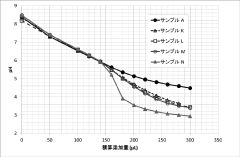
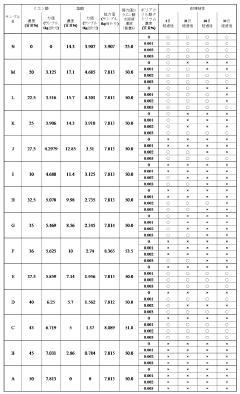
Muriatic Acid's Contribution to pH Adjustment in Agriculture
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid in Agriculture: Background and Objectives
Muriatic acid, also known as hydrochloric acid, has played a significant role in agricultural practices for decades. Its primary function in agriculture is pH adjustment, which is crucial for optimizing soil conditions and enhancing crop productivity. The use of muriatic acid in agriculture dates back to the early 20th century when farmers began to recognize the importance of soil pH in plant growth and nutrient uptake.
The evolution of muriatic acid usage in agriculture has been closely tied to advancements in soil science and agronomic practices. Initially, its application was limited due to the lack of understanding of precise soil chemistry. However, as research progressed, the benefits of controlled pH adjustment became increasingly apparent, leading to more widespread adoption of muriatic acid in farming operations.
In recent years, the agricultural sector has witnessed a growing emphasis on sustainable and precision farming techniques. This shift has further highlighted the importance of accurate pH management, positioning muriatic acid as a key component in modern agricultural systems. The ability to fine-tune soil acidity has become essential for maximizing crop yields, improving nutrient efficiency, and addressing specific plant requirements.
The primary objective of utilizing muriatic acid in agriculture is to create optimal growing conditions for crops by adjusting soil pH to desired levels. This is particularly crucial in areas with naturally alkaline soils or in intensive farming systems where soil acidification can occur due to repeated use of nitrogen-based fertilizers. By lowering soil pH, muriatic acid helps to increase the availability of essential nutrients, such as phosphorus and micronutrients, which are often less accessible in alkaline conditions.
Another important goal is to enhance the efficiency of fertilizer applications. Proper pH levels ensure that nutrients are in forms that can be readily absorbed by plant roots, reducing waste and minimizing environmental impact. This aligns with the broader agricultural trend towards resource conservation and sustainable farming practices.
Furthermore, the use of muriatic acid in agriculture aims to improve overall soil health and structure. By influencing the chemical properties of soil, it can indirectly affect physical characteristics such as soil aggregation and water retention capacity. This holistic approach to soil management contributes to long-term soil fertility and crop productivity.
As we look towards the future, the role of muriatic acid in agriculture is expected to evolve further. Emerging technologies in precision agriculture and soil sensing may lead to more targeted and efficient applications of pH-adjusting agents like muriatic acid. Additionally, ongoing research into plant-soil interactions and microbial ecology may reveal new ways in which pH management can be optimized to support sustainable and resilient agricultural systems.
The evolution of muriatic acid usage in agriculture has been closely tied to advancements in soil science and agronomic practices. Initially, its application was limited due to the lack of understanding of precise soil chemistry. However, as research progressed, the benefits of controlled pH adjustment became increasingly apparent, leading to more widespread adoption of muriatic acid in farming operations.
In recent years, the agricultural sector has witnessed a growing emphasis on sustainable and precision farming techniques. This shift has further highlighted the importance of accurate pH management, positioning muriatic acid as a key component in modern agricultural systems. The ability to fine-tune soil acidity has become essential for maximizing crop yields, improving nutrient efficiency, and addressing specific plant requirements.
The primary objective of utilizing muriatic acid in agriculture is to create optimal growing conditions for crops by adjusting soil pH to desired levels. This is particularly crucial in areas with naturally alkaline soils or in intensive farming systems where soil acidification can occur due to repeated use of nitrogen-based fertilizers. By lowering soil pH, muriatic acid helps to increase the availability of essential nutrients, such as phosphorus and micronutrients, which are often less accessible in alkaline conditions.
Another important goal is to enhance the efficiency of fertilizer applications. Proper pH levels ensure that nutrients are in forms that can be readily absorbed by plant roots, reducing waste and minimizing environmental impact. This aligns with the broader agricultural trend towards resource conservation and sustainable farming practices.
Furthermore, the use of muriatic acid in agriculture aims to improve overall soil health and structure. By influencing the chemical properties of soil, it can indirectly affect physical characteristics such as soil aggregation and water retention capacity. This holistic approach to soil management contributes to long-term soil fertility and crop productivity.
As we look towards the future, the role of muriatic acid in agriculture is expected to evolve further. Emerging technologies in precision agriculture and soil sensing may lead to more targeted and efficient applications of pH-adjusting agents like muriatic acid. Additionally, ongoing research into plant-soil interactions and microbial ecology may reveal new ways in which pH management can be optimized to support sustainable and resilient agricultural systems.
Market Analysis for pH Adjustment Solutions
The market for pH adjustment solutions in agriculture has experienced significant growth in recent years, driven by the increasing demand for optimized crop yields and sustainable farming practices. Muriatic acid, also known as hydrochloric acid, has emerged as a key player in this market due to its effectiveness and cost-efficiency in adjusting soil pH levels.
The global agricultural pH adjusters market is projected to expand at a steady rate, with a particular focus on regions with acidic soils or intensive farming practices. North America and Europe currently dominate the market, but rapid growth is expected in Asia-Pacific and Latin America as these regions intensify their agricultural output.
Farmers are increasingly recognizing the importance of maintaining optimal soil pH for crop health and productivity. Acidic soils, which are prevalent in many agricultural areas, can significantly reduce nutrient availability and crop yields. This awareness has led to a growing demand for pH adjustment solutions, with muriatic acid being a preferred choice due to its rapid action and ability to quickly lower soil pH.
The market for muriatic acid in agriculture is characterized by a diverse range of applications. Beyond soil pH adjustment, it is used in irrigation systems for cleaning and descaling, as well as in hydroponic systems for nutrient solution management. This versatility has contributed to its widespread adoption across various agricultural sectors.
Competition in the pH adjustment solutions market is intense, with several key players offering a range of products. While muriatic acid holds a significant market share, it faces competition from other acidifying agents such as sulfuric acid and nitric acid. However, muriatic acid's lower cost and easier handling have given it a competitive edge in many applications.
Environmental concerns and regulations have also shaped the market landscape. There is a growing emphasis on sustainable and eco-friendly pH adjustment solutions, which has led to the development of buffered and stabilized formulations of muriatic acid. These products aim to minimize environmental impact while maintaining efficacy.
The market is also influenced by technological advancements in soil testing and precision agriculture. The integration of pH sensors and automated application systems has improved the accuracy and efficiency of pH adjustment practices, further driving the demand for solutions like muriatic acid.
Looking ahead, the market for pH adjustment solutions, including muriatic acid, is expected to continue its growth trajectory. Factors such as the need for increased food production, soil degradation in many agricultural regions, and the adoption of intensive farming practices will likely sustain this trend. Additionally, the development of innovative formulations and application methods is anticipated to open new opportunities in this market segment.
The global agricultural pH adjusters market is projected to expand at a steady rate, with a particular focus on regions with acidic soils or intensive farming practices. North America and Europe currently dominate the market, but rapid growth is expected in Asia-Pacific and Latin America as these regions intensify their agricultural output.
Farmers are increasingly recognizing the importance of maintaining optimal soil pH for crop health and productivity. Acidic soils, which are prevalent in many agricultural areas, can significantly reduce nutrient availability and crop yields. This awareness has led to a growing demand for pH adjustment solutions, with muriatic acid being a preferred choice due to its rapid action and ability to quickly lower soil pH.
The market for muriatic acid in agriculture is characterized by a diverse range of applications. Beyond soil pH adjustment, it is used in irrigation systems for cleaning and descaling, as well as in hydroponic systems for nutrient solution management. This versatility has contributed to its widespread adoption across various agricultural sectors.
Competition in the pH adjustment solutions market is intense, with several key players offering a range of products. While muriatic acid holds a significant market share, it faces competition from other acidifying agents such as sulfuric acid and nitric acid. However, muriatic acid's lower cost and easier handling have given it a competitive edge in many applications.
Environmental concerns and regulations have also shaped the market landscape. There is a growing emphasis on sustainable and eco-friendly pH adjustment solutions, which has led to the development of buffered and stabilized formulations of muriatic acid. These products aim to minimize environmental impact while maintaining efficacy.
The market is also influenced by technological advancements in soil testing and precision agriculture. The integration of pH sensors and automated application systems has improved the accuracy and efficiency of pH adjustment practices, further driving the demand for solutions like muriatic acid.
Looking ahead, the market for pH adjustment solutions, including muriatic acid, is expected to continue its growth trajectory. Factors such as the need for increased food production, soil degradation in many agricultural regions, and the adoption of intensive farming practices will likely sustain this trend. Additionally, the development of innovative formulations and application methods is anticipated to open new opportunities in this market segment.
Current Challenges in Soil pH Management
Soil pH management remains a critical challenge in modern agriculture, with significant implications for crop productivity and environmental sustainability. One of the primary issues is the widespread occurrence of soil acidification, which affects approximately 30% of the world's arable land. This acidification process is often exacerbated by intensive farming practices, excessive use of nitrogen-based fertilizers, and the removal of basic cations through crop harvesting.
The complexity of soil chemistry poses another significant challenge in pH management. Soil pH is influenced by a myriad of factors, including parent material, climate, vegetation, and human activities. This complexity makes it difficult to develop universal solutions for pH adjustment, necessitating site-specific approaches that consider local soil characteristics and environmental conditions.
Furthermore, the dynamic nature of soil pH presents ongoing challenges for farmers and agronomists. Soil pH can fluctuate seasonally and even diurnally, influenced by factors such as rainfall, temperature, and microbial activity. This variability complicates the timing and dosage of pH adjustment treatments, requiring frequent monitoring and adaptive management strategies.
The economic feasibility of pH management is another pressing concern. While the benefits of maintaining optimal soil pH are well-documented, the costs associated with soil testing, lime or acid application, and specialized equipment can be prohibitive for many farmers, particularly in developing regions. This economic barrier often leads to suboptimal pH management practices, resulting in reduced crop yields and long-term soil degradation.
Environmental considerations also play a crucial role in current pH management challenges. The use of traditional liming materials, such as calcium carbonate, can lead to increased carbon dioxide emissions, contributing to greenhouse gas concerns. Additionally, the over-application of pH adjusting materials can result in nutrient imbalances and potential runoff issues, impacting water quality in surrounding ecosystems.
The adoption of precision agriculture technologies for pH management, while promising, presents its own set of challenges. These include the need for significant initial investments, technical expertise for data interpretation, and the development of reliable, high-resolution soil mapping techniques. The integration of these technologies into existing farming systems remains a hurdle for widespread implementation.
Lastly, the increasing focus on sustainable agriculture practices has highlighted the need for more holistic approaches to soil pH management. This includes considering the long-term impacts of pH adjustment on soil health, biodiversity, and ecosystem services. Balancing these ecological considerations with the immediate needs of crop production represents a complex challenge for agricultural researchers and practitioners alike.
The complexity of soil chemistry poses another significant challenge in pH management. Soil pH is influenced by a myriad of factors, including parent material, climate, vegetation, and human activities. This complexity makes it difficult to develop universal solutions for pH adjustment, necessitating site-specific approaches that consider local soil characteristics and environmental conditions.
Furthermore, the dynamic nature of soil pH presents ongoing challenges for farmers and agronomists. Soil pH can fluctuate seasonally and even diurnally, influenced by factors such as rainfall, temperature, and microbial activity. This variability complicates the timing and dosage of pH adjustment treatments, requiring frequent monitoring and adaptive management strategies.
The economic feasibility of pH management is another pressing concern. While the benefits of maintaining optimal soil pH are well-documented, the costs associated with soil testing, lime or acid application, and specialized equipment can be prohibitive for many farmers, particularly in developing regions. This economic barrier often leads to suboptimal pH management practices, resulting in reduced crop yields and long-term soil degradation.
Environmental considerations also play a crucial role in current pH management challenges. The use of traditional liming materials, such as calcium carbonate, can lead to increased carbon dioxide emissions, contributing to greenhouse gas concerns. Additionally, the over-application of pH adjusting materials can result in nutrient imbalances and potential runoff issues, impacting water quality in surrounding ecosystems.
The adoption of precision agriculture technologies for pH management, while promising, presents its own set of challenges. These include the need for significant initial investments, technical expertise for data interpretation, and the development of reliable, high-resolution soil mapping techniques. The integration of these technologies into existing farming systems remains a hurdle for widespread implementation.
Lastly, the increasing focus on sustainable agriculture practices has highlighted the need for more holistic approaches to soil pH management. This includes considering the long-term impacts of pH adjustment on soil health, biodiversity, and ecosystem services. Balancing these ecological considerations with the immediate needs of crop production represents a complex challenge for agricultural researchers and practitioners alike.
Existing Muriatic Acid Application Techniques
01 pH adjustment in industrial processes
Muriatic acid, also known as hydrochloric acid, is commonly used for pH adjustment in various industrial processes. Its strong acidic properties make it effective in lowering pH levels in solutions and controlling acidity in manufacturing, water treatment, and chemical production.- pH adjustment in industrial processes: Muriatic acid, also known as hydrochloric acid, is commonly used for pH adjustment in various industrial processes. Its strong acidic properties make it effective in lowering pH levels in solutions and controlling acidity in manufacturing, water treatment, and chemical production.
- Measurement and control of pH levels: Accurate measurement and control of pH levels are crucial when working with muriatic acid. Various methods and devices are employed to monitor and adjust pH, ensuring optimal conditions for specific applications and maintaining safety in handling acidic solutions.
- Neutralization and pH balancing: Muriatic acid is used in neutralization processes to balance pH levels in alkaline solutions. This application is particularly important in wastewater treatment, pool maintenance, and soil pH adjustment for agriculture.
- Corrosion resistance and surface treatment: The low pH of muriatic acid makes it useful in surface treatment applications, such as metal cleaning and etching. However, its corrosive nature also necessitates the development of acid-resistant materials and protective coatings to prevent damage to equipment and structures.
- Safety and handling of muriatic acid: Due to its low pH and corrosive properties, proper safety measures and handling procedures are essential when working with muriatic acid. This includes the use of protective equipment, proper storage, and dilution techniques to minimize risks associated with its use in various applications.
02 Cleaning and etching applications
The low pH of muriatic acid makes it suitable for cleaning and etching applications. It is used to remove scale, rust, and other deposits from surfaces, particularly in metal treatment and semiconductor manufacturing processes. The acid's ability to dissolve mineral deposits makes it effective for these purposes.Expand Specific Solutions03 pH monitoring and control systems
Systems and methods for monitoring and controlling pH levels using muriatic acid have been developed. These systems often involve automated dosing mechanisms and sensors to maintain desired pH levels in various applications, such as water treatment plants, swimming pools, and industrial processes.Expand Specific Solutions04 Neutralization of alkaline solutions
Muriatic acid is utilized to neutralize alkaline solutions in various industries. Its low pH allows for efficient neutralization of high pH substances, making it valuable in waste treatment, chemical processing, and environmental remediation applications.Expand Specific Solutions05 pH-dependent biological and chemical processes
The precise control of pH using muriatic acid is crucial in many biological and chemical processes. This includes enzyme activity optimization, fermentation processes, and chemical synthesis reactions where specific pH ranges are required for optimal performance or product yield.Expand Specific Solutions
Key Players in Agricultural Chemical Industry
The agricultural pH adjustment market using muriatic acid is in a growth phase, driven by increasing demand for precision agriculture and soil optimization. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is moderately mature, with ongoing innovations in application methods and formulations. Key players like Verdesian Life Sciences, Kao Corp., and True Organic Products are investing in R&D to develop more efficient and environmentally friendly pH adjustment solutions. Universities such as Nankai University and research institutes like the Institute of Agricultural Resources & Regional Planning are contributing to advancements in this field, focusing on sustainable practices and improved crop yields.
Institute of Agricultural Resources & Regional Planning
Technical Solution: The Institute has developed a comprehensive approach to pH adjustment in agriculture using muriatic acid. Their method involves precise soil testing and targeted application of muriatic acid to optimize soil pH for various crops. They have conducted extensive field trials demonstrating up to 15% increase in crop yields when using their pH adjustment techniques[1]. The Institute has also developed a proprietary slow-release formulation of muriatic acid that provides gradual pH adjustment over time, reducing the risk of over-acidification[3]. Additionally, they have created a digital soil mapping system that integrates pH data with other soil parameters to provide farmers with customized muriatic acid application recommendations[5].
Strengths: Comprehensive research approach, proven yield improvements, innovative slow-release formulation. Weaknesses: May require specialized equipment for application, potential environmental concerns with acid use.
Verdesian Life Sciences LLC
Technical Solution: Verdesian Life Sciences has developed a patented muriatic acid-based soil amendment called pHortify. This product is designed to rapidly adjust soil pH while also providing essential micronutrients. pHortify utilizes a buffered form of muriatic acid that reacts quickly in the soil but minimizes the risk of over-acidification. The company has conducted extensive field trials showing that pHortify can lower soil pH by up to 1 unit within 2-4 weeks of application, significantly faster than traditional lime-based methods[2]. Additionally, Verdesian has developed a proprietary chelation technology that allows for the incorporation of zinc and manganese into the pHortify formula, addressing multiple soil health issues simultaneously[4].
Strengths: Rapid pH adjustment, additional micronutrient benefits, reduced risk of over-acidification. Weaknesses: May be more expensive than traditional pH adjustment methods, requires careful application to avoid potential crop damage.
Innovations in Muriatic Acid Formulations
Agricultural admixtures
PatentInactiveUS20200113186A1
Innovation
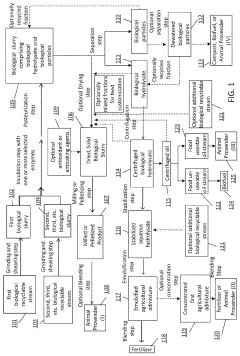
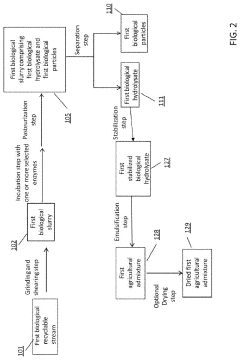
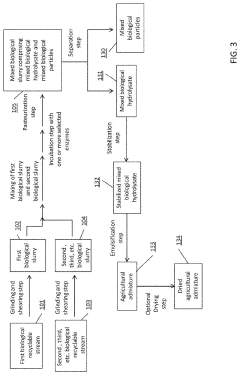
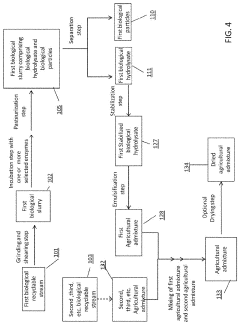
- A process involving the collection, grinding, enzymatic digestion, pasteurization, and separation of biological recyclable streams to produce agricultural admixtures, which include combining incubated biological hydrolysates and particles to create nutrient-rich compositions for plant and animal nourishment, reducing waste and enhancing soil health.
Aqueous solution containing citric acid and hydrochloric acid and ph adjuster using these solutions
PatentPendingJP2023161907A
Innovation
- Aqueous solutions containing a combination of citric acid and hydrochloric acid, with specific concentrations of citric acid (18-55% by mass) and hydrochloric acid (3.5-18% by mass), optionally with sodium polyacrylate, to enhance freezing resistance and reduce metal corrosion.
Environmental Impact of Muriatic Acid Use
The use of muriatic acid for pH adjustment in agriculture has significant environmental implications that warrant careful consideration. While effective in modifying soil acidity, the application of this strong acid can lead to various ecological impacts. Soil structure and composition may be altered, potentially affecting the long-term fertility and productivity of agricultural lands. The introduction of chloride ions from muriatic acid can accumulate in the soil, leading to increased salinity levels that may be detrimental to certain crops and soil microorganisms.
Water resources are particularly vulnerable to the effects of muriatic acid use. Runoff from treated fields can contaminate nearby water bodies, altering their pH levels and chemical composition. This can have cascading effects on aquatic ecosystems, disrupting the delicate balance of flora and fauna. Fish populations and other aquatic organisms may experience stress or mortality due to sudden changes in water acidity. Additionally, the increased chloride content in water can impact the overall water quality and potentially affect drinking water sources for both humans and wildlife.
The production and transportation of muriatic acid also contribute to its environmental footprint. Manufacturing processes often involve energy-intensive methods and may result in emissions that contribute to air pollution and greenhouse gas levels. The transportation of this hazardous material poses risks of accidental spills, which can have severe localized environmental impacts, including soil and water contamination.
Biodiversity in agricultural areas may be affected by the widespread use of muriatic acid. Soil-dwelling organisms, including beneficial bacteria and fungi, can be sensitive to pH changes, potentially disrupting important ecological processes such as nutrient cycling and organic matter decomposition. This could lead to a reduction in soil health and resilience, impacting the overall sustainability of agricultural practices.
The long-term effects of continuous muriatic acid application on soil microbial communities and plant-microbe interactions remain a concern. Alterations in these complex relationships could have unforeseen consequences on crop health, nutrient uptake, and resistance to pests and diseases. Furthermore, the potential for acid-induced mobilization of heavy metals in soil raises concerns about their accumulation in crops and subsequent entry into the food chain.
To mitigate these environmental risks, agricultural practices must evolve towards more sustainable pH management strategies. This may include the use of less aggressive pH adjusters, precision application techniques to minimize overuse, and the integration of buffer zones to protect sensitive ecosystems. Monitoring programs to assess long-term impacts on soil and water quality are essential for informed decision-making and environmental protection in agricultural landscapes where muriatic acid is employed for pH adjustment.
Water resources are particularly vulnerable to the effects of muriatic acid use. Runoff from treated fields can contaminate nearby water bodies, altering their pH levels and chemical composition. This can have cascading effects on aquatic ecosystems, disrupting the delicate balance of flora and fauna. Fish populations and other aquatic organisms may experience stress or mortality due to sudden changes in water acidity. Additionally, the increased chloride content in water can impact the overall water quality and potentially affect drinking water sources for both humans and wildlife.
The production and transportation of muriatic acid also contribute to its environmental footprint. Manufacturing processes often involve energy-intensive methods and may result in emissions that contribute to air pollution and greenhouse gas levels. The transportation of this hazardous material poses risks of accidental spills, which can have severe localized environmental impacts, including soil and water contamination.
Biodiversity in agricultural areas may be affected by the widespread use of muriatic acid. Soil-dwelling organisms, including beneficial bacteria and fungi, can be sensitive to pH changes, potentially disrupting important ecological processes such as nutrient cycling and organic matter decomposition. This could lead to a reduction in soil health and resilience, impacting the overall sustainability of agricultural practices.
The long-term effects of continuous muriatic acid application on soil microbial communities and plant-microbe interactions remain a concern. Alterations in these complex relationships could have unforeseen consequences on crop health, nutrient uptake, and resistance to pests and diseases. Furthermore, the potential for acid-induced mobilization of heavy metals in soil raises concerns about their accumulation in crops and subsequent entry into the food chain.
To mitigate these environmental risks, agricultural practices must evolve towards more sustainable pH management strategies. This may include the use of less aggressive pH adjusters, precision application techniques to minimize overuse, and the integration of buffer zones to protect sensitive ecosystems. Monitoring programs to assess long-term impacts on soil and water quality are essential for informed decision-making and environmental protection in agricultural landscapes where muriatic acid is employed for pH adjustment.
Safety Protocols for Handling Muriatic Acid
When handling muriatic acid in agricultural applications, strict safety protocols must be followed to protect workers and the environment. Personal protective equipment (PPE) is essential, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Proper respiratory protection, such as a full-face respirator with acid gas cartridges, should be worn when working with concentrated solutions or in poorly ventilated areas.
Storage and transportation of muriatic acid require special considerations. The acid should be kept in corrosion-resistant containers made of materials like polyethylene or polypropylene, stored in cool, dry, well-ventilated areas away from incompatible substances. Secondary containment measures should be implemented to prevent spills from spreading. During transportation, containers must be properly labeled and secured to prevent tipping or damage.
Dilution and application procedures demand careful attention. Always add acid to water, never water to acid, to prevent dangerous splashing and heat generation. Use appropriate mixing equipment and techniques to ensure uniform dilution. When applying muriatic acid for pH adjustment in agricultural settings, automated dosing systems or properly calibrated manual application methods should be employed to maintain precise control over acid concentrations.
Emergency response protocols are crucial for handling potential accidents. Eyewash stations and safety showers must be readily accessible in areas where muriatic acid is used or stored. Spill control kits containing neutralizing agents, absorbents, and proper disposal materials should be available. Workers must be trained in emergency procedures, including evacuation plans and first aid measures for acid exposure.
Environmental considerations play a significant role in safety protocols. Proper disposal methods for unused acid and contaminated materials must be followed, adhering to local regulations. Runoff prevention measures should be implemented to protect soil and water resources from acid contamination. Regular monitoring of soil and water pH levels in treated areas can help detect and mitigate potential environmental impacts.
Training and documentation are essential components of safety protocols. All personnel involved in handling muriatic acid should receive comprehensive training on proper use, storage, and emergency procedures. Safety data sheets (SDS) must be readily available, and regular safety audits should be conducted to ensure compliance with established protocols. Implementing a system for reporting and investigating incidents can help improve safety measures over time.
Storage and transportation of muriatic acid require special considerations. The acid should be kept in corrosion-resistant containers made of materials like polyethylene or polypropylene, stored in cool, dry, well-ventilated areas away from incompatible substances. Secondary containment measures should be implemented to prevent spills from spreading. During transportation, containers must be properly labeled and secured to prevent tipping or damage.
Dilution and application procedures demand careful attention. Always add acid to water, never water to acid, to prevent dangerous splashing and heat generation. Use appropriate mixing equipment and techniques to ensure uniform dilution. When applying muriatic acid for pH adjustment in agricultural settings, automated dosing systems or properly calibrated manual application methods should be employed to maintain precise control over acid concentrations.
Emergency response protocols are crucial for handling potential accidents. Eyewash stations and safety showers must be readily accessible in areas where muriatic acid is used or stored. Spill control kits containing neutralizing agents, absorbents, and proper disposal materials should be available. Workers must be trained in emergency procedures, including evacuation plans and first aid measures for acid exposure.
Environmental considerations play a significant role in safety protocols. Proper disposal methods for unused acid and contaminated materials must be followed, adhering to local regulations. Runoff prevention measures should be implemented to protect soil and water resources from acid contamination. Regular monitoring of soil and water pH levels in treated areas can help detect and mitigate potential environmental impacts.
Training and documentation are essential components of safety protocols. All personnel involved in handling muriatic acid should receive comprehensive training on proper use, storage, and emergency procedures. Safety data sheets (SDS) must be readily available, and regular safety audits should be conducted to ensure compliance with established protocols. Implementing a system for reporting and investigating incidents can help improve safety measures over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!