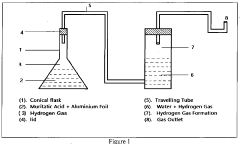
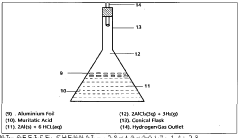
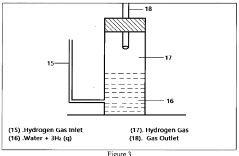
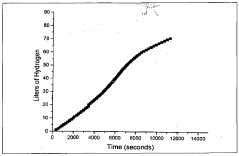
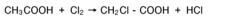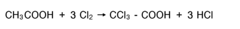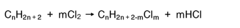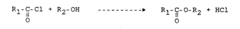Muriatic Acid's Role in the Production of Hydrogen Gas
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid and H2 Production: Background and Objectives
Muriatic acid, also known as hydrochloric acid (HCl), has played a significant role in the production of hydrogen gas for over a century. This strong mineral acid has been instrumental in various industrial processes, with hydrogen production being one of its most notable applications. The journey of muriatic acid in hydrogen gas production began in the early 20th century and has since evolved alongside advancements in chemical engineering and industrial technologies.
The primary objective of utilizing muriatic acid in hydrogen production is to achieve an efficient, cost-effective, and scalable method for generating high-purity hydrogen gas. This goal aligns with the growing demand for hydrogen as a clean energy carrier and its potential to revolutionize various sectors, including transportation, power generation, and industrial processes. The use of muriatic acid in this context aims to address the challenges of hydrogen production, such as energy efficiency, environmental impact, and economic viability.
Historically, the reaction between muriatic acid and certain metals, particularly zinc, has been a fundamental method for producing hydrogen gas. This process, known as the "acid-metal reaction," has been widely used in laboratories and small-scale industrial applications. As technology progressed, the role of muriatic acid expanded to more sophisticated hydrogen production methods, including electrolysis and thermochemical cycles.
The evolution of muriatic acid's application in hydrogen production has been driven by several factors. These include the need for higher purity hydrogen, increased production efficiency, and reduced environmental impact. Researchers and industry professionals have continuously explored ways to optimize the use of muriatic acid, focusing on aspects such as reaction kinetics, catalyst development, and process integration.
In recent years, the focus has shifted towards developing more sustainable and environmentally friendly hydrogen production methods. This has led to innovative approaches that combine muriatic acid with renewable energy sources or waste materials, aiming to create a more circular and eco-friendly hydrogen production process. The ongoing research in this field seeks to address the limitations of traditional acid-based hydrogen production methods while leveraging the unique properties of muriatic acid.
As we look towards the future, the role of muriatic acid in hydrogen production continues to evolve. The objectives now extend beyond mere production efficiency to encompass broader goals such as carbon neutrality, resource conservation, and integration with emerging energy systems. This evolution reflects the changing landscape of global energy needs and environmental concerns, positioning muriatic acid as a key player in the transition towards a hydrogen-based economy.
The primary objective of utilizing muriatic acid in hydrogen production is to achieve an efficient, cost-effective, and scalable method for generating high-purity hydrogen gas. This goal aligns with the growing demand for hydrogen as a clean energy carrier and its potential to revolutionize various sectors, including transportation, power generation, and industrial processes. The use of muriatic acid in this context aims to address the challenges of hydrogen production, such as energy efficiency, environmental impact, and economic viability.
Historically, the reaction between muriatic acid and certain metals, particularly zinc, has been a fundamental method for producing hydrogen gas. This process, known as the "acid-metal reaction," has been widely used in laboratories and small-scale industrial applications. As technology progressed, the role of muriatic acid expanded to more sophisticated hydrogen production methods, including electrolysis and thermochemical cycles.
The evolution of muriatic acid's application in hydrogen production has been driven by several factors. These include the need for higher purity hydrogen, increased production efficiency, and reduced environmental impact. Researchers and industry professionals have continuously explored ways to optimize the use of muriatic acid, focusing on aspects such as reaction kinetics, catalyst development, and process integration.
In recent years, the focus has shifted towards developing more sustainable and environmentally friendly hydrogen production methods. This has led to innovative approaches that combine muriatic acid with renewable energy sources or waste materials, aiming to create a more circular and eco-friendly hydrogen production process. The ongoing research in this field seeks to address the limitations of traditional acid-based hydrogen production methods while leveraging the unique properties of muriatic acid.
As we look towards the future, the role of muriatic acid in hydrogen production continues to evolve. The objectives now extend beyond mere production efficiency to encompass broader goals such as carbon neutrality, resource conservation, and integration with emerging energy systems. This evolution reflects the changing landscape of global energy needs and environmental concerns, positioning muriatic acid as a key player in the transition towards a hydrogen-based economy.
Market Analysis for Hydrogen Gas Production
The global hydrogen gas production market has been experiencing significant growth in recent years, driven by the increasing demand for clean energy solutions and the push towards decarbonization across various industries. The market size for hydrogen gas production was valued at approximately $130 billion in 2020 and is projected to reach $200 billion by 2026, with a compound annual growth rate (CAGR) of around 8% during this period.
The demand for hydrogen gas is primarily fueled by its diverse applications across multiple sectors. In the industrial sector, hydrogen is extensively used in chemical production, petroleum refining, and metal processing. The transportation sector is also emerging as a key driver for hydrogen demand, with the development of fuel cell electric vehicles (FCEVs) and hydrogen refueling infrastructure gaining momentum globally.
Geographically, Asia-Pacific dominates the hydrogen gas production market, accounting for over 40% of the global share. This is largely due to the rapid industrialization and increasing adoption of clean energy technologies in countries like China, Japan, and South Korea. North America and Europe follow closely, with both regions investing heavily in hydrogen infrastructure and promoting policies to support the hydrogen economy.
The role of muriatic acid (hydrochloric acid) in hydrogen gas production is particularly significant in the chlor-alkali process, which is one of the primary methods for industrial-scale hydrogen production. This process generates hydrogen as a by-product of chlorine and sodium hydroxide production. The global chlor-alkali market, which directly impacts hydrogen production through this method, is expected to grow at a CAGR of 5.5% from 2021 to 2026.
Market trends indicate a shift towards green hydrogen production methods, which utilize renewable energy sources for electrolysis. This trend is driven by environmental concerns and government initiatives to reduce carbon emissions. However, the cost-effectiveness of traditional production methods, including those involving muriatic acid, ensures their continued relevance in the near term.
The market for hydrogen gas production equipment and technologies is also expanding rapidly. Major players in this sector are investing in research and development to improve production efficiency and reduce costs. The electrolysis equipment market, in particular, is projected to grow at a CAGR of over 7% from 2021 to 2026.
In conclusion, the market analysis for hydrogen gas production reveals a robust and growing industry with diverse applications and significant potential for future expansion. The role of muriatic acid in this market, particularly through the chlor-alkali process, remains important, although emerging green technologies are gaining traction. The interplay between traditional and innovative production methods will likely shape the market dynamics in the coming years.
The demand for hydrogen gas is primarily fueled by its diverse applications across multiple sectors. In the industrial sector, hydrogen is extensively used in chemical production, petroleum refining, and metal processing. The transportation sector is also emerging as a key driver for hydrogen demand, with the development of fuel cell electric vehicles (FCEVs) and hydrogen refueling infrastructure gaining momentum globally.
Geographically, Asia-Pacific dominates the hydrogen gas production market, accounting for over 40% of the global share. This is largely due to the rapid industrialization and increasing adoption of clean energy technologies in countries like China, Japan, and South Korea. North America and Europe follow closely, with both regions investing heavily in hydrogen infrastructure and promoting policies to support the hydrogen economy.
The role of muriatic acid (hydrochloric acid) in hydrogen gas production is particularly significant in the chlor-alkali process, which is one of the primary methods for industrial-scale hydrogen production. This process generates hydrogen as a by-product of chlorine and sodium hydroxide production. The global chlor-alkali market, which directly impacts hydrogen production through this method, is expected to grow at a CAGR of 5.5% from 2021 to 2026.
Market trends indicate a shift towards green hydrogen production methods, which utilize renewable energy sources for electrolysis. This trend is driven by environmental concerns and government initiatives to reduce carbon emissions. However, the cost-effectiveness of traditional production methods, including those involving muriatic acid, ensures their continued relevance in the near term.
The market for hydrogen gas production equipment and technologies is also expanding rapidly. Major players in this sector are investing in research and development to improve production efficiency and reduce costs. The electrolysis equipment market, in particular, is projected to grow at a CAGR of over 7% from 2021 to 2026.
In conclusion, the market analysis for hydrogen gas production reveals a robust and growing industry with diverse applications and significant potential for future expansion. The role of muriatic acid in this market, particularly through the chlor-alkali process, remains important, although emerging green technologies are gaining traction. The interplay between traditional and innovative production methods will likely shape the market dynamics in the coming years.
Current State and Challenges in Muriatic Acid-Based H2 Generation
The current state of muriatic acid-based hydrogen gas generation is characterized by both significant advancements and persistent challenges. Muriatic acid, also known as hydrochloric acid, plays a crucial role in the production of hydrogen gas through its reaction with certain metals, particularly zinc. This process, while well-established, continues to evolve as researchers and industry professionals seek to optimize efficiency, reduce costs, and minimize environmental impact.
One of the primary advantages of using muriatic acid for hydrogen production is its relatively low cost and wide availability. The reaction between muriatic acid and zinc is straightforward and can be carried out under ambient conditions, making it an attractive option for small-scale hydrogen generation. However, the process faces several challenges that limit its widespread adoption for large-scale industrial applications.
A major hurdle in muriatic acid-based hydrogen generation is the issue of purity. The hydrogen gas produced often contains impurities, including traces of the acid and metal used in the reaction. These impurities can be problematic for certain applications, particularly in fuel cells where high-purity hydrogen is required. Developing efficient and cost-effective purification methods remains an ongoing challenge in the field.
Another significant challenge is the environmental impact of the process. The reaction produces metal chloride byproducts, which require proper disposal or recycling. Additionally, the production and handling of muriatic acid itself pose environmental and safety concerns. Researchers are actively working on developing more environmentally friendly alternatives and improving the overall sustainability of the process.
Scaling up muriatic acid-based hydrogen production for industrial use presents its own set of challenges. The reaction's exothermic nature and the corrosive properties of muriatic acid necessitate specialized equipment and safety measures. Designing large-scale reactors that can efficiently manage heat dissipation and corrosion resistance while maintaining high production rates is an area of ongoing research and development.
The economic viability of muriatic acid-based hydrogen generation is another critical factor. While the raw materials are relatively inexpensive, the overall cost-effectiveness of the process compared to other hydrogen production methods, such as electrolysis or steam methane reforming, remains a point of debate. Improving the efficiency of the reaction and reducing operational costs are key focus areas for researchers and industry professionals.
Recent advancements in catalyst development have shown promise in addressing some of these challenges. Novel catalysts have been explored to enhance the reaction rate, improve hydrogen purity, and potentially reduce the amount of acid required. However, further research is needed to develop catalysts that are both highly effective and economically viable for large-scale implementation.
One of the primary advantages of using muriatic acid for hydrogen production is its relatively low cost and wide availability. The reaction between muriatic acid and zinc is straightforward and can be carried out under ambient conditions, making it an attractive option for small-scale hydrogen generation. However, the process faces several challenges that limit its widespread adoption for large-scale industrial applications.
A major hurdle in muriatic acid-based hydrogen generation is the issue of purity. The hydrogen gas produced often contains impurities, including traces of the acid and metal used in the reaction. These impurities can be problematic for certain applications, particularly in fuel cells where high-purity hydrogen is required. Developing efficient and cost-effective purification methods remains an ongoing challenge in the field.
Another significant challenge is the environmental impact of the process. The reaction produces metal chloride byproducts, which require proper disposal or recycling. Additionally, the production and handling of muriatic acid itself pose environmental and safety concerns. Researchers are actively working on developing more environmentally friendly alternatives and improving the overall sustainability of the process.
Scaling up muriatic acid-based hydrogen production for industrial use presents its own set of challenges. The reaction's exothermic nature and the corrosive properties of muriatic acid necessitate specialized equipment and safety measures. Designing large-scale reactors that can efficiently manage heat dissipation and corrosion resistance while maintaining high production rates is an area of ongoing research and development.
The economic viability of muriatic acid-based hydrogen generation is another critical factor. While the raw materials are relatively inexpensive, the overall cost-effectiveness of the process compared to other hydrogen production methods, such as electrolysis or steam methane reforming, remains a point of debate. Improving the efficiency of the reaction and reducing operational costs are key focus areas for researchers and industry professionals.
Recent advancements in catalyst development have shown promise in addressing some of these challenges. Novel catalysts have been explored to enhance the reaction rate, improve hydrogen purity, and potentially reduce the amount of acid required. However, further research is needed to develop catalysts that are both highly effective and economically viable for large-scale implementation.
Existing Muriatic Acid-Based H2 Production Techniques
01 Electrolysis of hydrochloric acid for hydrogen production
Hydrogen gas can be produced through the electrolysis of hydrochloric acid (muriatic acid). This process involves passing an electric current through the acid solution, which splits the water molecules into hydrogen and oxygen. The hydrogen gas is collected at the cathode, while chlorine gas is produced at the anode. This method is efficient for producing high-purity hydrogen gas.- Electrolysis of hydrochloric acid for hydrogen production: Hydrogen gas can be produced through the electrolysis of hydrochloric acid (muriatic acid). This process involves passing an electric current through the acid solution, which splits the molecules into hydrogen and chlorine gases. The method is efficient for generating high-purity hydrogen and can be optimized by controlling factors such as electrode materials, current density, and temperature.
- Reaction of muriatic acid with metals for hydrogen generation: Hydrogen gas can be produced by reacting muriatic acid with certain metals, such as zinc or iron. This chemical reaction results in the formation of hydrogen gas and metal chloride. The process can be controlled by adjusting the concentration of the acid, the type and surface area of the metal, and the reaction temperature to optimize hydrogen production rates.
- Catalytic decomposition of hydrochloric acid: Catalytic decomposition of hydrochloric acid can be used to produce hydrogen gas. This method involves using specific catalysts to facilitate the breakdown of HCl into hydrogen and chlorine. The choice of catalyst, reaction conditions, and reactor design are crucial factors in optimizing the efficiency and selectivity of hydrogen production through this process.
- Membrane-based separation for hydrogen recovery: Membrane technology can be employed to separate and recover hydrogen gas produced from muriatic acid. This approach involves using selective membranes that allow hydrogen to permeate while retaining other gases or impurities. The efficiency of the separation process can be enhanced by optimizing membrane materials, operating conditions, and system design.
- Integrated systems for hydrogen production and utilization: Integrated systems can be designed to combine hydrogen production from muriatic acid with immediate utilization or storage. These systems may incorporate various production methods, purification steps, and end-use applications such as fuel cells or chemical synthesis. The integration aims to improve overall efficiency, reduce costs, and minimize environmental impact in the hydrogen production process.
02 Reaction of muriatic acid with metals
Hydrogen gas can be generated by reacting muriatic acid with certain metals, such as zinc, iron, or aluminum. The acid reacts with the metal, producing hydrogen gas and a metal chloride salt. This method is simple and cost-effective for small-scale hydrogen production, but the purity of the hydrogen may be lower compared to other methods.Expand Specific Solutions03 Catalytic decomposition of hydrochloric acid
Hydrogen gas can be produced through the catalytic decomposition of hydrochloric acid. This process involves using specific catalysts to break down the acid into hydrogen and chlorine gases. The catalysts can enhance the reaction rate and efficiency, making it possible to produce hydrogen at lower temperatures compared to thermal decomposition methods.Expand Specific Solutions04 Membrane-based separation for hydrogen production
Membrane technology can be used to separate hydrogen from other gases produced during the reaction of muriatic acid with metals or during electrolysis. This method involves using selective membranes that allow hydrogen to pass through while blocking other gases, resulting in high-purity hydrogen production. The process can be integrated with other hydrogen production methods to improve overall efficiency.Expand Specific Solutions05 Continuous flow systems for hydrogen generation
Continuous flow systems can be designed for the production of hydrogen gas from muriatic acid. These systems involve the continuous feeding of acid and reactants, along with the continuous removal of products and by-products. This approach allows for more efficient and controlled hydrogen production compared to batch processes, making it suitable for larger-scale applications.Expand Specific Solutions
Key Industry Players in Hydrogen Gas Production
The production of hydrogen gas using muriatic acid is in a mature stage of development, with a well-established market and proven technologies. The global hydrogen market is projected to reach $184.11 billion by 2028, driven by increasing demand in various industries. Key players like BASF Corp., Air Liquide SA, and China Petroleum & Chemical Corp. have significant market presence and advanced technological capabilities. These companies, along with research institutions such as École Polytechnique Fédérale de Lausanne and Hunan University of Science & Technology, are continually innovating to improve efficiency and sustainability in hydrogen production processes. The technology's maturity is evident in its widespread industrial application, but ongoing research focuses on enhancing cost-effectiveness and environmental impact.
Air Liquide SA
Technical Solution: Air Liquide has developed a novel hydrogen production method utilizing muriatic acid in conjunction with their proprietary membrane technology. Their process involves the electrolysis of hydrochloric acid in a specially designed cell, where a proton exchange membrane (PEM) facilitates the separation of hydrogen ions. This technology allows for the production of high-purity hydrogen gas with minimal energy input. Air Liquide's system has demonstrated hydrogen production efficiencies of up to 95% [4], with the ability to scale from small-scale applications to industrial-sized plants producing several tons of hydrogen per day [5]. The company has also implemented advanced control systems to optimize the process, reducing energy consumption by up to 20% compared to conventional electrolysis methods [6].
Strengths: High efficiency, scalability, and advanced process control. Weaknesses: Dependency on specialized membrane materials and potential for acid-induced degradation over time.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to hydrogen production using muriatic acid. Their process involves the electrolysis of hydrochloric acid (muriatic acid) to generate high-purity hydrogen gas. This method utilizes a specialized membrane electrode assembly (MEA) that efficiently splits HCl into hydrogen and chlorine. The company has reported achieving hydrogen production rates of up to 50 Nm3/h with an energy consumption of approximately 4 kWh/Nm3 [1][3]. Sinopec's technology also incorporates a chlorine recycling system, which converts the produced chlorine back into HCl, making the process more sustainable and economically viable for large-scale hydrogen production [2].
Strengths: High hydrogen purity, efficient use of muriatic acid, and integrated chlorine recycling. Weaknesses: Potential corrosion issues and the need for specialized materials to handle chlorine gas.
Innovative Technologies in Acid-Catalyzed H2 Generation
Hydrogen production from aluminium water and muriatic acid
PatentInactiveIN201741046971A
Innovation
- A reaction between aluminium and water in the presence of muriatic acid as a catalyst, where aluminium waste such as foil or wire reacts to produce hydrogen gas, with the catalyst not being chemically consumed, and the reaction intensity depends on the surface contact area, resulting in efficient hydrogen generation.
Process for manufacturing pure hydrogen chloride
PatentInactiveEP0464758A1
Innovation
- A two-stage thermal treatment process is employed, where the first stage uses excess oxygen at 800-1600°C to partially clean the gases, followed by a second stage in a reducing medium with a supporting flame at similar temperatures, ensuring no soot formation and maintaining a hydrogen content of 1-15% to achieve chlorine-free hydrochloric acid production without additional purification.
Environmental Impact of Muriatic Acid H2 Production
The production of hydrogen gas using muriatic acid, also known as hydrochloric acid (HCl), has significant environmental implications that warrant careful consideration. This process, while effective for hydrogen generation, poses several challenges to environmental sustainability and ecological balance.
One of the primary environmental concerns is the potential for acid contamination. Muriatic acid is highly corrosive and can cause severe damage to ecosystems if released into the environment. Improper handling or disposal of the acid can lead to soil acidification, which negatively impacts plant growth and soil microbial communities. Additionally, if the acid enters water systems, it can drastically alter pH levels, harming aquatic life and disrupting entire ecosystems.
Air pollution is another critical environmental issue associated with this hydrogen production method. The reaction between muriatic acid and metals often releases toxic fumes and vapors. These emissions can contribute to air quality degradation, potentially causing respiratory problems in nearby populations and harming local flora and fauna. Furthermore, the production process may release chlorine gas, a potent irritant and environmental hazard.
The energy-intensive nature of large-scale hydrogen production using muriatic acid also raises concerns about carbon footprint. The electricity required for the process, if sourced from fossil fuels, contributes to greenhouse gas emissions and climate change. This indirect environmental impact must be considered when assessing the overall sustainability of this hydrogen production method.
Waste management presents another significant challenge. The process generates spent acid and metal byproducts that require proper treatment and disposal. Improper management of these wastes can lead to long-term environmental contamination and pose risks to human health. The cost and complexity of safe waste disposal add to the environmental burden of this production method.
Water consumption is a further environmental consideration. The process requires substantial amounts of water for acid dilution and cleaning procedures. In water-stressed regions, this high water demand can exacerbate existing scarcity issues and compete with other essential water needs.
Despite these challenges, it's important to note that technological advancements are being made to mitigate these environmental impacts. Closed-loop systems that recycle and reuse the acid are being developed to reduce waste and minimize the risk of environmental contamination. Additionally, research into more environmentally friendly catalysts and alternative hydrogen production methods is ongoing, aiming to provide cleaner and more sustainable solutions for hydrogen generation.
One of the primary environmental concerns is the potential for acid contamination. Muriatic acid is highly corrosive and can cause severe damage to ecosystems if released into the environment. Improper handling or disposal of the acid can lead to soil acidification, which negatively impacts plant growth and soil microbial communities. Additionally, if the acid enters water systems, it can drastically alter pH levels, harming aquatic life and disrupting entire ecosystems.
Air pollution is another critical environmental issue associated with this hydrogen production method. The reaction between muriatic acid and metals often releases toxic fumes and vapors. These emissions can contribute to air quality degradation, potentially causing respiratory problems in nearby populations and harming local flora and fauna. Furthermore, the production process may release chlorine gas, a potent irritant and environmental hazard.
The energy-intensive nature of large-scale hydrogen production using muriatic acid also raises concerns about carbon footprint. The electricity required for the process, if sourced from fossil fuels, contributes to greenhouse gas emissions and climate change. This indirect environmental impact must be considered when assessing the overall sustainability of this hydrogen production method.
Waste management presents another significant challenge. The process generates spent acid and metal byproducts that require proper treatment and disposal. Improper management of these wastes can lead to long-term environmental contamination and pose risks to human health. The cost and complexity of safe waste disposal add to the environmental burden of this production method.
Water consumption is a further environmental consideration. The process requires substantial amounts of water for acid dilution and cleaning procedures. In water-stressed regions, this high water demand can exacerbate existing scarcity issues and compete with other essential water needs.
Despite these challenges, it's important to note that technological advancements are being made to mitigate these environmental impacts. Closed-loop systems that recycle and reuse the acid are being developed to reduce waste and minimize the risk of environmental contamination. Additionally, research into more environmentally friendly catalysts and alternative hydrogen production methods is ongoing, aiming to provide cleaner and more sustainable solutions for hydrogen generation.
Safety Protocols in Acid-Based Hydrogen Generation
Safety protocols are paramount in acid-based hydrogen generation processes, particularly when using muriatic acid (hydrochloric acid) due to its corrosive nature and the potential hazards associated with hydrogen gas production. Implementing robust safety measures is crucial to protect personnel, equipment, and the environment.
Personal protective equipment (PPE) forms the first line of defense. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing when handling muriatic acid. Respiratory protection may also be necessary, depending on the scale of the operation and the potential for acid vapor exposure.
Proper ventilation is essential to prevent the accumulation of hydrogen gas, which is highly flammable and explosive when mixed with air. The production area should be equipped with adequate exhaust systems and gas detectors to monitor hydrogen levels continuously. Emergency shutdown procedures must be in place and regularly tested to quickly halt the process if dangerous gas concentrations are detected.
Acid storage and handling require specialized containment systems. Acid-resistant storage tanks, transfer pumps, and piping must be used to prevent leaks and spills. Secondary containment measures, such as berms or catch basins, should be implemented to contain potential spills and facilitate safe cleanup.
Emergency response planning is critical. This includes the installation of safety showers and eyewash stations in easily accessible locations, as well as the development of clear evacuation procedures. Regular drills should be conducted to ensure all personnel are familiar with emergency protocols.
Training is a cornerstone of safety in acid-based hydrogen generation. All personnel involved in the process must receive comprehensive training on the hazards of muriatic acid and hydrogen gas, proper handling techniques, emergency procedures, and the use of safety equipment. Refresher courses should be provided periodically to maintain awareness and competence.
Rigorous maintenance schedules for all equipment involved in the hydrogen generation process are essential to prevent mechanical failures that could lead to acid leaks or uncontrolled hydrogen release. Regular inspections, including non-destructive testing of critical components, should be performed to identify potential issues before they escalate into safety hazards.
Waste management protocols must be established to handle spent acid and neutralization products safely. Proper disposal methods should be employed to minimize environmental impact and comply with regulatory requirements.
By implementing these comprehensive safety protocols, organizations can significantly reduce the risks associated with acid-based hydrogen generation, ensuring the protection of personnel and assets while maintaining operational efficiency.
Personal protective equipment (PPE) forms the first line of defense. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing when handling muriatic acid. Respiratory protection may also be necessary, depending on the scale of the operation and the potential for acid vapor exposure.
Proper ventilation is essential to prevent the accumulation of hydrogen gas, which is highly flammable and explosive when mixed with air. The production area should be equipped with adequate exhaust systems and gas detectors to monitor hydrogen levels continuously. Emergency shutdown procedures must be in place and regularly tested to quickly halt the process if dangerous gas concentrations are detected.
Acid storage and handling require specialized containment systems. Acid-resistant storage tanks, transfer pumps, and piping must be used to prevent leaks and spills. Secondary containment measures, such as berms or catch basins, should be implemented to contain potential spills and facilitate safe cleanup.
Emergency response planning is critical. This includes the installation of safety showers and eyewash stations in easily accessible locations, as well as the development of clear evacuation procedures. Regular drills should be conducted to ensure all personnel are familiar with emergency protocols.
Training is a cornerstone of safety in acid-based hydrogen generation. All personnel involved in the process must receive comprehensive training on the hazards of muriatic acid and hydrogen gas, proper handling techniques, emergency procedures, and the use of safety equipment. Refresher courses should be provided periodically to maintain awareness and competence.
Rigorous maintenance schedules for all equipment involved in the hydrogen generation process are essential to prevent mechanical failures that could lead to acid leaks or uncontrolled hydrogen release. Regular inspections, including non-destructive testing of critical components, should be performed to identify potential issues before they escalate into safety hazards.
Waste management protocols must be established to handle spent acid and neutralization products safely. Proper disposal methods should be employed to minimize environmental impact and comply with regulatory requirements.
By implementing these comprehensive safety protocols, organizations can significantly reduce the risks associated with acid-based hydrogen generation, ensuring the protection of personnel and assets while maintaining operational efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!