Muriatic Acid's Use in Oil Refining Processes
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid in Refining: Background and Objectives
Muriatic acid, also known as hydrochloric acid, has been a crucial component in oil refining processes for decades. Its use in the petroleum industry dates back to the early 20th century when refineries began to recognize its effectiveness in various stages of crude oil processing. The evolution of muriatic acid's application in refining has been closely tied to the advancements in petroleum technology and the increasing demand for higher-quality fuel products.
Initially, muriatic acid was primarily used for cleaning and descaling equipment in refineries. However, as the understanding of its chemical properties deepened, its role expanded to more sophisticated applications within the refining process. The acid's ability to react with and remove unwanted impurities from crude oil became a cornerstone of modern refining techniques.
The primary objective of using muriatic acid in oil refining is to enhance the quality and yield of petroleum products. This is achieved through several key processes, including the removal of inorganic contaminants, pH adjustment in various refining stages, and catalytic support in certain chemical reactions. The acid's strong corrosive properties make it particularly effective in dissolving metal salts and other inorganic compounds that can interfere with refining efficiency.
In recent years, the focus has shifted towards optimizing the use of muriatic acid to meet increasingly stringent environmental regulations and improve overall process efficiency. Refineries are now exploring ways to minimize acid consumption while maximizing its benefits, leading to the development of more sophisticated acid management systems and recovery techniques.
The technological trajectory of muriatic acid use in oil refining is closely aligned with broader industry trends towards cleaner fuels and more sustainable refining practices. As such, current research and development efforts are aimed at finding innovative applications for muriatic acid that can contribute to reducing the environmental footprint of refining operations while maintaining or improving product quality.
Looking ahead, the objectives for muriatic acid use in oil refining include developing more precise application methods, improving acid recovery and recycling processes, and exploring potential synergies with other refining chemicals. There is also a growing interest in finding environmentally friendly alternatives that can match or exceed the performance of muriatic acid in certain applications, driven by the industry's commitment to sustainability and regulatory compliance.
Initially, muriatic acid was primarily used for cleaning and descaling equipment in refineries. However, as the understanding of its chemical properties deepened, its role expanded to more sophisticated applications within the refining process. The acid's ability to react with and remove unwanted impurities from crude oil became a cornerstone of modern refining techniques.
The primary objective of using muriatic acid in oil refining is to enhance the quality and yield of petroleum products. This is achieved through several key processes, including the removal of inorganic contaminants, pH adjustment in various refining stages, and catalytic support in certain chemical reactions. The acid's strong corrosive properties make it particularly effective in dissolving metal salts and other inorganic compounds that can interfere with refining efficiency.
In recent years, the focus has shifted towards optimizing the use of muriatic acid to meet increasingly stringent environmental regulations and improve overall process efficiency. Refineries are now exploring ways to minimize acid consumption while maximizing its benefits, leading to the development of more sophisticated acid management systems and recovery techniques.
The technological trajectory of muriatic acid use in oil refining is closely aligned with broader industry trends towards cleaner fuels and more sustainable refining practices. As such, current research and development efforts are aimed at finding innovative applications for muriatic acid that can contribute to reducing the environmental footprint of refining operations while maintaining or improving product quality.
Looking ahead, the objectives for muriatic acid use in oil refining include developing more precise application methods, improving acid recovery and recycling processes, and exploring potential synergies with other refining chemicals. There is also a growing interest in finding environmentally friendly alternatives that can match or exceed the performance of muriatic acid in certain applications, driven by the industry's commitment to sustainability and regulatory compliance.
Market Demand Analysis for Muriatic Acid in Oil Refining
The market demand for muriatic acid, also known as hydrochloric acid, in oil refining processes has been steadily growing due to its crucial role in various stages of petroleum processing. The oil and gas industry represents a significant portion of the global hydrochloric acid market, with refineries being major consumers.
In recent years, the increasing complexity of crude oil compositions and the need for more efficient refining processes have driven the demand for muriatic acid. As refineries process heavier and more sour crude oils, the requirement for acid treatment has intensified. This trend is particularly evident in regions with expanding refining capacities, such as Asia-Pacific and the Middle East.
The primary application of muriatic acid in oil refining is in the acidizing process, which enhances well productivity by dissolving formation damage near the wellbore. This process has become increasingly important as oil companies seek to maximize output from existing wells and explore unconventional oil sources.
Furthermore, muriatic acid plays a vital role in catalyst activation and regeneration processes within refineries. As the global push for cleaner fuels continues, refineries are adopting more sophisticated catalytic processes, which in turn drives the demand for high-purity hydrochloric acid.
The market for muriatic acid in oil refining is also influenced by environmental regulations. Stricter emission standards have led to increased use of acid in flue gas treatment systems, further boosting demand. Additionally, the growing emphasis on recycling and proper disposal of spent acid has created new market opportunities for acid recovery and regeneration services.
Geographically, North America and the Middle East are key markets for muriatic acid in oil refining. The shale oil boom in the United States has significantly contributed to the increased demand for acidizing treatments. Meanwhile, the expanding refining sector in the Middle East, driven by the region's oil-rich resources, continues to be a major consumer of muriatic acid.
Looking ahead, the market demand for muriatic acid in oil refining is expected to continue its upward trajectory. Factors such as the ongoing exploration of new oil fields, the need for enhanced oil recovery techniques, and the continuous optimization of refining processes will sustain this growth. However, the market may face challenges from the global shift towards renewable energy sources and the potential long-term decline in fossil fuel consumption.
In recent years, the increasing complexity of crude oil compositions and the need for more efficient refining processes have driven the demand for muriatic acid. As refineries process heavier and more sour crude oils, the requirement for acid treatment has intensified. This trend is particularly evident in regions with expanding refining capacities, such as Asia-Pacific and the Middle East.
The primary application of muriatic acid in oil refining is in the acidizing process, which enhances well productivity by dissolving formation damage near the wellbore. This process has become increasingly important as oil companies seek to maximize output from existing wells and explore unconventional oil sources.
Furthermore, muriatic acid plays a vital role in catalyst activation and regeneration processes within refineries. As the global push for cleaner fuels continues, refineries are adopting more sophisticated catalytic processes, which in turn drives the demand for high-purity hydrochloric acid.
The market for muriatic acid in oil refining is also influenced by environmental regulations. Stricter emission standards have led to increased use of acid in flue gas treatment systems, further boosting demand. Additionally, the growing emphasis on recycling and proper disposal of spent acid has created new market opportunities for acid recovery and regeneration services.
Geographically, North America and the Middle East are key markets for muriatic acid in oil refining. The shale oil boom in the United States has significantly contributed to the increased demand for acidizing treatments. Meanwhile, the expanding refining sector in the Middle East, driven by the region's oil-rich resources, continues to be a major consumer of muriatic acid.
Looking ahead, the market demand for muriatic acid in oil refining is expected to continue its upward trajectory. Factors such as the ongoing exploration of new oil fields, the need for enhanced oil recovery techniques, and the continuous optimization of refining processes will sustain this growth. However, the market may face challenges from the global shift towards renewable energy sources and the potential long-term decline in fossil fuel consumption.
Current Challenges in Muriatic Acid Application
The application of muriatic acid in oil refining processes faces several significant challenges that require careful consideration and innovative solutions. One of the primary issues is the corrosive nature of muriatic acid, which can lead to severe damage to equipment and infrastructure. This corrosion not only reduces the lifespan of refinery components but also poses safety risks to personnel and the environment.
Another challenge lies in the handling and storage of muriatic acid. Its highly reactive properties necessitate specialized containment systems and stringent safety protocols. Refineries must invest in corrosion-resistant materials and implement robust safety measures to prevent leaks or spills, which can result in hazardous situations and environmental contamination.
The disposal of spent muriatic acid presents a significant environmental challenge. The acid, often contaminated with various hydrocarbons and heavy metals after use in refining processes, requires careful treatment before disposal. This process is not only costly but also energy-intensive, contributing to the overall environmental footprint of refining operations.
Efficiency in acid utilization is another area of concern. Current processes may not fully optimize the use of muriatic acid, leading to unnecessary waste and increased operational costs. Improving the efficiency of acid application while maintaining or enhancing process effectiveness remains a key challenge for refineries.
The variability in crude oil composition also poses challenges in muriatic acid application. Different types of crude oil require varying amounts and concentrations of acid for effective treatment, making it difficult to standardize processes across different refining operations. This variability necessitates adaptive strategies and potentially more complex acid management systems.
Health and safety concerns for refinery workers handling muriatic acid continue to be a significant challenge. Despite protective measures, the risk of exposure to acid fumes or direct contact with the acid remains a constant threat. Developing safer handling methods and improving personal protective equipment are ongoing challenges in the industry.
Regulatory compliance adds another layer of complexity to muriatic acid use in oil refining. Stringent environmental regulations and safety standards require refineries to continuously update their processes and infrastructure, often at significant cost. Balancing regulatory requirements with operational efficiency and cost-effectiveness is an ongoing challenge for the industry.
Another challenge lies in the handling and storage of muriatic acid. Its highly reactive properties necessitate specialized containment systems and stringent safety protocols. Refineries must invest in corrosion-resistant materials and implement robust safety measures to prevent leaks or spills, which can result in hazardous situations and environmental contamination.
The disposal of spent muriatic acid presents a significant environmental challenge. The acid, often contaminated with various hydrocarbons and heavy metals after use in refining processes, requires careful treatment before disposal. This process is not only costly but also energy-intensive, contributing to the overall environmental footprint of refining operations.
Efficiency in acid utilization is another area of concern. Current processes may not fully optimize the use of muriatic acid, leading to unnecessary waste and increased operational costs. Improving the efficiency of acid application while maintaining or enhancing process effectiveness remains a key challenge for refineries.
The variability in crude oil composition also poses challenges in muriatic acid application. Different types of crude oil require varying amounts and concentrations of acid for effective treatment, making it difficult to standardize processes across different refining operations. This variability necessitates adaptive strategies and potentially more complex acid management systems.
Health and safety concerns for refinery workers handling muriatic acid continue to be a significant challenge. Despite protective measures, the risk of exposure to acid fumes or direct contact with the acid remains a constant threat. Developing safer handling methods and improving personal protective equipment are ongoing challenges in the industry.
Regulatory compliance adds another layer of complexity to muriatic acid use in oil refining. Stringent environmental regulations and safety standards require refineries to continuously update their processes and infrastructure, often at significant cost. Balancing regulatory requirements with operational efficiency and cost-effectiveness is an ongoing challenge for the industry.
Existing Muriatic Acid Application Techniques
01 Chemical properties and applications
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.- Chemical properties and applications: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.
- Use in metal treatment and surface preparation: Muriatic acid is widely used in metal treatment processes, including pickling, etching, and surface preparation. It effectively removes oxide layers and contaminants from metal surfaces, improving adhesion for subsequent coatings or treatments. The acid is also used in the production of various metal compounds.
- Environmental and safety considerations: Due to its corrosive nature, handling and disposal of muriatic acid require careful consideration of environmental and safety factors. Proper containment, neutralization, and waste management practices are essential to prevent environmental contamination and ensure worker safety. Specialized equipment and protective measures are often necessary when working with this acid.
- Production and purification methods: Various methods exist for the production and purification of muriatic acid. These include the reaction of sodium chloride with sulfuric acid, direct synthesis from hydrogen and chlorine, and recovery from industrial processes. Purification techniques may involve distillation, membrane separation, or other chemical treatments to remove impurities and achieve desired concentrations.
- Specialized formulations and mixtures: Muriatic acid is often formulated into specialized mixtures or solutions for specific applications. These may include cleaning products, etching solutions, or industrial process chemicals. The acid can be combined with other compounds to enhance its effectiveness or modify its properties for particular uses, such as improving its stability or reducing its corrosiveness.
02 Production and manufacturing processes
Various methods are employed to produce muriatic acid, including the reaction of sodium chloride with sulfuric acid and the direct synthesis from hydrogen and chlorine. Manufacturing processes may involve specialized equipment and safety measures to handle the corrosive nature of the acid.Expand Specific Solutions03 Use in metal treatment and surface preparation
Muriatic acid is widely used in metal treatment processes, including pickling, etching, and surface preparation. It effectively removes oxides, scale, and other impurities from metal surfaces, improving their properties and preparing them for further processing or coating applications.Expand Specific Solutions04 Environmental and safety considerations
Handling and disposal of muriatic acid require careful attention to environmental and safety regulations. Proper storage, transportation, and waste management practices are essential to minimize risks associated with its corrosive nature and potential environmental impact.Expand Specific Solutions05 Specialized applications in various industries
Muriatic acid finds specialized applications in diverse industries, including water treatment, oil and gas, pharmaceuticals, and food processing. It is used for pH adjustment, chlorine production, and as a catalyst in various chemical reactions. The acid's unique properties make it valuable in specific industrial processes and product formulations.Expand Specific Solutions
Key Players in Muriatic Acid and Oil Refining Industries
The competitive landscape for muriatic acid's use in oil refining processes is characterized by a mature industry with established players and steady market growth. The global market size for this application is estimated to be in the billions of dollars, driven by ongoing demand in petroleum refining. Technologically, the process is well-developed, with major oil companies like ExxonMobil, Shell, and TotalEnergies leading innovation. Chinese firms such as PetroChina and Sinopec are also significant players, leveraging their large-scale operations. Research institutes affiliated with these companies, along with specialized chemical suppliers like Arkema and Henkel, continue to refine and optimize the use of muriatic acid in refining processes, focusing on efficiency and environmental impact reduction.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) employs muriatic acid (hydrochloric acid) in various oil refining processes. In their alkylation units, muriatic acid is used as a catalyst to produce high-octane gasoline blending components[1]. Sinopec has developed an improved HCl regeneration process that reduces acid consumption and enhances catalyst efficiency[2]. They also utilize muriatic acid in their desalting operations to neutralize basic compounds and prevent corrosion in downstream units[3]. Additionally, Sinopec has implemented advanced acid management systems to optimize muriatic acid usage and minimize environmental impact across their refineries[4].
Strengths: Extensive experience in large-scale refining operations, advanced acid management systems, and improved HCl regeneration process. Weaknesses: Potential environmental concerns associated with acid handling and disposal.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil Technology & Engineering Co. has developed innovative applications for muriatic acid in oil refining. They use a proprietary HCl-based catalyst system in their alkylation units, which offers improved selectivity and reduced acid consumption compared to traditional sulfuric acid processes[5]. ExxonMobil has also implemented advanced corrosion mitigation techniques, utilizing controlled amounts of muriatic acid to form protective iron sulfide films in refinery equipment[6]. Their research has led to the development of novel acid recovery and recycling systems, significantly reducing fresh acid requirements and waste generation[7]. Furthermore, ExxonMobil employs muriatic acid in their hydroprocessing units to remove metal contaminants from crude oil, enhancing downstream catalyst performance[8].
Strengths: Proprietary HCl-based catalyst system, advanced corrosion mitigation techniques, and innovative acid recovery systems. Weaknesses: Potential higher initial implementation costs for proprietary technologies.
Innovative Muriatic Acid Utilization Methods
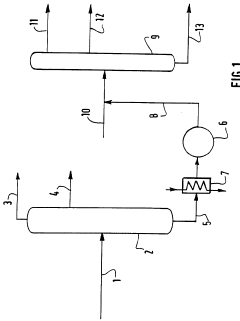
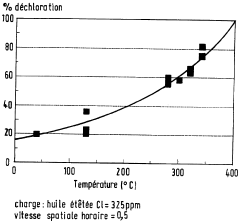
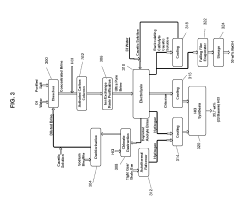
Process for dechlorination of a spent oil fraction
PatentWO1996008546A1
Innovation
- A process involving the use of solid composite products, such as alumina or metal oxides and hydroxides, to adsorb chlorine from used oils before refinery recycling, effectively reducing chlorine content through a dechlorination step at elevated temperatures, allowing for safer and more cost-effective recycling.
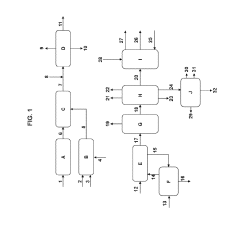
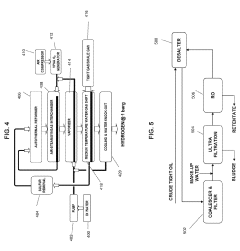
Modular processes for the production of tight gas and tight oil and for tight oil refining
PatentActiveUS10240098B2
Innovation
- A modular system for producing HCl, including a purified salt production facility and a regional modular HCl production facility, which utilizes purified salt to generate HCl through electrolysis, and an autothermal reformer to produce excess hydrogen, integrated with optimized hydraulic fracturing and refining processes for tight gas and tight oil, ensuring efficient operation and reduced transportation requirements.
Environmental Impact of Muriatic Acid in Refining
The use of muriatic acid, also known as hydrochloric acid, in oil refining processes has significant environmental implications that warrant careful consideration. This strong mineral acid plays a crucial role in various stages of petroleum refining, particularly in the removal of impurities and the enhancement of product quality. However, its corrosive nature and potential for environmental contamination pose substantial challenges.
When released into the environment, muriatic acid can have severe impacts on ecosystems. It can dramatically lower the pH of soil and water bodies, leading to acidification. This acidification can harm aquatic life, disrupt plant growth, and affect the overall balance of ecosystems. In extreme cases, it may result in fish kills and the destruction of sensitive habitats.
The atmospheric release of muriatic acid vapors during refining processes contributes to air pollution. These emissions can form acid rain when they interact with moisture in the atmosphere, leading to widespread environmental damage. Acid rain can affect large areas, damaging forests, crops, and infrastructure far from the original source of pollution.
Proper handling and disposal of muriatic acid are critical to mitigating its environmental impact. Refineries must implement robust containment systems and treatment processes to neutralize the acid before its release. This often involves the use of alkaline substances to adjust pH levels and render the acid less harmful. However, the neutralization process itself can generate significant amounts of salt byproducts, which may pose their own environmental challenges if not managed properly.
The potential for accidental spills or leaks of muriatic acid in refineries presents a serious environmental risk. Such incidents can lead to immediate and severe damage to local ecosystems, contaminate groundwater, and pose health risks to nearby communities. Consequently, stringent safety protocols and emergency response plans are essential components of responsible refinery operations.
Regulatory frameworks play a crucial role in managing the environmental impact of muriatic acid use in oil refining. Many countries have implemented strict guidelines for the handling, storage, and disposal of this acid, as well as monitoring requirements for air and water quality in the vicinity of refineries. Compliance with these regulations is not only a legal obligation but also a key factor in maintaining the industry's social license to operate.
As the oil refining industry continues to evolve, there is a growing emphasis on developing more environmentally friendly alternatives to traditional acid-based processes. Research into green chemistry solutions and innovative refining technologies aims to reduce reliance on harsh chemicals like muriatic acid, potentially mitigating long-term environmental impacts while maintaining or improving refining efficiency.
When released into the environment, muriatic acid can have severe impacts on ecosystems. It can dramatically lower the pH of soil and water bodies, leading to acidification. This acidification can harm aquatic life, disrupt plant growth, and affect the overall balance of ecosystems. In extreme cases, it may result in fish kills and the destruction of sensitive habitats.
The atmospheric release of muriatic acid vapors during refining processes contributes to air pollution. These emissions can form acid rain when they interact with moisture in the atmosphere, leading to widespread environmental damage. Acid rain can affect large areas, damaging forests, crops, and infrastructure far from the original source of pollution.
Proper handling and disposal of muriatic acid are critical to mitigating its environmental impact. Refineries must implement robust containment systems and treatment processes to neutralize the acid before its release. This often involves the use of alkaline substances to adjust pH levels and render the acid less harmful. However, the neutralization process itself can generate significant amounts of salt byproducts, which may pose their own environmental challenges if not managed properly.
The potential for accidental spills or leaks of muriatic acid in refineries presents a serious environmental risk. Such incidents can lead to immediate and severe damage to local ecosystems, contaminate groundwater, and pose health risks to nearby communities. Consequently, stringent safety protocols and emergency response plans are essential components of responsible refinery operations.
Regulatory frameworks play a crucial role in managing the environmental impact of muriatic acid use in oil refining. Many countries have implemented strict guidelines for the handling, storage, and disposal of this acid, as well as monitoring requirements for air and water quality in the vicinity of refineries. Compliance with these regulations is not only a legal obligation but also a key factor in maintaining the industry's social license to operate.
As the oil refining industry continues to evolve, there is a growing emphasis on developing more environmentally friendly alternatives to traditional acid-based processes. Research into green chemistry solutions and innovative refining technologies aims to reduce reliance on harsh chemicals like muriatic acid, potentially mitigating long-term environmental impacts while maintaining or improving refining efficiency.
Safety Protocols for Muriatic Acid Handling
Safety protocols for handling muriatic acid in oil refining processes are of paramount importance due to the highly corrosive and hazardous nature of this chemical. Proper handling procedures must be implemented to protect workers, equipment, and the environment from potential harm.
Personal protective equipment (PPE) is the first line of defense when working with muriatic acid. Workers must wear chemical-resistant gloves, protective eyewear, face shields, and acid-resistant clothing. Respiratory protection may also be necessary, depending on the concentration and potential for vapor exposure. All PPE should be regularly inspected and replaced as needed to ensure its effectiveness.
Storage and transportation of muriatic acid require specialized containers made of corrosion-resistant materials such as polyethylene or fiberglass-reinforced plastic. These containers should be properly labeled and stored in well-ventilated areas away from incompatible substances. Secondary containment systems should be in place to prevent spills from spreading in case of a leak or container failure.
Proper training is essential for all personnel involved in handling muriatic acid. This includes education on the chemical properties, potential hazards, proper handling techniques, and emergency response procedures. Regular refresher courses should be conducted to ensure that all workers maintain up-to-date knowledge and skills.
Engineering controls play a crucial role in minimizing exposure risks. These may include the use of closed systems for acid transfer, local exhaust ventilation to remove acid vapors, and automated dosing systems to reduce the need for manual handling. Implementing these controls can significantly reduce the potential for accidents and exposure.
Emergency response planning is a critical component of safety protocols. This includes the installation of emergency showers and eyewash stations in easily accessible locations, as well as the development of clear procedures for spill containment and neutralization. A well-stocked first aid kit specifically designed for acid exposure should be readily available, and personnel should be trained in its use.
Regular maintenance and inspection of all equipment and systems that come into contact with muriatic acid is essential. This includes checking for signs of corrosion, wear, or damage that could lead to leaks or failures. Implementing a preventive maintenance schedule can help identify and address potential issues before they become serious problems.
Proper disposal of muriatic acid and contaminated materials is also a critical safety consideration. This may involve neutralization processes, specialized waste treatment facilities, or recycling programs. Compliance with local, state, and federal regulations regarding hazardous waste disposal is mandatory.
By implementing comprehensive safety protocols for muriatic acid handling in oil refining processes, companies can significantly reduce the risks associated with this hazardous substance and create a safer working environment for their employees.
Personal protective equipment (PPE) is the first line of defense when working with muriatic acid. Workers must wear chemical-resistant gloves, protective eyewear, face shields, and acid-resistant clothing. Respiratory protection may also be necessary, depending on the concentration and potential for vapor exposure. All PPE should be regularly inspected and replaced as needed to ensure its effectiveness.
Storage and transportation of muriatic acid require specialized containers made of corrosion-resistant materials such as polyethylene or fiberglass-reinforced plastic. These containers should be properly labeled and stored in well-ventilated areas away from incompatible substances. Secondary containment systems should be in place to prevent spills from spreading in case of a leak or container failure.
Proper training is essential for all personnel involved in handling muriatic acid. This includes education on the chemical properties, potential hazards, proper handling techniques, and emergency response procedures. Regular refresher courses should be conducted to ensure that all workers maintain up-to-date knowledge and skills.
Engineering controls play a crucial role in minimizing exposure risks. These may include the use of closed systems for acid transfer, local exhaust ventilation to remove acid vapors, and automated dosing systems to reduce the need for manual handling. Implementing these controls can significantly reduce the potential for accidents and exposure.
Emergency response planning is a critical component of safety protocols. This includes the installation of emergency showers and eyewash stations in easily accessible locations, as well as the development of clear procedures for spill containment and neutralization. A well-stocked first aid kit specifically designed for acid exposure should be readily available, and personnel should be trained in its use.
Regular maintenance and inspection of all equipment and systems that come into contact with muriatic acid is essential. This includes checking for signs of corrosion, wear, or damage that could lead to leaks or failures. Implementing a preventive maintenance schedule can help identify and address potential issues before they become serious problems.
Proper disposal of muriatic acid and contaminated materials is also a critical safety consideration. This may involve neutralization processes, specialized waste treatment facilities, or recycling programs. Compliance with local, state, and federal regulations regarding hazardous waste disposal is mandatory.
By implementing comprehensive safety protocols for muriatic acid handling in oil refining processes, companies can significantly reduce the risks associated with this hazardous substance and create a safer working environment for their employees.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!