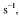What are the Long-Term Environmental Effects of Muriatic Acid?
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid Overview and Research Objectives
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with significant industrial and commercial applications. This corrosive substance has been utilized for centuries in various sectors, including manufacturing, construction, and household cleaning. As we delve into the long-term environmental effects of muriatic acid, it is crucial to establish a comprehensive understanding of its properties, uses, and potential impacts on ecosystems.
The primary objective of this research is to evaluate the prolonged consequences of muriatic acid exposure on the environment. This investigation aims to assess the acid's persistence in soil and water systems, its interactions with flora and fauna, and its potential to alter ecosystem dynamics over extended periods. By examining these aspects, we seek to provide valuable insights for environmental management strategies and regulatory frameworks.
Muriatic acid is characterized by its high reactivity and ability to dissolve various materials. In its pure form, it is a colorless, highly corrosive liquid with a pungent odor. The acid's chemical formula, HCl, indicates its composition of hydrogen and chlorine atoms. Its strong acidic nature makes it effective for numerous applications but also raises concerns about its environmental impact when released into natural systems.
The research objectives encompass several key areas of investigation. Firstly, we aim to analyze the acid's behavior in different environmental matrices, including soil, water bodies, and atmospheric conditions. This includes studying its dissociation patterns, mobility, and potential for bioaccumulation in various organisms. Secondly, we will explore the acid's effects on soil chemistry, focusing on changes in pH levels, nutrient availability, and microbial communities essential for ecosystem health.
Furthermore, our investigation will extend to aquatic environments, examining how muriatic acid influences water quality, aquatic life, and the overall balance of freshwater and marine ecosystems. We will also consider the potential for long-term alterations in habitat structures and food chains resulting from prolonged exposure to the acid.
Another critical aspect of our research is to evaluate the acid's interaction with other pollutants and its role in exacerbating or mitigating existing environmental issues. This includes assessing its potential to mobilize heavy metals in soil and sediments, which could lead to increased bioavailability and toxicity of these contaminants.
By addressing these research objectives, we aim to contribute to a more comprehensive understanding of muriatic acid's long-term environmental effects. This knowledge will be instrumental in developing more effective environmental protection measures, improving industrial practices, and informing policy decisions related to the use and disposal of this widely used acid.
The primary objective of this research is to evaluate the prolonged consequences of muriatic acid exposure on the environment. This investigation aims to assess the acid's persistence in soil and water systems, its interactions with flora and fauna, and its potential to alter ecosystem dynamics over extended periods. By examining these aspects, we seek to provide valuable insights for environmental management strategies and regulatory frameworks.
Muriatic acid is characterized by its high reactivity and ability to dissolve various materials. In its pure form, it is a colorless, highly corrosive liquid with a pungent odor. The acid's chemical formula, HCl, indicates its composition of hydrogen and chlorine atoms. Its strong acidic nature makes it effective for numerous applications but also raises concerns about its environmental impact when released into natural systems.
The research objectives encompass several key areas of investigation. Firstly, we aim to analyze the acid's behavior in different environmental matrices, including soil, water bodies, and atmospheric conditions. This includes studying its dissociation patterns, mobility, and potential for bioaccumulation in various organisms. Secondly, we will explore the acid's effects on soil chemistry, focusing on changes in pH levels, nutrient availability, and microbial communities essential for ecosystem health.
Furthermore, our investigation will extend to aquatic environments, examining how muriatic acid influences water quality, aquatic life, and the overall balance of freshwater and marine ecosystems. We will also consider the potential for long-term alterations in habitat structures and food chains resulting from prolonged exposure to the acid.
Another critical aspect of our research is to evaluate the acid's interaction with other pollutants and its role in exacerbating or mitigating existing environmental issues. This includes assessing its potential to mobilize heavy metals in soil and sediments, which could lead to increased bioavailability and toxicity of these contaminants.
By addressing these research objectives, we aim to contribute to a more comprehensive understanding of muriatic acid's long-term environmental effects. This knowledge will be instrumental in developing more effective environmental protection measures, improving industrial practices, and informing policy decisions related to the use and disposal of this widely used acid.
Environmental Impact Assessment
The long-term environmental effects of muriatic acid, also known as hydrochloric acid, are significant and multifaceted. When released into the environment, this strong mineral acid can cause severe damage to ecosystems, particularly aquatic environments. In water bodies, muriatic acid lowers the pH, leading to acidification that can harm or kill fish, plants, and other aquatic organisms. This acidification can persist for extended periods, disrupting the delicate balance of aquatic ecosystems.
Soil contamination is another major concern. When muriatic acid seeps into the ground, it can alter soil chemistry, affecting plant growth and microbial communities essential for soil health. The acid can also mobilize heavy metals present in the soil, making them more bioavailable and potentially toxic to plants and animals. This mobilization can lead to the accumulation of harmful substances in the food chain, posing risks to higher-level consumers, including humans.
The impact on air quality should not be overlooked. While muriatic acid is not highly volatile, its vapors can contribute to air pollution, particularly in industrial settings where large quantities are used. These vapors can cause respiratory irritation in humans and animals and may contribute to acid rain formation when released in significant amounts.
Long-term exposure to muriatic acid in the environment can lead to the degradation of infrastructure. Buildings, bridges, and other structures exposed to acid-contaminated air or water may experience accelerated corrosion, leading to structural weaknesses and increased maintenance costs. This effect is particularly pronounced in urban areas with high industrial activity.
The impact on biodiversity is a critical long-term concern. Ecosystems exposed to muriatic acid pollution may experience shifts in species composition, favoring acid-tolerant organisms and potentially leading to a loss of sensitive species. This can result in reduced biodiversity and altered ecosystem functions, which may take decades to recover, if at all.
Groundwater contamination is another significant long-term effect. Muriatic acid can percolate through soil layers and eventually reach groundwater aquifers. Once contaminated, these water sources can remain unsafe for consumption or agricultural use for extended periods, potentially affecting human health and food security in affected areas.
Addressing these long-term environmental effects requires comprehensive monitoring, stringent regulations on industrial use and disposal, and innovative remediation techniques. Developing and implementing sustainable alternatives to muriatic acid in various applications is crucial for mitigating its environmental impact and ensuring the long-term health of ecosystems and human communities.
Soil contamination is another major concern. When muriatic acid seeps into the ground, it can alter soil chemistry, affecting plant growth and microbial communities essential for soil health. The acid can also mobilize heavy metals present in the soil, making them more bioavailable and potentially toxic to plants and animals. This mobilization can lead to the accumulation of harmful substances in the food chain, posing risks to higher-level consumers, including humans.
The impact on air quality should not be overlooked. While muriatic acid is not highly volatile, its vapors can contribute to air pollution, particularly in industrial settings where large quantities are used. These vapors can cause respiratory irritation in humans and animals and may contribute to acid rain formation when released in significant amounts.
Long-term exposure to muriatic acid in the environment can lead to the degradation of infrastructure. Buildings, bridges, and other structures exposed to acid-contaminated air or water may experience accelerated corrosion, leading to structural weaknesses and increased maintenance costs. This effect is particularly pronounced in urban areas with high industrial activity.
The impact on biodiversity is a critical long-term concern. Ecosystems exposed to muriatic acid pollution may experience shifts in species composition, favoring acid-tolerant organisms and potentially leading to a loss of sensitive species. This can result in reduced biodiversity and altered ecosystem functions, which may take decades to recover, if at all.
Groundwater contamination is another significant long-term effect. Muriatic acid can percolate through soil layers and eventually reach groundwater aquifers. Once contaminated, these water sources can remain unsafe for consumption or agricultural use for extended periods, potentially affecting human health and food security in affected areas.
Addressing these long-term environmental effects requires comprehensive monitoring, stringent regulations on industrial use and disposal, and innovative remediation techniques. Developing and implementing sustainable alternatives to muriatic acid in various applications is crucial for mitigating its environmental impact and ensuring the long-term health of ecosystems and human communities.
Current Challenges in Acid Pollution Monitoring
Monitoring acid pollution presents significant challenges in environmental science and management. The complexity of acid pollution stems from its diverse sources, ranging from industrial emissions to agricultural runoff, making comprehensive monitoring a daunting task. One of the primary challenges is the wide geographical spread of acid pollution, which requires extensive monitoring networks to capture accurate data across various ecosystems.
The dynamic nature of acid pollution further complicates monitoring efforts. Seasonal variations, weather patterns, and human activities can cause rapid fluctuations in acid levels, necessitating continuous and real-time monitoring systems. However, implementing such systems on a large scale is both technically challenging and resource-intensive.
Another critical challenge lies in the detection and measurement of different acid types. While some acids, like sulfuric acid from industrial emissions, are well-known and easier to detect, others, such as organic acids from natural sources, can be more elusive. This diversity requires sophisticated analytical techniques and equipment, which may not be readily available or feasible for widespread deployment.
The long-term effects of acid pollution, particularly from substances like muriatic acid, pose a unique monitoring challenge. These effects often manifest gradually over extended periods, making it difficult to establish clear cause-and-effect relationships. Monitoring programs must be designed to capture both acute and chronic impacts, requiring sustained, long-term commitment and funding.
Standardization of monitoring methods and data interpretation across different regions and countries is another significant hurdle. The lack of uniform protocols can lead to inconsistencies in data collection and analysis, hampering global efforts to address acid pollution effectively.
Furthermore, the interaction of acid pollution with other environmental factors, such as climate change and biodiversity loss, adds layers of complexity to monitoring efforts. Understanding these interactions requires interdisciplinary approaches and advanced modeling techniques, which are still evolving.
Lastly, the translation of monitoring data into actionable policy and mitigation strategies remains a challenge. Effective communication of complex scientific data to policymakers and the public is crucial for driving meaningful change in acid pollution management. Overcoming these challenges requires innovative technologies, collaborative research efforts, and sustained commitment from governments, industries, and scientific communities worldwide.
The dynamic nature of acid pollution further complicates monitoring efforts. Seasonal variations, weather patterns, and human activities can cause rapid fluctuations in acid levels, necessitating continuous and real-time monitoring systems. However, implementing such systems on a large scale is both technically challenging and resource-intensive.
Another critical challenge lies in the detection and measurement of different acid types. While some acids, like sulfuric acid from industrial emissions, are well-known and easier to detect, others, such as organic acids from natural sources, can be more elusive. This diversity requires sophisticated analytical techniques and equipment, which may not be readily available or feasible for widespread deployment.
The long-term effects of acid pollution, particularly from substances like muriatic acid, pose a unique monitoring challenge. These effects often manifest gradually over extended periods, making it difficult to establish clear cause-and-effect relationships. Monitoring programs must be designed to capture both acute and chronic impacts, requiring sustained, long-term commitment and funding.
Standardization of monitoring methods and data interpretation across different regions and countries is another significant hurdle. The lack of uniform protocols can lead to inconsistencies in data collection and analysis, hampering global efforts to address acid pollution effectively.
Furthermore, the interaction of acid pollution with other environmental factors, such as climate change and biodiversity loss, adds layers of complexity to monitoring efforts. Understanding these interactions requires interdisciplinary approaches and advanced modeling techniques, which are still evolving.
Lastly, the translation of monitoring data into actionable policy and mitigation strategies remains a challenge. Effective communication of complex scientific data to policymakers and the public is crucial for driving meaningful change in acid pollution management. Overcoming these challenges requires innovative technologies, collaborative research efforts, and sustained commitment from governments, industries, and scientific communities worldwide.
Existing Mitigation Strategies
01 Acid neutralization and waste treatment
Muriatic acid's environmental effects can be mitigated through neutralization processes and waste treatment methods. These techniques involve converting the acid into less harmful substances and properly managing the resulting waste to minimize environmental impact.- Aquatic ecosystem impact: Muriatic acid, also known as hydrochloric acid, can have significant effects on aquatic ecosystems. When released into water bodies, it can lower the pH, potentially harming aquatic life and disrupting the balance of marine environments. The acid can also react with minerals in water, affecting water quality and potentially releasing harmful substances.
- Atmospheric pollution: The release of muriatic acid vapors into the atmosphere can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming acid rain or other harmful substances. This can have far-reaching effects on both terrestrial and aquatic ecosystems, as well as human health.
- Soil contamination: When muriatic acid is spilled or improperly disposed of, it can contaminate soil. This can lead to changes in soil pH, affecting plant growth and soil microorganisms. The acid can also mobilize heavy metals in the soil, potentially increasing their bioavailability and toxicity to plants and animals.
- Waste management and recycling: Proper management and recycling of muriatic acid waste are crucial to mitigate its environmental impact. This includes neutralization processes, treatment methods, and recycling techniques to reduce the amount of acid released into the environment. Effective waste management can significantly decrease the potential for environmental harm.
- Monitoring and remediation: Environmental monitoring systems and remediation techniques are essential for assessing and mitigating the effects of muriatic acid on the environment. This includes the development of sensors for detecting acid levels in various environmental media, as well as methods for neutralizing or removing acid contamination from affected areas.
02 Corrosion prevention in industrial applications
When used in industrial processes, muriatic acid can contribute to corrosion. Implementing corrosion prevention techniques and using appropriate materials can help reduce the environmental impact of acid-induced degradation in industrial settings.Expand Specific Solutions03 Aquatic ecosystem impact and monitoring
Muriatic acid can have significant effects on aquatic ecosystems. Monitoring techniques and environmental assessment methods are crucial for understanding and mitigating the acid's impact on water bodies and aquatic life.Expand Specific Solutions04 Soil contamination and remediation
Muriatic acid can contaminate soil, affecting its pH and nutrient composition. Soil remediation techniques and strategies for managing acid-contaminated land are essential for minimizing long-term environmental damage.Expand Specific Solutions05 Air quality and emissions control
The use and production of muriatic acid can result in air pollution through emissions and vapors. Implementing effective air quality control measures and emission reduction technologies is crucial for minimizing the acid's impact on atmospheric conditions.Expand Specific Solutions
Key Stakeholders in Acid Management
The environmental effects of muriatic acid present a complex competitive landscape, reflecting an industry in transition. The market is experiencing moderate growth, driven by increasing industrial applications and environmental concerns. While the technology for acid production is mature, innovations in containment, neutralization, and ecological impact mitigation are emerging. Companies like Corning, Inc. and POSCO Holdings are at the forefront, developing advanced materials and processes to address long-term environmental challenges. Academic institutions such as McGill University and China Agricultural University are contributing valuable research on ecological impacts and remediation strategies, fostering collaborations between industry and academia to advance sustainable solutions in this field.
Corning, Inc.
Technical Solution: Corning, Inc. has developed specialized glass and ceramic materials resistant to muriatic acid corrosion, which can help mitigate long-term environmental effects. Their approach includes: 1) Creating acid-resistant linings for storage tanks and pipelines, reducing the risk of leaks and spills[4]. 2) Developing advanced filtration membranes that can effectively remove acid contaminants from wastewater[5]. 3) Producing corrosion-resistant sensors for real-time monitoring of acid levels in industrial processes and environmental settings. These innovations help prevent accidental releases and enable more efficient acid management, thereby reducing potential environmental impacts over time.
Strengths: Durable and long-lasting solutions, reduces risk of accidental environmental contamination. Weaknesses: Limited to preventive measures, does not address existing contamination directly.
WM Intellectual Property Holdings LLC
Technical Solution: WM Intellectual Property Holdings LLC, associated with Waste Management, has developed innovative waste treatment and disposal methods for muriatic acid and its byproducts. Their approach includes: 1) Advanced chemical stabilization techniques to render acid waste less reactive and harmful[6]. 2) Engineered landfill designs with multiple protective layers to prevent acid leachate from contaminating soil and groundwater[7]. 3) Closed-loop recycling systems for industrial processes using muriatic acid, minimizing waste generation. 4) Development of biodegradable alternatives to muriatic acid for certain applications, reducing long-term environmental impact. These strategies aim to minimize the acid's potential for long-term environmental damage through proper handling, treatment, and disposal.
Strengths: Comprehensive waste management approach, addresses multiple stages of acid lifecycle. Weaknesses: Some methods may be energy-intensive or costly to implement on a large scale.
Innovative Detection and Neutralization Methods
Using synthetic acid compositions as alternatives to conventional acids in the oil and gas industry
PatentWO2017165954A1
Innovation
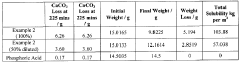
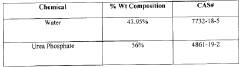
- A synthetic acid composition comprising urea and a phosphoric acid derivative in a specific molar ratio, which is chrome-friendly, non-fuming, and stable across a wide temperature range, reducing corrosion and environmental impact while maintaining effectiveness in scaling treatments and fluid compatibility.
Stabilizing aqueous lysine-hcl compositions
PatentWO2023139538A1
Innovation
- A method to stabilize an aqueous composition of lysine monohydrochloride by adding a predetermined amount of hydrochloric acid, creating a reconstituted modified acid composition that maintains stability at temperatures below 7 °C and can be shipped in water tanks, allowing for a safer and more effective acid solution for oil and gas operations.
Regulatory Framework for Industrial Acid Use
The regulatory framework for industrial acid use, particularly concerning muriatic acid (hydrochloric acid), is a complex and evolving landscape designed to mitigate long-term environmental impacts. At the federal level in the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating the use, storage, and disposal of industrial acids under various statutes, including the Clean Air Act, Clean Water Act, and Resource Conservation and Recovery Act.
The Occupational Safety and Health Administration (OSHA) complements these efforts by establishing workplace safety standards for handling acids, including muriatic acid. These regulations mandate proper storage, handling procedures, and personal protective equipment to minimize risks to workers and potential environmental contamination.
State-level regulations often build upon federal guidelines, sometimes imposing stricter controls. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm, which includes certain industrial acids.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those of industrial acids. This system has been adopted by many countries, facilitating consistent global management of chemical risks.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework that impacts the use of industrial acids. It requires companies to register chemical substances and provide safety data, promoting the responsible use of potentially harmful substances.
Specific to muriatic acid, regulations often focus on its corrosive properties and potential for environmental harm. Guidelines typically address proper containment, neutralization procedures for spills, and disposal methods to prevent soil and water contamination. Many jurisdictions require detailed record-keeping of acid use and disposal, as well as regular environmental impact assessments for facilities using significant quantities of acids.
As scientific understanding of long-term environmental effects evolves, regulatory frameworks are periodically updated. Recent trends include increased emphasis on lifecycle management of chemicals, stricter emissions controls, and more comprehensive environmental monitoring requirements. These evolving regulations aim to balance industrial needs with long-term environmental protection, recognizing the persistent nature of acid-related environmental impacts.
The Occupational Safety and Health Administration (OSHA) complements these efforts by establishing workplace safety standards for handling acids, including muriatic acid. These regulations mandate proper storage, handling procedures, and personal protective equipment to minimize risks to workers and potential environmental contamination.
State-level regulations often build upon federal guidelines, sometimes imposing stricter controls. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm, which includes certain industrial acids.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those of industrial acids. This system has been adopted by many countries, facilitating consistent global management of chemical risks.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework that impacts the use of industrial acids. It requires companies to register chemical substances and provide safety data, promoting the responsible use of potentially harmful substances.
Specific to muriatic acid, regulations often focus on its corrosive properties and potential for environmental harm. Guidelines typically address proper containment, neutralization procedures for spills, and disposal methods to prevent soil and water contamination. Many jurisdictions require detailed record-keeping of acid use and disposal, as well as regular environmental impact assessments for facilities using significant quantities of acids.
As scientific understanding of long-term environmental effects evolves, regulatory frameworks are periodically updated. Recent trends include increased emphasis on lifecycle management of chemicals, stricter emissions controls, and more comprehensive environmental monitoring requirements. These evolving regulations aim to balance industrial needs with long-term environmental protection, recognizing the persistent nature of acid-related environmental impacts.
Ecosystem Resilience and Recovery Studies
Long-term studies on ecosystem resilience and recovery following muriatic acid exposure are crucial for understanding the environmental impacts of this corrosive substance. Research has shown that ecosystems exposed to muriatic acid can exhibit varying degrees of resilience, depending on factors such as the concentration of acid, duration of exposure, and the specific characteristics of the affected ecosystem.
Aquatic ecosystems, particularly freshwater systems, have been the focus of numerous recovery studies. These investigations have revealed that while initial acid exposure can cause significant harm to aquatic life, many ecosystems demonstrate remarkable recovery potential over time. The recovery process typically begins with the recolonization of acid-tolerant species, followed by a gradual return of more sensitive organisms as pH levels stabilize.
Soil ecosystems have also been extensively studied in the context of acid recovery. Research has shown that soil microorganisms play a crucial role in ecosystem resilience, with some microbial communities adapting to acidic conditions and contributing to the neutralization of soil pH over time. However, the recovery of soil ecosystems can be a slow process, often taking years or even decades to return to pre-exposure conditions.
Vegetation recovery studies have provided insights into the long-term effects of muriatic acid on plant communities. While acute exposure can lead to immediate plant death, long-term studies have demonstrated that some plant species can adapt to moderately acidic conditions. These acid-tolerant plants often play a pivotal role in ecosystem recovery by stabilizing soil and creating microclimates that support the return of less tolerant species.
Researchers have also investigated the cascading effects of muriatic acid exposure on food webs and ecosystem functions. These studies have revealed that while some ecosystem components may recover relatively quickly, others may experience long-lasting impacts. For example, the loss of key species due to acid exposure can lead to altered predator-prey relationships and changes in nutrient cycling, potentially resulting in long-term shifts in ecosystem structure and function.
Recovery studies have also highlighted the importance of external factors in ecosystem resilience. Factors such as climate, surrounding land use, and the presence of pollution from other sources can significantly influence an ecosystem's ability to recover from muriatic acid exposure. This underscores the need for holistic approaches to environmental management and restoration efforts.
Aquatic ecosystems, particularly freshwater systems, have been the focus of numerous recovery studies. These investigations have revealed that while initial acid exposure can cause significant harm to aquatic life, many ecosystems demonstrate remarkable recovery potential over time. The recovery process typically begins with the recolonization of acid-tolerant species, followed by a gradual return of more sensitive organisms as pH levels stabilize.
Soil ecosystems have also been extensively studied in the context of acid recovery. Research has shown that soil microorganisms play a crucial role in ecosystem resilience, with some microbial communities adapting to acidic conditions and contributing to the neutralization of soil pH over time. However, the recovery of soil ecosystems can be a slow process, often taking years or even decades to return to pre-exposure conditions.
Vegetation recovery studies have provided insights into the long-term effects of muriatic acid on plant communities. While acute exposure can lead to immediate plant death, long-term studies have demonstrated that some plant species can adapt to moderately acidic conditions. These acid-tolerant plants often play a pivotal role in ecosystem recovery by stabilizing soil and creating microclimates that support the return of less tolerant species.
Researchers have also investigated the cascading effects of muriatic acid exposure on food webs and ecosystem functions. These studies have revealed that while some ecosystem components may recover relatively quickly, others may experience long-lasting impacts. For example, the loss of key species due to acid exposure can lead to altered predator-prey relationships and changes in nutrient cycling, potentially resulting in long-term shifts in ecosystem structure and function.
Recovery studies have also highlighted the importance of external factors in ecosystem resilience. Factors such as climate, surrounding land use, and the presence of pollution from other sources can significantly influence an ecosystem's ability to recover from muriatic acid exposure. This underscores the need for holistic approaches to environmental management and restoration efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!