Environmental Impact of Muriatic Acid Use in pH Regulation
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid pH Regulation Background and Objectives
Muriatic acid, also known as hydrochloric acid, has been a cornerstone in pH regulation across various industries for decades. Its use in controlling acidity levels has evolved significantly since its initial industrial applications in the early 20th century. The primary objective of utilizing muriatic acid for pH regulation is to maintain optimal chemical conditions in processes ranging from water treatment to manufacturing.
The development of pH regulation techniques using muriatic acid has been driven by the increasing demand for precise control in industrial processes. As environmental concerns have grown, the focus has shifted towards not only achieving desired pH levels but also minimizing the ecological impact of acid usage. This dual objective has spurred innovations in application methods and dosing systems.
In recent years, the environmental implications of muriatic acid use have come under scrutiny. While effective in pH adjustment, its corrosive nature and potential for harmful emissions have led to a reevaluation of its role in sustainable industrial practices. The industry now faces the challenge of balancing the efficacy of muriatic acid with the need for environmentally responsible solutions.
The current technological landscape is characterized by efforts to optimize muriatic acid usage through advanced monitoring systems and automated dosing equipment. These developments aim to reduce overall acid consumption while maintaining or improving pH regulation efficiency. Concurrently, research is being conducted into alternative pH regulation methods that could potentially replace or complement muriatic acid use, with a focus on minimizing environmental impact.
As we look towards the future, the objectives for muriatic acid use in pH regulation are multifaceted. There is a pressing need to develop more sustainable practices that reduce the ecological footprint of acid-based pH control. This includes improving containment and handling protocols to prevent accidental releases, as well as exploring recycling and neutralization techniques to mitigate long-term environmental effects.
Additionally, there is a growing emphasis on understanding the lifecycle impact of muriatic acid, from production to disposal. This holistic approach aims to identify areas where environmental improvements can be made throughout the entire process chain. The ultimate goal is to establish pH regulation methods that are not only effective and economically viable but also align with global sustainability targets and stringent environmental regulations.
The development of pH regulation techniques using muriatic acid has been driven by the increasing demand for precise control in industrial processes. As environmental concerns have grown, the focus has shifted towards not only achieving desired pH levels but also minimizing the ecological impact of acid usage. This dual objective has spurred innovations in application methods and dosing systems.
In recent years, the environmental implications of muriatic acid use have come under scrutiny. While effective in pH adjustment, its corrosive nature and potential for harmful emissions have led to a reevaluation of its role in sustainable industrial practices. The industry now faces the challenge of balancing the efficacy of muriatic acid with the need for environmentally responsible solutions.
The current technological landscape is characterized by efforts to optimize muriatic acid usage through advanced monitoring systems and automated dosing equipment. These developments aim to reduce overall acid consumption while maintaining or improving pH regulation efficiency. Concurrently, research is being conducted into alternative pH regulation methods that could potentially replace or complement muriatic acid use, with a focus on minimizing environmental impact.
As we look towards the future, the objectives for muriatic acid use in pH regulation are multifaceted. There is a pressing need to develop more sustainable practices that reduce the ecological footprint of acid-based pH control. This includes improving containment and handling protocols to prevent accidental releases, as well as exploring recycling and neutralization techniques to mitigate long-term environmental effects.
Additionally, there is a growing emphasis on understanding the lifecycle impact of muriatic acid, from production to disposal. This holistic approach aims to identify areas where environmental improvements can be made throughout the entire process chain. The ultimate goal is to establish pH regulation methods that are not only effective and economically viable but also align with global sustainability targets and stringent environmental regulations.
Market Analysis for Eco-friendly pH Regulators
The market for eco-friendly pH regulators has been experiencing significant growth in recent years, driven by increasing environmental concerns and stricter regulations on chemical usage. As industries seek alternatives to traditional pH control methods, particularly those involving muriatic acid, the demand for sustainable solutions has surged.
The global market for eco-friendly pH regulators is projected to expand at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026. This growth is primarily attributed to the rising awareness of environmental issues and the push for sustainable practices across various sectors, including water treatment, agriculture, and industrial processes.
Key market segments for eco-friendly pH regulators include municipal water treatment, industrial wastewater management, and agricultural applications. The water treatment sector, in particular, has shown robust demand due to the increasing need for clean water and the implementation of stringent water quality standards worldwide.
Geographically, North America and Europe lead the market for eco-friendly pH regulators, owing to their stringent environmental regulations and high adoption rates of sustainable technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, urbanization, and increasing environmental awareness in countries like China and India.
The market is characterized by a mix of established players and innovative startups. Major companies in this space are investing heavily in research and development to create more effective and cost-efficient eco-friendly pH regulation solutions. Collaborations between industry players and research institutions are also becoming more common, fostering innovation in this field.
Consumer preferences are shifting towards products that offer not only environmental benefits but also improved performance and cost-effectiveness. This trend is pushing manufacturers to develop multi-functional eco-friendly pH regulators that can address various industrial needs while minimizing environmental impact.
Challenges in the market include the higher initial costs of eco-friendly alternatives compared to traditional pH regulators and the need for extensive education and awareness campaigns to promote adoption. However, as economies of scale improve and regulations tighten, these barriers are expected to diminish over time.
The future outlook for the eco-friendly pH regulator market remains positive, with potential for further growth in emerging economies and new application areas. As technology advances and environmental concerns continue to shape industrial practices, the demand for sustainable pH regulation solutions is likely to increase, presenting significant opportunities for market expansion and innovation.
The global market for eco-friendly pH regulators is projected to expand at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026. This growth is primarily attributed to the rising awareness of environmental issues and the push for sustainable practices across various sectors, including water treatment, agriculture, and industrial processes.
Key market segments for eco-friendly pH regulators include municipal water treatment, industrial wastewater management, and agricultural applications. The water treatment sector, in particular, has shown robust demand due to the increasing need for clean water and the implementation of stringent water quality standards worldwide.
Geographically, North America and Europe lead the market for eco-friendly pH regulators, owing to their stringent environmental regulations and high adoption rates of sustainable technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, urbanization, and increasing environmental awareness in countries like China and India.
The market is characterized by a mix of established players and innovative startups. Major companies in this space are investing heavily in research and development to create more effective and cost-efficient eco-friendly pH regulation solutions. Collaborations between industry players and research institutions are also becoming more common, fostering innovation in this field.
Consumer preferences are shifting towards products that offer not only environmental benefits but also improved performance and cost-effectiveness. This trend is pushing manufacturers to develop multi-functional eco-friendly pH regulators that can address various industrial needs while minimizing environmental impact.
Challenges in the market include the higher initial costs of eco-friendly alternatives compared to traditional pH regulators and the need for extensive education and awareness campaigns to promote adoption. However, as economies of scale improve and regulations tighten, these barriers are expected to diminish over time.
The future outlook for the eco-friendly pH regulator market remains positive, with potential for further growth in emerging economies and new application areas. As technology advances and environmental concerns continue to shape industrial practices, the demand for sustainable pH regulation solutions is likely to increase, presenting significant opportunities for market expansion and innovation.
Current Challenges in Muriatic Acid Usage
The use of muriatic acid (hydrochloric acid) for pH regulation in various industries presents several significant challenges. One of the primary concerns is the potential for environmental contamination. When improperly handled or disposed of, muriatic acid can lead to soil and water pollution, causing severe damage to ecosystems and posing risks to human health. The acid's corrosive nature can also result in the degradation of infrastructure and equipment, leading to increased maintenance costs and potential safety hazards.
Another challenge lies in the transportation and storage of muriatic acid. Due to its highly corrosive properties, specialized containers and handling procedures are required, which can be costly and logistically complex. There is always a risk of accidental spills or leaks during transport or storage, which could have immediate and severe environmental consequences.
The use of muriatic acid also raises occupational health and safety concerns. Workers handling the acid are at risk of chemical burns, respiratory issues, and other health problems if proper safety protocols are not strictly followed. This necessitates comprehensive training programs and the use of appropriate personal protective equipment, adding to operational costs and complexity.
Furthermore, the production of muriatic acid itself has environmental implications. The manufacturing process can result in the release of chlorine gas and other potentially harmful byproducts, contributing to air pollution and greenhouse gas emissions. This raises questions about the overall sustainability of using muriatic acid for pH regulation, especially in light of growing environmental awareness and stricter regulations.
There are also challenges related to the precise control of pH levels when using muriatic acid. Over-application can lead to rapid and potentially harmful shifts in pH, which can be particularly problematic in sensitive applications such as water treatment or food processing. This requires careful monitoring and control systems, adding to the complexity and cost of operations.
Lastly, there is growing public concern and regulatory scrutiny regarding the use of strong acids in industrial processes. This has led to increased pressure on industries to find safer, more environmentally friendly alternatives for pH regulation. The challenge lies in identifying and implementing alternatives that are as effective and economically viable as muriatic acid, while also meeting stringent environmental and safety standards.
Another challenge lies in the transportation and storage of muriatic acid. Due to its highly corrosive properties, specialized containers and handling procedures are required, which can be costly and logistically complex. There is always a risk of accidental spills or leaks during transport or storage, which could have immediate and severe environmental consequences.
The use of muriatic acid also raises occupational health and safety concerns. Workers handling the acid are at risk of chemical burns, respiratory issues, and other health problems if proper safety protocols are not strictly followed. This necessitates comprehensive training programs and the use of appropriate personal protective equipment, adding to operational costs and complexity.
Furthermore, the production of muriatic acid itself has environmental implications. The manufacturing process can result in the release of chlorine gas and other potentially harmful byproducts, contributing to air pollution and greenhouse gas emissions. This raises questions about the overall sustainability of using muriatic acid for pH regulation, especially in light of growing environmental awareness and stricter regulations.
There are also challenges related to the precise control of pH levels when using muriatic acid. Over-application can lead to rapid and potentially harmful shifts in pH, which can be particularly problematic in sensitive applications such as water treatment or food processing. This requires careful monitoring and control systems, adding to the complexity and cost of operations.
Lastly, there is growing public concern and regulatory scrutiny regarding the use of strong acids in industrial processes. This has led to increased pressure on industries to find safer, more environmentally friendly alternatives for pH regulation. The challenge lies in identifying and implementing alternatives that are as effective and economically viable as muriatic acid, while also meeting stringent environmental and safety standards.
Existing Muriatic Acid Alternatives
01 Environmental monitoring and assessment
Systems and methods for monitoring and assessing the environmental impact of muriatic acid, including its effects on air and water quality. These approaches involve data collection, analysis, and reporting to evaluate the acid's ecological footprint and potential risks to ecosystems.- Environmental impact assessment of muriatic acid: Evaluating the environmental effects of muriatic acid, also known as hydrochloric acid, involves assessing its potential impacts on air, water, and soil quality. This includes analyzing emissions, waste management, and potential ecological risks associated with its production and use in various industries.
- Waste management and disposal methods: Proper waste management and disposal techniques for muriatic acid are crucial to minimize its environmental impact. This includes neutralization processes, treatment methods, and safe disposal practices to prevent contamination of water bodies and soil.
- Monitoring and control systems for acid-related processes: Implementation of advanced monitoring and control systems in industrial processes involving muriatic acid can help reduce environmental risks. These systems can optimize acid usage, detect leaks, and ensure compliance with environmental regulations.
- Eco-friendly alternatives and substitutes: Research and development of environmentally friendly alternatives or substitutes for muriatic acid in various applications can help mitigate its environmental impact. This includes exploring less harmful chemicals or processes that can achieve similar results with reduced ecological footprint.
- Lifecycle assessment and sustainability practices: Conducting comprehensive lifecycle assessments of muriatic acid production and use can identify areas for improving sustainability. This involves analyzing the entire supply chain, from raw material extraction to end-of-life disposal, to implement more environmentally responsible practices.
02 Waste management and disposal techniques
Innovative strategies for managing and disposing of muriatic acid waste to minimize environmental harm. This includes neutralization processes, recycling methods, and safe disposal practices that reduce the acid's impact on soil and water resources.Expand Specific Solutions03 Eco-friendly alternatives and substitutes
Research and development of environmentally friendly alternatives to muriatic acid for various industrial applications. These substitutes aim to reduce the environmental footprint while maintaining effectiveness in cleaning, etching, and other processes.Expand Specific Solutions04 Mitigation of acid rain and atmospheric pollution
Technologies and methods to mitigate the contribution of muriatic acid to acid rain formation and atmospheric pollution. This includes emission control systems, improved manufacturing processes, and strategies to neutralize acidic emissions before they enter the environment.Expand Specific Solutions05 Lifecycle assessment and sustainability practices
Comprehensive lifecycle assessments of muriatic acid production, use, and disposal to identify areas for improving sustainability. This involves analyzing energy consumption, resource utilization, and environmental impacts throughout the acid's lifecycle to develop more sustainable practices in its production and application.Expand Specific Solutions
Key Players in pH Regulation Industry
The environmental impact of muriatic acid in pH regulation is a complex issue within a mature industry, yet with growing concerns about sustainability. The market for pH regulation chemicals is substantial, driven by diverse industrial applications. Technologically, companies like BASF Corp., Evoqua Water Technologies, and Fluid Energy Group are at the forefront, developing more environmentally friendly alternatives. Research institutions such as Penn State Research Foundation and SISSA are contributing to advancements in this field. The competition is intense, with established players like Dorf Ketal Chemicals and ChemTreat competing against innovative startups, all striving to balance effectiveness with reduced environmental impact.
Evoqua Water Technologies LLC
Technical Solution: Evoqua Water Technologies LLC has pioneered an electrochemical approach to pH regulation that significantly reduces the need for muriatic acid. Their system utilizes electrolysis to generate hydrogen ions in situ, effectively lowering pH without the addition of external acids[2]. This process is controlled by advanced sensors and automation, ensuring precise pH adjustment. The technology also incorporates a closed-loop system that recycles and reuses water, minimizing waste and reducing the overall environmental impact[4]. Additionally, Evoqua has developed specialized electrode materials that enhance the efficiency of the electrolysis process, further reducing energy consumption and improving the system's sustainability[5].
Strengths: Elimination of acid storage and handling, reduced chemical transportation, and improved safety. Weaknesses: Higher energy consumption and potential limitations in large-scale applications.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative solutions for pH regulation that minimize the environmental impact of muriatic acid use. Their approach involves the use of advanced polymer-based pH adjusters that can effectively regulate pH levels while significantly reducing the amount of muriatic acid required. This technology incorporates specially designed polymeric compounds that act as buffers, maintaining stable pH levels over extended periods[1]. The polymers are biodegradable and have a lower environmental footprint compared to traditional mineral acids. BASF's solution also includes a smart dosing system that optimizes the application of pH regulators, further reducing chemical consumption and potential environmental contamination[3].
Strengths: Reduced chemical usage, lower environmental impact, and improved pH stability. Weaknesses: Higher initial costs and potential need for system modifications to implement the technology.
Innovations in Eco-friendly pH Regulation
Stabilized modified acid pre-blends
PatentWO2023214360A1
Innovation
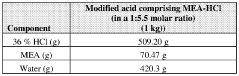
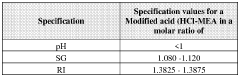
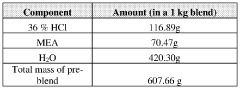
- A method to stabilize an aqueous composition of alkanolamine by adding a first acidic component, maintaining a pH between 7 and 11, and adjusting the molar ratio of alkanolamine to hydrochloric acid to achieve a stable modified acid composition that is resistant to temperature fluctuations, reduces corrosion, and is biodegradable, allowing for safer handling and more effective use in a wide range of oil and gas applications.
Synthetic acid compositions and uses thereof
PatentActiveCA2925142A1
Innovation
- A synthetic acid composition comprising urea and hydrogen chloride in a specific molar ratio, combined with metal iodides, alcohols, and phosphonic acids, which reduces corrosion rates, is non-fuming, non-toxic, and biodegradable, offering improved safety and environmental compatibility.
Environmental Regulations on Chemical Usage
The use of muriatic acid (hydrochloric acid) in pH regulation is subject to stringent environmental regulations due to its potential hazards and environmental impacts. These regulations are designed to protect human health, ecosystems, and water resources from the adverse effects of chemical pollution.
At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the regulation of muriatic acid under various statutes. The Toxic Substances Control Act (TSCA) requires manufacturers and importers to report information on chemical production and potential risks. The Clean Water Act (CWA) regulates the discharge of muriatic acid into water bodies, setting limits on pH levels and requiring permits for industrial effluents.
The Resource Conservation and Recovery Act (RCRA) governs the proper disposal of hazardous waste, including spent muriatic acid. Facilities using large quantities of muriatic acid must comply with the Emergency Planning and Community Right-to-Know Act (EPCRA), which mandates reporting of chemical inventories and releases to local authorities.
Occupational safety regulations, enforced by the Occupational Safety and Health Administration (OSHA), require proper handling, storage, and use of muriatic acid in workplace settings. This includes providing personal protective equipment, safety training, and maintaining safety data sheets.
At the state and local levels, additional regulations may apply. Many states have adopted more stringent requirements for chemical usage and disposal. Some local jurisdictions have implemented ordinances restricting the sale or use of muriatic acid due to its potential for misuse in illegal drug manufacturing.
Internationally, the use of muriatic acid is regulated under various agreements and conventions. The Stockholm Convention on Persistent Organic Pollutants, while not directly addressing muriatic acid, influences the broader landscape of chemical regulation. The Rotterdam Convention governs the international trade of hazardous chemicals, including certain formulations containing hydrochloric acid.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires manufacturers and importers to register chemical substances and provide safety information. This comprehensive approach to chemical management has influenced global standards for chemical regulation.
Compliance with these regulations often requires businesses to implement robust environmental management systems, conduct regular monitoring and reporting, and invest in pollution control technologies. As environmental concerns continue to grow, it is likely that regulations governing the use of muriatic acid and other potentially harmful chemicals will become increasingly stringent, driving innovation in safer alternatives and more sustainable practices for pH regulation.
At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the regulation of muriatic acid under various statutes. The Toxic Substances Control Act (TSCA) requires manufacturers and importers to report information on chemical production and potential risks. The Clean Water Act (CWA) regulates the discharge of muriatic acid into water bodies, setting limits on pH levels and requiring permits for industrial effluents.
The Resource Conservation and Recovery Act (RCRA) governs the proper disposal of hazardous waste, including spent muriatic acid. Facilities using large quantities of muriatic acid must comply with the Emergency Planning and Community Right-to-Know Act (EPCRA), which mandates reporting of chemical inventories and releases to local authorities.
Occupational safety regulations, enforced by the Occupational Safety and Health Administration (OSHA), require proper handling, storage, and use of muriatic acid in workplace settings. This includes providing personal protective equipment, safety training, and maintaining safety data sheets.
At the state and local levels, additional regulations may apply. Many states have adopted more stringent requirements for chemical usage and disposal. Some local jurisdictions have implemented ordinances restricting the sale or use of muriatic acid due to its potential for misuse in illegal drug manufacturing.
Internationally, the use of muriatic acid is regulated under various agreements and conventions. The Stockholm Convention on Persistent Organic Pollutants, while not directly addressing muriatic acid, influences the broader landscape of chemical regulation. The Rotterdam Convention governs the international trade of hazardous chemicals, including certain formulations containing hydrochloric acid.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires manufacturers and importers to register chemical substances and provide safety information. This comprehensive approach to chemical management has influenced global standards for chemical regulation.
Compliance with these regulations often requires businesses to implement robust environmental management systems, conduct regular monitoring and reporting, and invest in pollution control technologies. As environmental concerns continue to grow, it is likely that regulations governing the use of muriatic acid and other potentially harmful chemicals will become increasingly stringent, driving innovation in safer alternatives and more sustainable practices for pH regulation.
Life Cycle Assessment of pH Regulators
Life Cycle Assessment (LCA) is a crucial tool for evaluating the environmental impacts of pH regulators throughout their entire lifecycle, from raw material extraction to disposal. In the context of muriatic acid (hydrochloric acid) used for pH regulation, LCA provides valuable insights into its environmental footprint compared to alternative pH regulators.
The assessment begins with the production phase, where muriatic acid is typically manufactured as a byproduct of chlorine production through the chloralkali process. This process involves the electrolysis of sodium chloride solution, which requires significant energy input and may result in emissions of chlorine gas and mercury if not properly controlled. The transportation of raw materials and finished products also contributes to the overall environmental impact through fuel consumption and associated emissions.
During the use phase, muriatic acid's high reactivity and corrosive nature necessitate careful handling and storage. Proper containment systems and safety measures are essential to prevent accidental releases, which could have severe environmental consequences. The acid's interaction with water bodies and soil can lead to localized pH changes, potentially affecting aquatic ecosystems and soil chemistry.
The disposal of muriatic acid and its byproducts presents another critical stage in its lifecycle. Neutralization is often required before disposal, which involves additional chemical inputs and energy consumption. Improper disposal can lead to soil and water contamination, highlighting the importance of adherence to environmental regulations and best practices.
When comparing muriatic acid to alternative pH regulators, such as sulfuric acid or carbon dioxide, LCA reveals trade-offs in environmental impacts. While muriatic acid may have advantages in certain applications due to its effectiveness and relatively low cost, its production and handling may result in higher environmental burdens in some categories, such as acidification potential and ecotoxicity.
The LCA also considers the potential for recycling and reuse of muriatic acid in industrial processes, which can significantly reduce its overall environmental impact. Closed-loop systems and recovery technologies can extend the useful life of the acid, minimizing waste and the need for new production.
In conclusion, a comprehensive LCA of muriatic acid as a pH regulator provides decision-makers with a holistic view of its environmental implications. This assessment enables informed choices between different pH regulation strategies, considering not only immediate effectiveness but also long-term sustainability and environmental stewardship.
The assessment begins with the production phase, where muriatic acid is typically manufactured as a byproduct of chlorine production through the chloralkali process. This process involves the electrolysis of sodium chloride solution, which requires significant energy input and may result in emissions of chlorine gas and mercury if not properly controlled. The transportation of raw materials and finished products also contributes to the overall environmental impact through fuel consumption and associated emissions.
During the use phase, muriatic acid's high reactivity and corrosive nature necessitate careful handling and storage. Proper containment systems and safety measures are essential to prevent accidental releases, which could have severe environmental consequences. The acid's interaction with water bodies and soil can lead to localized pH changes, potentially affecting aquatic ecosystems and soil chemistry.
The disposal of muriatic acid and its byproducts presents another critical stage in its lifecycle. Neutralization is often required before disposal, which involves additional chemical inputs and energy consumption. Improper disposal can lead to soil and water contamination, highlighting the importance of adherence to environmental regulations and best practices.
When comparing muriatic acid to alternative pH regulators, such as sulfuric acid or carbon dioxide, LCA reveals trade-offs in environmental impacts. While muriatic acid may have advantages in certain applications due to its effectiveness and relatively low cost, its production and handling may result in higher environmental burdens in some categories, such as acidification potential and ecotoxicity.
The LCA also considers the potential for recycling and reuse of muriatic acid in industrial processes, which can significantly reduce its overall environmental impact. Closed-loop systems and recovery technologies can extend the useful life of the acid, minimizing waste and the need for new production.
In conclusion, a comprehensive LCA of muriatic acid as a pH regulator provides decision-makers with a holistic view of its environmental implications. This assessment enables informed choices between different pH regulation strategies, considering not only immediate effectiveness but also long-term sustainability and environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!