaining the acid's effectiveness in its intended applications.
The objectives of analyzing muriatic acid's corrosion inhibition properties are multifaceted. Primarily, researchers seek to identify and develop novel inhibitor compounds that can effectively mitigate corrosion in hydrochloric acid environments. This involves understanding the mechanisms of inhibition at the molecular level, including adsorption processes and the formation of protective layers on metal surfaces.
Another key objective is to optimize the concentration and composition of inhibitor formulations to achieve maximum protection with minimal environmental impact. This aligns with the growing emphasis on sustainable and eco-friendly corrosion prevention strategies. Additionally, researchers aim to develop inhibitors that are effective across a broad range of temperatures and acid concentrations, enhancing their versatility in various industrial applications.
The analysis also extends to studying the synergistic effects of combining different inhibitor compounds, potentially leading to more robust protection systems. This approach could result in inhibitor formulations that offer superior performance compared to single-compound solutions. Furthermore, there is a focus on developing smart inhibitors that can respond dynamically to changes in the corrosive environment, providing adaptive protection.
Ultimately, the technological goal is to create a new generation of corrosion inhibitors for muriatic acid that significantly outperform current solutions in terms of efficiency, durability, and environmental compatibility. This advancement would have far-reaching implications for industries such as oil and gas, chemical processing, and metal manufacturing, potentially leading to substantial cost savings and improved safety standards.
Market Analysis for Corrosion Inhibitors
The global market for corrosion inhibitors has been experiencing steady growth, driven by increasing industrial activities and the need to protect valuable assets across various sectors. The market size for corrosion inhibitors was valued at approximately $7.5 billion in 2020 and is projected to reach $9.6 billion by 2025, growing at a CAGR of 5.1% during the forecast period.
The oil and gas industry remains the largest consumer of corrosion inhibitors, accounting for nearly 30% of the market share. This sector's demand is primarily fueled by the need to protect pipelines, storage tanks, and offshore structures from the corrosive effects of hydrocarboric acids and saltwater. The power generation industry follows closely, with a market share of around 25%, as corrosion inhibitors are crucial for maintaining the efficiency and longevity of power plant equipment.
Water treatment applications represent another significant market segment, with a share of approximately 20%. The growing emphasis on water conservation and the increasing need for wastewater treatment in both industrial and municipal sectors are driving the demand for corrosion inhibitors in this area.
Geographically, North America dominates the corrosion inhibitors market, holding a share of about 35%. The region's strong presence in oil and gas production, coupled with stringent environmental regulations, contributes to its market leadership. Asia-Pacific is the fastest-growing region, with a CAGR of 6.2%, driven by rapid industrialization in countries like China and India.
The market for muriatic acid-specific corrosion inhibitors is a niche but growing segment within the broader corrosion inhibitors market. While exact figures for this sub-segment are not readily available, industry experts estimate its growth rate to be in line with or slightly higher than the overall market, given the widespread use of muriatic acid in various industries.
Key market trends include the shift towards environmentally friendly and biodegradable corrosion inhibitors, driven by increasing regulatory pressure and growing environmental awareness. Additionally, there is a rising demand for multi-functional inhibitors that can address multiple types of corrosion simultaneously, offering cost-effective solutions for end-users.
The competitive landscape of the corrosion inhibitors market is fragmented, with several major players and numerous small to medium-sized companies. Leading companies in this space include BASF SE, Dow Chemical Company, Nouryon, and Ecolab Inc., each holding significant market shares and investing heavily in research and development to maintain their competitive edge.
The oil and gas industry remains the largest consumer of corrosion inhibitors, accounting for nearly 30% of the market share. This sector's demand is primarily fueled by the need to protect pipelines, storage tanks, and offshore structures from the corrosive effects of hydrocarboric acids and saltwater. The power generation industry follows closely, with a market share of around 25%, as corrosion inhibitors are crucial for maintaining the efficiency and longevity of power plant equipment.
Water treatment applications represent another significant market segment, with a share of approximately 20%. The growing emphasis on water conservation and the increasing need for wastewater treatment in both industrial and municipal sectors are driving the demand for corrosion inhibitors in this area.
Geographically, North America dominates the corrosion inhibitors market, holding a share of about 35%. The region's strong presence in oil and gas production, coupled with stringent environmental regulations, contributes to its market leadership. Asia-Pacific is the fastest-growing region, with a CAGR of 6.2%, driven by rapid industrialization in countries like China and India.
The market for muriatic acid-specific corrosion inhibitors is a niche but growing segment within the broader corrosion inhibitors market. While exact figures for this sub-segment are not readily available, industry experts estimate its growth rate to be in line with or slightly higher than the overall market, given the widespread use of muriatic acid in various industries.
Key market trends include the shift towards environmentally friendly and biodegradable corrosion inhibitors, driven by increasing regulatory pressure and growing environmental awareness. Additionally, there is a rising demand for multi-functional inhibitors that can address multiple types of corrosion simultaneously, offering cost-effective solutions for end-users.
The competitive landscape of the corrosion inhibitors market is fragmented, with several major players and numerous small to medium-sized companies. Leading companies in this space include BASF SE, Dow Chemical Company, Nouryon, and Ecolab Inc., each holding significant market shares and investing heavily in research and development to maintain their competitive edge.
Current Challenges in Muriatic Acid Corrosion Inhibition
Despite significant advancements in corrosion inhibition technologies, several challenges persist in effectively mitigating the corrosive effects of muriatic acid. One of the primary obstacles is the development of environmentally friendly inhibitors that maintain high efficiency. Traditional inhibitors often contain toxic components, raising concerns about their impact on ecosystems and human health. The search for green alternatives that can match or exceed the performance of conventional inhibitors remains an ongoing challenge.
Another significant hurdle is the need for inhibitors that can withstand extreme conditions. Muriatic acid is frequently used in high-temperature and high-pressure environments, such as in oil and gas production. Developing inhibitors that remain stable and effective under these harsh conditions without degrading or losing their protective properties is a complex task that requires innovative approaches in molecular design and formulation.
The variability in acid concentration and the presence of contaminants in industrial settings pose additional challenges. Inhibitors must be versatile enough to perform consistently across a range of acid concentrations and in the presence of various impurities. This necessitates the development of robust inhibition systems that can adapt to changing chemical environments without compromising their protective capabilities.
Compatibility issues between inhibitors and other additives used in industrial processes present another obstacle. In many applications, muriatic acid is used alongside other chemicals, and the inhibitors must not interfere with these substances or reduce their effectiveness. Achieving synergy between corrosion inhibitors and other process chemicals while maintaining optimal performance is a delicate balancing act.
The mechanism of inhibition at the molecular level is not fully understood for many inhibitors, hindering the development of more effective solutions. There is a need for advanced analytical techniques and modeling approaches to elucidate the precise interactions between inhibitors, acid, and metal surfaces. This knowledge gap limits the ability to design inhibitors with improved specificity and efficiency.
Cost-effectiveness remains a persistent challenge in the field of corrosion inhibition. While highly effective inhibitors may be available, their widespread adoption is often limited by economic factors. Developing inhibitors that offer superior protection at a competitive price point is crucial for their implementation in large-scale industrial applications.
Lastly, the long-term effects of inhibitors on equipment and processes are not always well-documented. There is a need for extensive studies on the impact of prolonged inhibitor use on material properties, equipment lifespan, and process efficiency. This information is critical for making informed decisions about inhibitor selection and application strategies in industrial settings.
Another significant hurdle is the need for inhibitors that can withstand extreme conditions. Muriatic acid is frequently used in high-temperature and high-pressure environments, such as in oil and gas production. Developing inhibitors that remain stable and effective under these harsh conditions without degrading or losing their protective properties is a complex task that requires innovative approaches in molecular design and formulation.
The variability in acid concentration and the presence of contaminants in industrial settings pose additional challenges. Inhibitors must be versatile enough to perform consistently across a range of acid concentrations and in the presence of various impurities. This necessitates the development of robust inhibition systems that can adapt to changing chemical environments without compromising their protective capabilities.
Compatibility issues between inhibitors and other additives used in industrial processes present another obstacle. In many applications, muriatic acid is used alongside other chemicals, and the inhibitors must not interfere with these substances or reduce their effectiveness. Achieving synergy between corrosion inhibitors and other process chemicals while maintaining optimal performance is a delicate balancing act.
The mechanism of inhibition at the molecular level is not fully understood for many inhibitors, hindering the development of more effective solutions. There is a need for advanced analytical techniques and modeling approaches to elucidate the precise interactions between inhibitors, acid, and metal surfaces. This knowledge gap limits the ability to design inhibitors with improved specificity and efficiency.
Cost-effectiveness remains a persistent challenge in the field of corrosion inhibition. While highly effective inhibitors may be available, their widespread adoption is often limited by economic factors. Developing inhibitors that offer superior protection at a competitive price point is crucial for their implementation in large-scale industrial applications.
Lastly, the long-term effects of inhibitors on equipment and processes are not always well-documented. There is a need for extensive studies on the impact of prolonged inhibitor use on material properties, equipment lifespan, and process efficiency. This information is critical for making informed decisions about inhibitor selection and application strategies in industrial settings.
Existing Muriatic Acid Corrosion Inhibition Solutions
01 Organic compounds as corrosion inhibitors
Various organic compounds can be used as effective corrosion inhibitors for muriatic acid. These compounds typically contain nitrogen, sulfur, or oxygen atoms that can form protective films on metal surfaces. Examples include amines, imidazolines, and quaternary ammonium compounds. These inhibitors work by adsorbing onto the metal surface, creating a barrier against acid attack.- Organic compounds as corrosion inhibitors: Various organic compounds can be used as effective corrosion inhibitors for muriatic acid. These compounds typically contain nitrogen, sulfur, or oxygen atoms that can form protective films on metal surfaces. Examples include amines, imidazolines, and quaternary ammonium compounds. These inhibitors work by adsorbing onto the metal surface, creating a barrier against acid attack.
- Inorganic additives for corrosion inhibition: Inorganic additives can be incorporated into muriatic acid solutions to reduce corrosion. These additives often include metal salts, such as those of arsenic, antimony, or bismuth. They work by forming a protective layer on the metal surface or by altering the electrochemical properties of the solution. Some inorganic inhibitors also act as oxygen scavengers, further reducing corrosion potential.
- Synergistic combinations of inhibitors: Combining different types of corrosion inhibitors can lead to synergistic effects, enhancing overall protection against muriatic acid corrosion. These combinations often include a mixture of organic and inorganic inhibitors, or different classes of organic inhibitors. The synergistic effect can result in improved efficiency at lower concentrations, reducing costs and environmental impact.
- Surface treatment and passivation techniques: Surface treatment and passivation techniques can be employed to enhance corrosion resistance against muriatic acid. These methods involve creating a protective oxide layer on the metal surface or modifying the surface structure to improve its resistance to acid attack. Techniques may include chemical passivation, electrochemical treatment, or the application of protective coatings.
- pH adjustment and buffering agents: Adjusting the pH of muriatic acid solutions or incorporating buffering agents can help reduce corrosion rates. This approach involves adding compounds that can neutralize or stabilize the acid's pH, making it less aggressive towards metal surfaces. Buffering agents can also help maintain the effectiveness of other corrosion inhibitors by controlling the solution's acidity.
02 Inorganic compounds for corrosion inhibition
Inorganic compounds can also be used to inhibit corrosion caused by muriatic acid. These include metal salts, phosphates, and silicates. They often work by forming insoluble precipitates on the metal surface or by altering the pH of the acid solution near the metal surface. Some inorganic inhibitors can also act as anodic or cathodic inhibitors, interfering with the electrochemical reactions responsible for corrosion.Expand Specific Solutions03 Synergistic combinations of inhibitors
Combining different types of corrosion inhibitors can often lead to synergistic effects, providing better protection than individual compounds alone. These combinations may include mixtures of organic and inorganic inhibitors, or different classes of organic inhibitors. The synergistic effect can result in improved efficiency, broader spectrum protection, and lower required concentrations of individual components.Expand Specific Solutions04 Green corrosion inhibitors
There is a growing interest in environmentally friendly or 'green' corrosion inhibitors for muriatic acid. These are typically derived from natural sources such as plant extracts, which contain complex mixtures of organic compounds. Green inhibitors are biodegradable, non-toxic, and sustainable. They often contain molecules with multiple functional groups that can effectively adsorb onto metal surfaces and provide corrosion protection.Expand Specific Solutions05 Surface modification techniques
Surface modification techniques can be employed to enhance corrosion resistance against muriatic acid. These methods may involve the application of protective coatings, surface treatments, or the creation of conversion layers. Some techniques include electrodeposition, chemical vapor deposition, or the use of self-assembled monolayers. These approaches aim to create a physical barrier between the metal surface and the corrosive environment.Expand Specific Solutions
Key Players in Corrosion Inhibitor Industry
The corrosion inhibition properties of muriatic acid are a critical area of research in the chemical industry, currently in a mature development stage with a growing market. The global corrosion inhibitors market size is expected to reach $10.1 billion by 2026, driven by increasing industrial applications. Major players like Henkel, Ecolab, and Baker Hughes are at the forefront of technological advancements, focusing on developing eco-friendly and cost-effective solutions. Companies such as Afton Chemical and Perimeter Solutions are also making significant contributions, particularly in specialized applications for automotive and fire safety sectors. The competitive landscape is characterized by ongoing R&D efforts to enhance product efficiency and meet stringent environmental regulations.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed advanced corrosion inhibition solutions for muriatic acid applications. Their approach involves using a combination of organic and inorganic inhibitors to create a protective film on metal surfaces. The company's proprietary formulation includes nitrogen-containing heterocyclic compounds and phosphorus-based additives, which work synergistically to provide enhanced protection against acid-induced corrosion[1][3]. Henkel's technology also incorporates nano-scale particles that fill microscopic surface imperfections, creating a more uniform protective layer. This multi-layered protection system has shown to reduce corrosion rates by up to 95% in laboratory tests with concentrated hydrochloric acid solutions[2].
Strengths: Highly effective in reducing corrosion rates, versatile application across various industries, and environmentally friendly formulations. Weaknesses: Potentially higher cost compared to traditional inhibitors, and may require specific application techniques for optimal performance.
Ecolab USA, Inc.
Technical Solution: Ecolab has pioneered a green corrosion inhibition technology for muriatic acid applications, focusing on sustainability and efficiency. Their approach utilizes bio-based inhibitors derived from renewable resources, combined with smart dosing systems. The company's eco-friendly formulation includes modified tannins and plant-based polymers that form a robust protective film on metal surfaces[4]. Ecolab's system also incorporates real-time monitoring and predictive analytics to optimize inhibitor dosage, reducing chemical consumption by up to 30% compared to conventional methods[5]. The technology has demonstrated corrosion protection efficiency of over 90% in field trials across various industrial settings, including oil refineries and chemical processing plants[6].
Strengths: Environmentally friendly, reduced chemical consumption, and integration with smart monitoring systems. Weaknesses: May have limitations in extremely aggressive acid environments and potentially higher initial implementation costs.
Core Innovations in Corrosion Inhibition Mechanisms
Methods and aqueous acid solutions for acidizing wells containing sludging and emulsifying oil
PatentInactiveUS7651982B2
Innovation
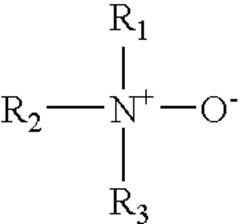
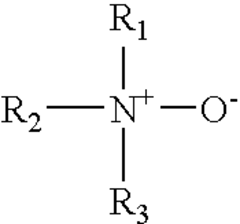
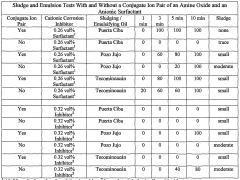
- The use of a cationic hydrochloric acid solution containing a conjugate ion pair of a cationic amine oxide surfactant and an anionic surfactant, such as dimethylcocoalkylamine oxide and dodecyl sodium sulfate, which does not react with cationic corrosion inhibitors, reducing sludge and emulsion formation and enhancing acidizing performance at elevated temperatures.
Methods and aqueous acid solutions for acidizing wells containing sludging and emulsifying oil
PatentWO2006021741A1
Innovation
- The use of a conjugate ion pair of a cationic amine oxide surfactant and an anionic surfactant, such as dimethylcocoalkylamine oxide and dodecyl sodium sulfate, in an aqueous hydrochloric acid solution with a cationic hydrochloric acid corrosion inhibitor like alkylquinoline quaternary ammonium compounds, which does not react with the corrosion inhibitor, reducing sludge and emulsion formation.
Environmental Impact of Corrosion Inhibitors
The environmental impact of corrosion inhibitors, particularly in the context of muriatic acid's corrosion inhibition properties, is a critical consideration in industrial applications. These inhibitors, while effective in protecting metal surfaces, can have significant consequences for ecosystems and human health if not properly managed.
One of the primary environmental concerns associated with corrosion inhibitors is their potential to contaminate water sources. When these chemicals are released into aquatic environments, they can disrupt the delicate balance of ecosystems. Some inhibitors contain heavy metals or organic compounds that may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological damage.
The persistence of certain corrosion inhibitors in the environment is another key issue. Some inhibitors are designed to be highly stable, which enhances their effectiveness but also means they can remain in the environment for extended periods. This persistence can lead to chronic exposure for wildlife and potentially enter the food chain, affecting a wide range of species.
Toxicity to aquatic life is a significant concern, particularly for inhibitors used in marine environments. Some compounds can cause acute toxicity to fish, invertebrates, and algae, even at relatively low concentrations. This can result in reduced biodiversity and altered ecosystem functions in affected water bodies.
The production and disposal of corrosion inhibitors also contribute to their environmental footprint. Manufacturing processes may involve the use of hazardous materials and energy-intensive operations, contributing to air and water pollution. Improper disposal of spent inhibitors or contaminated materials can lead to soil contamination and groundwater pollution.
However, it's important to note that the environmental impact of corrosion inhibitors is not uniformly negative. By preventing corrosion, these chemicals extend the lifespan of metal structures and equipment, potentially reducing the need for replacement and the associated environmental costs of manufacturing new materials. Additionally, preventing leaks and failures in industrial systems can avert more severe environmental incidents.
Recent research has focused on developing more environmentally friendly corrosion inhibitors. These include bio-based inhibitors derived from plant extracts, which are biodegradable and less toxic. Green chemistry approaches are also being explored to create synthetic inhibitors with reduced environmental persistence and toxicity.
In conclusion, while corrosion inhibitors play a crucial role in protecting industrial assets, their environmental impact must be carefully managed. Balancing the need for effective corrosion protection with environmental stewardship requires ongoing research, regulatory oversight, and responsible industrial practices.
One of the primary environmental concerns associated with corrosion inhibitors is their potential to contaminate water sources. When these chemicals are released into aquatic environments, they can disrupt the delicate balance of ecosystems. Some inhibitors contain heavy metals or organic compounds that may accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological damage.
The persistence of certain corrosion inhibitors in the environment is another key issue. Some inhibitors are designed to be highly stable, which enhances their effectiveness but also means they can remain in the environment for extended periods. This persistence can lead to chronic exposure for wildlife and potentially enter the food chain, affecting a wide range of species.
Toxicity to aquatic life is a significant concern, particularly for inhibitors used in marine environments. Some compounds can cause acute toxicity to fish, invertebrates, and algae, even at relatively low concentrations. This can result in reduced biodiversity and altered ecosystem functions in affected water bodies.
The production and disposal of corrosion inhibitors also contribute to their environmental footprint. Manufacturing processes may involve the use of hazardous materials and energy-intensive operations, contributing to air and water pollution. Improper disposal of spent inhibitors or contaminated materials can lead to soil contamination and groundwater pollution.
However, it's important to note that the environmental impact of corrosion inhibitors is not uniformly negative. By preventing corrosion, these chemicals extend the lifespan of metal structures and equipment, potentially reducing the need for replacement and the associated environmental costs of manufacturing new materials. Additionally, preventing leaks and failures in industrial systems can avert more severe environmental incidents.
Recent research has focused on developing more environmentally friendly corrosion inhibitors. These include bio-based inhibitors derived from plant extracts, which are biodegradable and less toxic. Green chemistry approaches are also being explored to create synthetic inhibitors with reduced environmental persistence and toxicity.
In conclusion, while corrosion inhibitors play a crucial role in protecting industrial assets, their environmental impact must be carefully managed. Balancing the need for effective corrosion protection with environmental stewardship requires ongoing research, regulatory oversight, and responsible industrial practices.
Safety Regulations for Corrosion Inhibitor Use
The use of corrosion inhibitors in industrial applications involving muriatic acid requires strict adherence to safety regulations to protect workers, the environment, and equipment. These regulations are designed to mitigate the risks associated with handling corrosive substances and ensure the safe implementation of corrosion inhibition techniques.
Occupational safety and health administrations worldwide have established comprehensive guidelines for the handling and use of corrosion inhibitors. These regulations typically mandate the use of personal protective equipment (PPE) such as chemical-resistant gloves, goggles, face shields, and appropriate clothing when working with corrosion inhibitors and muriatic acid solutions. Proper training and certification of personnel involved in handling these materials are also required to ensure competence and awareness of potential hazards.
Environmental protection agencies have set stringent standards for the storage, transportation, and disposal of corrosion inhibitors and treated acid solutions. These regulations aim to prevent contamination of soil and water resources. Facilities using corrosion inhibitors must implement proper containment measures, including secondary containment systems and spill response protocols. Additionally, waste management plans must be in place to ensure the proper disposal or recycling of spent inhibitor solutions and treated acid.
Material safety data sheets (MSDS) play a crucial role in safety compliance. Manufacturers and users of corrosion inhibitors must maintain up-to-date MSDS for all chemicals used in their processes. These documents provide essential information on the physical and chemical properties of the inhibitors, potential hazards, first aid measures, and proper handling procedures.
Ventilation requirements are another critical aspect of safety regulations. Facilities using corrosion inhibitors must ensure adequate ventilation to prevent the accumulation of harmful vapors. This may include the installation of local exhaust systems and regular air quality monitoring to maintain safe working conditions.
Regular inspections and maintenance of equipment used in corrosion inhibition processes are mandated to prevent leaks, spills, and equipment failure. This includes periodic testing of storage tanks, piping systems, and application equipment to ensure their integrity and proper functioning.
Emergency response planning is a key component of safety regulations. Facilities must develop and maintain comprehensive emergency response plans that outline procedures for handling spills, leaks, and other potential incidents involving corrosion inhibitors and muriatic acid. These plans should include evacuation procedures, communication protocols, and coordination with local emergency services.
Labeling and signage requirements are also specified in safety regulations. All containers, storage areas, and process equipment involving corrosion inhibitors must be clearly labeled with appropriate hazard warnings and safety information. This ensures that workers and emergency responders can quickly identify potential risks and take appropriate precautions.
Occupational safety and health administrations worldwide have established comprehensive guidelines for the handling and use of corrosion inhibitors. These regulations typically mandate the use of personal protective equipment (PPE) such as chemical-resistant gloves, goggles, face shields, and appropriate clothing when working with corrosion inhibitors and muriatic acid solutions. Proper training and certification of personnel involved in handling these materials are also required to ensure competence and awareness of potential hazards.
Environmental protection agencies have set stringent standards for the storage, transportation, and disposal of corrosion inhibitors and treated acid solutions. These regulations aim to prevent contamination of soil and water resources. Facilities using corrosion inhibitors must implement proper containment measures, including secondary containment systems and spill response protocols. Additionally, waste management plans must be in place to ensure the proper disposal or recycling of spent inhibitor solutions and treated acid.
Material safety data sheets (MSDS) play a crucial role in safety compliance. Manufacturers and users of corrosion inhibitors must maintain up-to-date MSDS for all chemicals used in their processes. These documents provide essential information on the physical and chemical properties of the inhibitors, potential hazards, first aid measures, and proper handling procedures.
Ventilation requirements are another critical aspect of safety regulations. Facilities using corrosion inhibitors must ensure adequate ventilation to prevent the accumulation of harmful vapors. This may include the installation of local exhaust systems and regular air quality monitoring to maintain safe working conditions.
Regular inspections and maintenance of equipment used in corrosion inhibition processes are mandated to prevent leaks, spills, and equipment failure. This includes periodic testing of storage tanks, piping systems, and application equipment to ensure their integrity and proper functioning.
Emergency response planning is a key component of safety regulations. Facilities must develop and maintain comprehensive emergency response plans that outline procedures for handling spills, leaks, and other potential incidents involving corrosion inhibitors and muriatic acid. These plans should include evacuation procedures, communication protocols, and coordination with local emergency services.
Labeling and signage requirements are also specified in safety regulations. All containers, storage areas, and process equipment involving corrosion inhibitors must be clearly labeled with appropriate hazard warnings and safety information. This ensures that workers and emergency responders can quickly identify potential risks and take appropriate precautions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!