How Muriatic Acid is Used in the Manufacture of Rubber
JUL 18, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid in Rubber Manufacturing: Background and Objectives
Muriatic acid, also known as hydrochloric acid, has played a significant role in the rubber manufacturing industry for decades. This strong mineral acid has been instrumental in various stages of rubber production, from raw material processing to final product refinement. The use of muriatic acid in rubber manufacturing dates back to the early 20th century when the rubber industry began to expand rapidly due to the growing demand for automobile tires and other rubber products.
The primary objective of using muriatic acid in rubber manufacturing is to enhance the quality and performance of rubber products while optimizing production processes. One of the key applications is in the coagulation of latex, where muriatic acid helps to separate rubber particles from the aqueous solution, facilitating the production of solid rubber. This process is crucial for creating the raw material used in various rubber products.
Another important goal of utilizing muriatic acid is to improve the adhesion properties of rubber. By treating rubber surfaces with muriatic acid, manufacturers can create a more receptive surface for bonding with other materials, such as metal or fabric. This is particularly valuable in the production of complex rubber products like tires, conveyor belts, and industrial seals.
Muriatic acid also plays a vital role in the cleaning and preparation of molds used in rubber manufacturing. The acid's corrosive properties make it effective in removing residues and contaminants from mold surfaces, ensuring high-quality finished products with precise dimensions and smooth surfaces.
In recent years, the rubber industry has been focusing on developing more sustainable and environmentally friendly manufacturing processes. This has led to research into optimizing the use of muriatic acid, exploring alternatives, and implementing more efficient acid recovery and neutralization systems. The goal is to maintain the benefits of muriatic acid in rubber production while minimizing its environmental impact and reducing waste.
As the rubber industry continues to evolve, the use of muriatic acid is expected to adapt to new technologies and environmental regulations. Future objectives may include developing novel applications for muriatic acid in advanced rubber compounds, improving acid handling and safety protocols, and integrating its use with emerging manufacturing techniques such as 3D printing of rubber products.
The primary objective of using muriatic acid in rubber manufacturing is to enhance the quality and performance of rubber products while optimizing production processes. One of the key applications is in the coagulation of latex, where muriatic acid helps to separate rubber particles from the aqueous solution, facilitating the production of solid rubber. This process is crucial for creating the raw material used in various rubber products.
Another important goal of utilizing muriatic acid is to improve the adhesion properties of rubber. By treating rubber surfaces with muriatic acid, manufacturers can create a more receptive surface for bonding with other materials, such as metal or fabric. This is particularly valuable in the production of complex rubber products like tires, conveyor belts, and industrial seals.
Muriatic acid also plays a vital role in the cleaning and preparation of molds used in rubber manufacturing. The acid's corrosive properties make it effective in removing residues and contaminants from mold surfaces, ensuring high-quality finished products with precise dimensions and smooth surfaces.
In recent years, the rubber industry has been focusing on developing more sustainable and environmentally friendly manufacturing processes. This has led to research into optimizing the use of muriatic acid, exploring alternatives, and implementing more efficient acid recovery and neutralization systems. The goal is to maintain the benefits of muriatic acid in rubber production while minimizing its environmental impact and reducing waste.
As the rubber industry continues to evolve, the use of muriatic acid is expected to adapt to new technologies and environmental regulations. Future objectives may include developing novel applications for muriatic acid in advanced rubber compounds, improving acid handling and safety protocols, and integrating its use with emerging manufacturing techniques such as 3D printing of rubber products.
Market Analysis for Muriatic Acid in Rubber Industry
The global market for muriatic acid in the rubber industry has shown steady growth in recent years, driven by the increasing demand for rubber products across various sectors. Muriatic acid, also known as hydrochloric acid, plays a crucial role in the rubber manufacturing process, particularly in the production of chloroprene rubber and other specialty elastomers.
The rubber industry's demand for muriatic acid is primarily influenced by the automotive sector, which accounts for a significant portion of rubber consumption. As the automotive industry continues to expand, especially in emerging markets, the demand for muriatic acid in rubber production is expected to rise correspondingly. Additionally, the construction and industrial sectors contribute to the market growth, as rubber products are widely used in seals, gaskets, and other applications.
Market analysis indicates that the Asia-Pacific region dominates the global market for muriatic acid in the rubber industry. This is largely due to the presence of major rubber-producing countries such as Thailand, Indonesia, and Malaysia, as well as the rapid industrialization in China and India. The region's robust manufacturing sector and increasing automotive production further bolster the demand for muriatic acid in rubber manufacturing.
North America and Europe also represent significant markets for muriatic acid in the rubber industry, albeit with more moderate growth rates. These regions are characterized by a mature rubber industry and a focus on high-performance and specialty rubber products, which often require muriatic acid in their production processes.
The market for muriatic acid in the rubber industry is relatively fragmented, with several key players competing for market share. Major chemical companies supply muriatic acid to rubber manufacturers, often as part of their broader product portfolios. The market is also influenced by the availability and pricing of raw materials, as well as environmental regulations governing the use and disposal of acidic substances.
Looking ahead, the market for muriatic acid in the rubber industry is expected to continue its growth trajectory. Factors such as the increasing adoption of synthetic rubber, the development of new rubber compounds for specialized applications, and the ongoing expansion of the automotive and construction sectors in emerging economies are likely to drive demand. However, the market may face challenges from environmental concerns and the push for more sustainable manufacturing processes, which could lead to the exploration of alternative materials or production methods in the rubber industry.
The rubber industry's demand for muriatic acid is primarily influenced by the automotive sector, which accounts for a significant portion of rubber consumption. As the automotive industry continues to expand, especially in emerging markets, the demand for muriatic acid in rubber production is expected to rise correspondingly. Additionally, the construction and industrial sectors contribute to the market growth, as rubber products are widely used in seals, gaskets, and other applications.
Market analysis indicates that the Asia-Pacific region dominates the global market for muriatic acid in the rubber industry. This is largely due to the presence of major rubber-producing countries such as Thailand, Indonesia, and Malaysia, as well as the rapid industrialization in China and India. The region's robust manufacturing sector and increasing automotive production further bolster the demand for muriatic acid in rubber manufacturing.
North America and Europe also represent significant markets for muriatic acid in the rubber industry, albeit with more moderate growth rates. These regions are characterized by a mature rubber industry and a focus on high-performance and specialty rubber products, which often require muriatic acid in their production processes.
The market for muriatic acid in the rubber industry is relatively fragmented, with several key players competing for market share. Major chemical companies supply muriatic acid to rubber manufacturers, often as part of their broader product portfolios. The market is also influenced by the availability and pricing of raw materials, as well as environmental regulations governing the use and disposal of acidic substances.
Looking ahead, the market for muriatic acid in the rubber industry is expected to continue its growth trajectory. Factors such as the increasing adoption of synthetic rubber, the development of new rubber compounds for specialized applications, and the ongoing expansion of the automotive and construction sectors in emerging economies are likely to drive demand. However, the market may face challenges from environmental concerns and the push for more sustainable manufacturing processes, which could lead to the exploration of alternative materials or production methods in the rubber industry.
Current Applications and Challenges in Rubber Production
Muriatic acid, also known as hydrochloric acid, plays a crucial role in various stages of rubber production. Its primary application is in the coagulation process of latex, where it helps convert liquid latex into solid rubber. The acid's ability to lower pH levels efficiently makes it an ideal choice for this purpose, ensuring consistent quality in rubber production.
In the manufacturing of synthetic rubber, muriatic acid is used as a catalyst in polymerization reactions. It facilitates the formation of long polymer chains, which are essential for the rubber's structural integrity and performance characteristics. The acid's controlled use in these reactions allows manufacturers to fine-tune the properties of the final rubber product.
Another significant application of muriatic acid in rubber production is in the cleaning and preparation of molds and equipment. Its corrosive properties make it effective in removing residual rubber, contaminants, and scale buildup from production machinery. This maintenance process is crucial for ensuring the longevity of equipment and maintaining product quality.
Despite its widespread use, the application of muriatic acid in rubber production faces several challenges. One of the primary concerns is the safety and handling of the acid. Its corrosive nature poses risks to workers and requires stringent safety protocols, including proper personal protective equipment and specialized storage facilities.
Environmental considerations also present challenges in the use of muriatic acid. The disposal of acid waste and the potential for harmful emissions during the production process necessitate careful management and compliance with environmental regulations. This has led to increased focus on developing more environmentally friendly alternatives or optimizing acid usage to minimize waste.
Quality control is another significant challenge in the application of muriatic acid. The concentration and purity of the acid can significantly impact the rubber production process and the final product quality. Maintaining consistent acid quality across batches and ensuring proper dilution for various applications requires sophisticated monitoring and control systems.
The rubber industry is also facing pressure to reduce its reliance on harsh chemicals, including muriatic acid. This has spurred research into alternative coagulation methods and catalysts that could potentially replace or reduce the use of muriatic acid in rubber production. However, finding substitutes that match the efficiency and cost-effectiveness of muriatic acid remains a significant challenge.
In the manufacturing of synthetic rubber, muriatic acid is used as a catalyst in polymerization reactions. It facilitates the formation of long polymer chains, which are essential for the rubber's structural integrity and performance characteristics. The acid's controlled use in these reactions allows manufacturers to fine-tune the properties of the final rubber product.
Another significant application of muriatic acid in rubber production is in the cleaning and preparation of molds and equipment. Its corrosive properties make it effective in removing residual rubber, contaminants, and scale buildup from production machinery. This maintenance process is crucial for ensuring the longevity of equipment and maintaining product quality.
Despite its widespread use, the application of muriatic acid in rubber production faces several challenges. One of the primary concerns is the safety and handling of the acid. Its corrosive nature poses risks to workers and requires stringent safety protocols, including proper personal protective equipment and specialized storage facilities.
Environmental considerations also present challenges in the use of muriatic acid. The disposal of acid waste and the potential for harmful emissions during the production process necessitate careful management and compliance with environmental regulations. This has led to increased focus on developing more environmentally friendly alternatives or optimizing acid usage to minimize waste.
Quality control is another significant challenge in the application of muriatic acid. The concentration and purity of the acid can significantly impact the rubber production process and the final product quality. Maintaining consistent acid quality across batches and ensuring proper dilution for various applications requires sophisticated monitoring and control systems.
The rubber industry is also facing pressure to reduce its reliance on harsh chemicals, including muriatic acid. This has spurred research into alternative coagulation methods and catalysts that could potentially replace or reduce the use of muriatic acid in rubber production. However, finding substitutes that match the efficiency and cost-effectiveness of muriatic acid remains a significant challenge.
Existing Muriatic Acid Processes in Rubber Manufacturing
01 Chemical properties and applications
Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.- Production and purification of muriatic acid: Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods often involve the reaction of chlorine with hydrogen or the treatment of salt (sodium chloride) with sulfuric acid. Purification techniques may include distillation or membrane separation to remove impurities and achieve desired concentrations.
- Applications in metal treatment and surface cleaning: Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It effectively removes rust, scale, and other contaminants from metal surfaces, preparing them for further processing or coating. The acid's strong reactivity makes it suitable for various industrial cleaning applications.
- Use in construction and building materials: In the construction industry, muriatic acid is utilized for cleaning masonry, concrete, and other building materials. It can remove efflorescence, mortar residues, and stains from surfaces. Additionally, it is used in the production of certain construction materials and for adjusting the pH of concrete mixtures.
- Environmental and safety considerations: Handling and disposal of muriatic acid require careful attention to environmental and safety regulations. Proper storage, transportation, and neutralization methods are essential to prevent accidents and minimize environmental impact. Specialized equipment and procedures are often employed to ensure safe handling and use of the acid in various applications.
- Alternative formulations and substitutes: Research has been conducted to develop alternative formulations or substitutes for muriatic acid in certain applications. These alternatives aim to reduce the environmental impact, improve safety, or enhance performance in specific use cases. Some formulations may include additives or modified chemical compositions to achieve desired properties while mitigating the drawbacks of traditional muriatic acid.
02 Production and manufacturing processes
Various methods are employed to produce muriatic acid, including the reaction of sodium chloride with sulfuric acid and the direct synthesis from hydrogen and chlorine. Industrial processes often involve the recovery of hydrochloric acid as a byproduct from other chemical reactions.Expand Specific Solutions03 Safety and handling considerations
Due to its corrosive nature, proper safety measures are crucial when handling muriatic acid. This includes using appropriate personal protective equipment, proper storage containers, and following specific disposal procedures. Neutralization techniques are often employed to mitigate potential hazards.Expand Specific Solutions04 Environmental impact and waste treatment
The use and disposal of muriatic acid can have significant environmental implications. Various treatment methods are employed to neutralize or recover the acid from waste streams, minimizing its impact on ecosystems. Recycling and reuse strategies are also implemented in industrial settings.Expand Specific Solutions05 Specialized applications in industry
Muriatic acid finds specialized applications in various industries, including metal processing, water treatment, and oil and gas production. It is used for pickling steel, adjusting pH in swimming pools, and as a stimulation fluid in oil well operations. The acid's unique properties make it valuable in these specific industrial processes.Expand Specific Solutions
Key Players in Muriatic Acid and Rubber Production
The competitive landscape for muriatic acid usage in rubber manufacturing is characterized by a mature industry with established players and steady market growth. The global rubber industry, valued at over $100 billion, continues to expand due to increasing demand in automotive, construction, and industrial sectors. Technologically, the process is well-developed, with major tire manufacturers like Goodyear, Bridgestone, and Michelin leading innovation. These companies, along with chemical giants such as Bayer and Sumitomo Chemical, invest heavily in R&D to improve efficiency and sustainability of rubber production processes, including the use of muriatic acid. The market also sees participation from specialized rubber chemical suppliers like ARLANXEO and JSR Corporation, contributing to a competitive and diverse ecosystem.
Goodyear Tire & Rubber Co.
Technical Solution: Goodyear utilizes muriatic acid (hydrochloric acid) in various stages of rubber manufacturing. In the initial processing, muriatic acid is used to clean and prepare natural rubber by removing impurities and coagulating latex. During vulcanization, it acts as a catalyst to accelerate the cross-linking process, improving the rubber's strength and elasticity[1]. Goodyear has developed a proprietary acid treatment process that enhances the bonding between rubber compounds and reinforcing materials like steel cords, resulting in improved tire durability and performance[2]. The company also employs muriatic acid in the production of chlorinated rubber, which is used in specialized rubber compounds for improved chemical resistance and adhesion properties[3].
Strengths: Improved tire durability and performance, enhanced bonding between rubber and reinforcing materials, specialized rubber compounds with superior properties. Weaknesses: Potential environmental concerns due to acid usage, need for careful handling and disposal of acid waste.
Bridgestone Corp.
Technical Solution: Bridgestone incorporates muriatic acid in its rubber manufacturing processes to enhance various properties of its products. The company uses a controlled acid treatment to modify the surface of carbon black fillers, improving their dispersion in rubber compounds and resulting in better wear resistance and reduced rolling resistance in tires[4]. Bridgestone has also developed an innovative acid-based process for recycling rubber from end-of-life tires, where muriatic acid is used to break down the vulcanized rubber structure, allowing for the recovery and reuse of rubber materials in new products[5]. Additionally, the company employs muriatic acid in the production of chloroprene rubber, which offers excellent resistance to weathering, ozone, and oil, making it suitable for specialized applications[6].
Strengths: Improved tire performance through enhanced filler dispersion, innovative rubber recycling process, production of specialized rubber types. Weaknesses: Acid handling safety concerns, potential for increased production costs due to specialized processes.
Innovative Techniques for Muriatic Acid Application
Method for producing rubber composition, and rubber composition
PatentActiveUS20170267818A1
Innovation
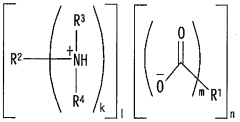
- Incorporating a monohydrazide compound supported on a solid, specifically represented by the general formula R—CONHNH2, during the compounding step of natural rubber and carbon black, which suppresses energy consumption and improves workability without requiring a mastication step or the use of stable viscosity natural rubber, thereby enhancing carbon black dispersibility.
Hydroxy acid amine salt, method for producing the same, and rubber composition containing the same
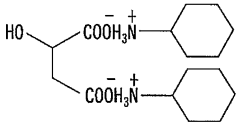
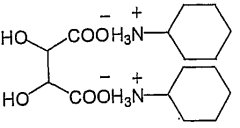
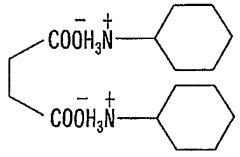
PatentWO2009014235A1
Innovation
- A hydroxy acid amine salt, represented by a specific general formula, is introduced and blended with rubber compositions to improve vulcanization efficiency and viscoelastic properties, which is produced through a reaction between a hydroxy acid and an amine in the presence or absence of a solvent, forming a high-yield amine salt that enhances rubber composition performance.
Environmental Impact and Regulations
The use of muriatic acid in rubber manufacturing processes has significant environmental implications and is subject to various regulations. The production, handling, and disposal of this corrosive substance require careful management to minimize ecological impact and ensure worker safety.
Muriatic acid, also known as hydrochloric acid, can potentially harm aquatic ecosystems if released into water bodies. It can alter pH levels, affecting fish and other aquatic organisms. To mitigate this risk, manufacturers must implement robust wastewater treatment systems and adhere to strict discharge limits set by environmental protection agencies.
Air emissions from rubber manufacturing facilities using muriatic acid are another concern. Acid fumes can contribute to air pollution and pose respiratory risks to nearby communities. Consequently, companies are required to install and maintain effective air scrubbing systems to capture and neutralize acid vapors before release.
Regulatory bodies, such as the Environmental Protection Agency (EPA) in the United States, have established guidelines for the safe use and disposal of muriatic acid in industrial processes. These regulations often mandate regular monitoring, reporting, and implementation of best management practices to prevent spills and leaks.
The Occupational Safety and Health Administration (OSHA) enforces stringent workplace safety standards for handling muriatic acid. This includes requirements for personal protective equipment, emergency response procedures, and employee training programs to minimize the risk of accidents and exposure.
Many countries have adopted the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), which standardizes hazard communication for chemicals like muriatic acid. This system ensures consistent labeling and safety data sheets across international borders, facilitating safer handling and transport.
As environmental concerns grow, there is increasing pressure on the rubber industry to explore greener alternatives to muriatic acid. Some manufacturers are investigating less hazardous substances or developing closed-loop systems to minimize acid consumption and waste generation.
Compliance with these regulations and adoption of environmentally friendly practices not only reduces ecological impact but also enhances corporate reputation and can lead to cost savings through improved efficiency and reduced waste management expenses.
Muriatic acid, also known as hydrochloric acid, can potentially harm aquatic ecosystems if released into water bodies. It can alter pH levels, affecting fish and other aquatic organisms. To mitigate this risk, manufacturers must implement robust wastewater treatment systems and adhere to strict discharge limits set by environmental protection agencies.
Air emissions from rubber manufacturing facilities using muriatic acid are another concern. Acid fumes can contribute to air pollution and pose respiratory risks to nearby communities. Consequently, companies are required to install and maintain effective air scrubbing systems to capture and neutralize acid vapors before release.
Regulatory bodies, such as the Environmental Protection Agency (EPA) in the United States, have established guidelines for the safe use and disposal of muriatic acid in industrial processes. These regulations often mandate regular monitoring, reporting, and implementation of best management practices to prevent spills and leaks.
The Occupational Safety and Health Administration (OSHA) enforces stringent workplace safety standards for handling muriatic acid. This includes requirements for personal protective equipment, emergency response procedures, and employee training programs to minimize the risk of accidents and exposure.
Many countries have adopted the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), which standardizes hazard communication for chemicals like muriatic acid. This system ensures consistent labeling and safety data sheets across international borders, facilitating safer handling and transport.
As environmental concerns grow, there is increasing pressure on the rubber industry to explore greener alternatives to muriatic acid. Some manufacturers are investigating less hazardous substances or developing closed-loop systems to minimize acid consumption and waste generation.
Compliance with these regulations and adoption of environmentally friendly practices not only reduces ecological impact but also enhances corporate reputation and can lead to cost savings through improved efficiency and reduced waste management expenses.
Safety Protocols and Handling Procedures
The use of muriatic acid in rubber manufacturing necessitates stringent safety protocols and handling procedures to protect workers and the environment. Personal protective equipment (PPE) is paramount, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Proper respiratory protection, such as acid gas respirators, must be worn in areas where acid vapors may be present.
Workplace ventilation systems play a crucial role in maintaining air quality. Local exhaust ventilation should be installed near acid handling areas to capture and remove any fumes or vapors. Regular maintenance and testing of these systems are essential to ensure their effectiveness.
Storage of muriatic acid requires specialized containment measures. Acid-resistant storage tanks or containers should be used, with secondary containment systems in place to prevent spills from spreading. Storage areas must be well-ventilated, cool, and away from incompatible materials or heat sources.
Emergency response procedures are vital for addressing potential acid spills or exposures. Eyewash stations and safety showers should be readily accessible in all areas where acid is handled. Spill kits containing neutralizing agents, absorbents, and proper disposal materials must be strategically placed throughout the facility.
Training is a critical component of safety protocols. All personnel involved in handling muriatic acid should receive comprehensive training on proper handling techniques, PPE usage, spill response, and first aid procedures. Regular refresher courses and safety drills help maintain a high level of preparedness.
Proper dilution and mixing procedures are essential when working with muriatic acid. The acid should always be added to water, never the reverse, to prevent dangerous splashing. Mixing should be performed in well-ventilated areas using appropriate equipment and following precise ratios.
Waste management and disposal of muriatic acid and its byproducts require careful consideration. Neutralization processes should be employed before disposal, and all waste must be handled in compliance with local environmental regulations.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
By implementing and strictly adhering to these safety protocols and handling procedures, rubber manufacturers can significantly reduce the risks associated with muriatic acid use, ensuring a safer work environment and minimizing the potential for accidents or environmental incidents.
Workplace ventilation systems play a crucial role in maintaining air quality. Local exhaust ventilation should be installed near acid handling areas to capture and remove any fumes or vapors. Regular maintenance and testing of these systems are essential to ensure their effectiveness.
Storage of muriatic acid requires specialized containment measures. Acid-resistant storage tanks or containers should be used, with secondary containment systems in place to prevent spills from spreading. Storage areas must be well-ventilated, cool, and away from incompatible materials or heat sources.
Emergency response procedures are vital for addressing potential acid spills or exposures. Eyewash stations and safety showers should be readily accessible in all areas where acid is handled. Spill kits containing neutralizing agents, absorbents, and proper disposal materials must be strategically placed throughout the facility.
Training is a critical component of safety protocols. All personnel involved in handling muriatic acid should receive comprehensive training on proper handling techniques, PPE usage, spill response, and first aid procedures. Regular refresher courses and safety drills help maintain a high level of preparedness.
Proper dilution and mixing procedures are essential when working with muriatic acid. The acid should always be added to water, never the reverse, to prevent dangerous splashing. Mixing should be performed in well-ventilated areas using appropriate equipment and following precise ratios.
Waste management and disposal of muriatic acid and its byproducts require careful consideration. Neutralization processes should be employed before disposal, and all waste must be handled in compliance with local environmental regulations.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
By implementing and strictly adhering to these safety protocols and handling procedures, rubber manufacturers can significantly reduce the risks associated with muriatic acid use, ensuring a safer work environment and minimizing the potential for accidents or environmental incidents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!