Muriatic Acid in Swimming Pool Chemistry: Balancing pH Levels
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pool Chemistry Fundamentals and Objectives
Swimming pool chemistry is a complex interplay of various chemical components, with pH balance being a critical factor in maintaining a safe and comfortable aquatic environment. The fundamental objective of pool chemistry management is to create a stable, hygienic, and enjoyable swimming experience for users while protecting the pool infrastructure and equipment from chemical damage.
At the core of pool chemistry is the concept of pH, which measures the acidity or alkalinity of the water. The ideal pH range for swimming pools is typically between 7.2 and 7.8, with 7.4 being the optimal level. This range closely mimics the pH of human tears and mucous membranes, minimizing eye and skin irritation for swimmers. Maintaining the correct pH is crucial not only for swimmer comfort but also for the effectiveness of other pool chemicals, particularly chlorine.
Muriatic acid, also known as hydrochloric acid, plays a vital role in pH adjustment. It is a strong acid commonly used to lower the pH when pool water becomes too alkaline. The primary objective of using muriatic acid is to bring the pH back into the ideal range, ensuring that other pool chemicals function optimally and preventing issues such as scaling and cloudy water.
Understanding the relationship between pH and chlorine effectiveness is essential. When pH levels are too high, chlorine's sanitizing power is significantly reduced, leading to inadequate disinfection and potential health risks for swimmers. Conversely, when pH is too low, chlorine becomes overly aggressive, potentially causing corrosion of pool equipment and irritation to swimmers.
The goal of balancing pH levels extends beyond immediate water quality. It also aims to protect the pool's structural integrity and equipment longevity. Proper pH balance helps prevent the formation of scale on pool surfaces and equipment, which can lead to reduced efficiency and increased maintenance costs over time.
Another fundamental aspect of pool chemistry is total alkalinity, which acts as a buffer for pH. Maintaining the correct total alkalinity (typically between 80-120 ppm) helps stabilize pH levels, making it easier to keep the pH within the desired range. Muriatic acid is also used to adjust total alkalinity when necessary.
In summary, the objectives of pool chemistry management, particularly concerning muriatic acid and pH balancing, are multifaceted. They include ensuring swimmer safety and comfort, optimizing the effectiveness of sanitizing chemicals, protecting pool infrastructure, and minimizing maintenance requirements. Achieving these objectives requires a thorough understanding of chemical interactions and careful monitoring and adjustment of pool water parameters.
At the core of pool chemistry is the concept of pH, which measures the acidity or alkalinity of the water. The ideal pH range for swimming pools is typically between 7.2 and 7.8, with 7.4 being the optimal level. This range closely mimics the pH of human tears and mucous membranes, minimizing eye and skin irritation for swimmers. Maintaining the correct pH is crucial not only for swimmer comfort but also for the effectiveness of other pool chemicals, particularly chlorine.
Muriatic acid, also known as hydrochloric acid, plays a vital role in pH adjustment. It is a strong acid commonly used to lower the pH when pool water becomes too alkaline. The primary objective of using muriatic acid is to bring the pH back into the ideal range, ensuring that other pool chemicals function optimally and preventing issues such as scaling and cloudy water.
Understanding the relationship between pH and chlorine effectiveness is essential. When pH levels are too high, chlorine's sanitizing power is significantly reduced, leading to inadequate disinfection and potential health risks for swimmers. Conversely, when pH is too low, chlorine becomes overly aggressive, potentially causing corrosion of pool equipment and irritation to swimmers.
The goal of balancing pH levels extends beyond immediate water quality. It also aims to protect the pool's structural integrity and equipment longevity. Proper pH balance helps prevent the formation of scale on pool surfaces and equipment, which can lead to reduced efficiency and increased maintenance costs over time.
Another fundamental aspect of pool chemistry is total alkalinity, which acts as a buffer for pH. Maintaining the correct total alkalinity (typically between 80-120 ppm) helps stabilize pH levels, making it easier to keep the pH within the desired range. Muriatic acid is also used to adjust total alkalinity when necessary.
In summary, the objectives of pool chemistry management, particularly concerning muriatic acid and pH balancing, are multifaceted. They include ensuring swimmer safety and comfort, optimizing the effectiveness of sanitizing chemicals, protecting pool infrastructure, and minimizing maintenance requirements. Achieving these objectives requires a thorough understanding of chemical interactions and careful monitoring and adjustment of pool water parameters.
Market Analysis for Pool Maintenance Products
The swimming pool maintenance product market has experienced steady growth in recent years, driven by increasing pool ownership and a growing emphasis on water quality and safety. The global market for pool chemicals and equipment is estimated to reach $25 billion by 2025, with a compound annual growth rate of 7.2% from 2020 to 2025. Within this market, pH balancing products, including muriatic acid, play a crucial role in maintaining proper water chemistry.
Muriatic acid, also known as hydrochloric acid, is widely used in pool maintenance for its effectiveness in lowering pH levels and its cost-efficiency compared to other pH-reducing chemicals. The demand for muriatic acid in the pool maintenance sector is closely tied to the overall growth of the swimming pool industry, which has seen a surge in residential pool installations, particularly in the wake of the COVID-19 pandemic.
The market for pH balancing products is characterized by a mix of established chemical manufacturers and specialized pool care companies. Major players in this segment include Occidental Petroleum Corporation, Olin Corporation, and Poolmaster, among others. These companies offer a range of muriatic acid-based products, from pure acid solutions to pre-diluted formulations designed for ease of use by pool owners.
Consumer trends indicate a growing preference for user-friendly and safer pool maintenance solutions. This has led to the development of alternative pH-lowering products, such as sodium bisulfate, which compete with traditional muriatic acid. However, muriatic acid remains a popular choice due to its effectiveness and lower cost.
The market for pool maintenance products, including pH balancers, is influenced by seasonal factors, with peak demand occurring during summer months in temperate regions. Geographic distribution of the market shows strong sales in regions with high pool density, such as the southern United States, Mediterranean countries, and Australia.
Regulatory factors play a significant role in shaping the market for pool chemicals. Stringent safety standards and environmental regulations impact product formulations and packaging. There is an increasing focus on developing eco-friendly alternatives and improving the safety profile of existing products, which may influence future market dynamics for muriatic acid and other pH-balancing chemicals.
E-commerce and digital platforms have emerged as important sales channels for pool maintenance products, offering convenience and a wide product selection to consumers. This trend has accelerated during the pandemic and is expected to continue, potentially reshaping traditional distribution models in the industry.
Muriatic acid, also known as hydrochloric acid, is widely used in pool maintenance for its effectiveness in lowering pH levels and its cost-efficiency compared to other pH-reducing chemicals. The demand for muriatic acid in the pool maintenance sector is closely tied to the overall growth of the swimming pool industry, which has seen a surge in residential pool installations, particularly in the wake of the COVID-19 pandemic.
The market for pH balancing products is characterized by a mix of established chemical manufacturers and specialized pool care companies. Major players in this segment include Occidental Petroleum Corporation, Olin Corporation, and Poolmaster, among others. These companies offer a range of muriatic acid-based products, from pure acid solutions to pre-diluted formulations designed for ease of use by pool owners.
Consumer trends indicate a growing preference for user-friendly and safer pool maintenance solutions. This has led to the development of alternative pH-lowering products, such as sodium bisulfate, which compete with traditional muriatic acid. However, muriatic acid remains a popular choice due to its effectiveness and lower cost.
The market for pool maintenance products, including pH balancers, is influenced by seasonal factors, with peak demand occurring during summer months in temperate regions. Geographic distribution of the market shows strong sales in regions with high pool density, such as the southern United States, Mediterranean countries, and Australia.
Regulatory factors play a significant role in shaping the market for pool chemicals. Stringent safety standards and environmental regulations impact product formulations and packaging. There is an increasing focus on developing eco-friendly alternatives and improving the safety profile of existing products, which may influence future market dynamics for muriatic acid and other pH-balancing chemicals.
E-commerce and digital platforms have emerged as important sales channels for pool maintenance products, offering convenience and a wide product selection to consumers. This trend has accelerated during the pandemic and is expected to continue, potentially reshaping traditional distribution models in the industry.
Current Challenges in Pool pH Management
Maintaining proper pH levels in swimming pools is a critical aspect of pool chemistry, yet it remains one of the most challenging tasks for pool owners and operators. The current challenges in pool pH management stem from various factors, including environmental influences, user behavior, and the limitations of existing pH control methods.
One of the primary challenges is the dynamic nature of pool water chemistry. Factors such as rainfall, evaporation, and the introduction of contaminants from swimmers can cause rapid fluctuations in pH levels. These changes often occur faster than traditional pH adjustment methods can respond, leading to periods where the pool water is outside the optimal pH range.
The use of muriatic acid for pH adjustment, while effective, presents its own set of challenges. Accurate dosing of muriatic acid is crucial, as overdosing can lead to dangerously low pH levels, potentially causing skin and eye irritation for swimmers, as well as damage to pool equipment and surfaces. Conversely, underdosing may fail to adequately lower pH, leaving the pool water alkaline and reducing the effectiveness of chlorine sanitizers.
Safety concerns associated with handling and storing muriatic acid pose another significant challenge. Pool operators must be trained in proper handling techniques and safety protocols to prevent accidents and ensure compliance with regulatory requirements. This necessity for specialized knowledge and training can be a barrier for many residential pool owners.
The frequency of pH testing and adjustment is another ongoing challenge. Ideally, pool pH should be tested and adjusted daily, but this level of maintenance is often impractical for many pool owners. Infrequent testing can lead to prolonged periods of imbalanced pH, potentially compromising water quality and swimmer comfort.
Automated pH control systems, while offering a potential solution, come with their own set of challenges. These systems can be expensive to install and maintain, and they may not always provide the level of precision required for optimal pH management. Additionally, they can malfunction or require frequent calibration, leading to periods of ineffective pH control.
The impact of other pool chemicals on pH levels adds another layer of complexity to pH management. For example, the addition of chlorine can affect pH levels, requiring careful balancing of multiple chemical parameters simultaneously. This interdependence of various pool chemistry factors makes it challenging to maintain stable pH levels over time.
Environmental factors, such as high bather loads during peak swimming seasons or the presence of organic matter from surrounding vegetation, can rapidly alter pool water chemistry. These factors necessitate more frequent pH testing and adjustment, placing additional demands on pool maintenance routines.
In conclusion, while muriatic acid remains a widely used tool for pH management in swimming pools, the current challenges in maintaining optimal pH levels are multifaceted and persistent. Addressing these challenges requires a combination of improved technologies, enhanced education for pool operators, and more robust, user-friendly pH control strategies.
One of the primary challenges is the dynamic nature of pool water chemistry. Factors such as rainfall, evaporation, and the introduction of contaminants from swimmers can cause rapid fluctuations in pH levels. These changes often occur faster than traditional pH adjustment methods can respond, leading to periods where the pool water is outside the optimal pH range.
The use of muriatic acid for pH adjustment, while effective, presents its own set of challenges. Accurate dosing of muriatic acid is crucial, as overdosing can lead to dangerously low pH levels, potentially causing skin and eye irritation for swimmers, as well as damage to pool equipment and surfaces. Conversely, underdosing may fail to adequately lower pH, leaving the pool water alkaline and reducing the effectiveness of chlorine sanitizers.
Safety concerns associated with handling and storing muriatic acid pose another significant challenge. Pool operators must be trained in proper handling techniques and safety protocols to prevent accidents and ensure compliance with regulatory requirements. This necessity for specialized knowledge and training can be a barrier for many residential pool owners.
The frequency of pH testing and adjustment is another ongoing challenge. Ideally, pool pH should be tested and adjusted daily, but this level of maintenance is often impractical for many pool owners. Infrequent testing can lead to prolonged periods of imbalanced pH, potentially compromising water quality and swimmer comfort.
Automated pH control systems, while offering a potential solution, come with their own set of challenges. These systems can be expensive to install and maintain, and they may not always provide the level of precision required for optimal pH management. Additionally, they can malfunction or require frequent calibration, leading to periods of ineffective pH control.
The impact of other pool chemicals on pH levels adds another layer of complexity to pH management. For example, the addition of chlorine can affect pH levels, requiring careful balancing of multiple chemical parameters simultaneously. This interdependence of various pool chemistry factors makes it challenging to maintain stable pH levels over time.
Environmental factors, such as high bather loads during peak swimming seasons or the presence of organic matter from surrounding vegetation, can rapidly alter pool water chemistry. These factors necessitate more frequent pH testing and adjustment, placing additional demands on pool maintenance routines.
In conclusion, while muriatic acid remains a widely used tool for pH management in swimming pools, the current challenges in maintaining optimal pH levels are multifaceted and persistent. Addressing these challenges requires a combination of improved technologies, enhanced education for pool operators, and more robust, user-friendly pH control strategies.
Muriatic Acid Application Techniques
01 pH adjustment in industrial processes
Muriatic acid, also known as hydrochloric acid, is used to adjust pH levels in various industrial processes. The acid's concentration and application can be controlled to achieve desired pH levels for specific manufacturing or treatment purposes. This is particularly important in water treatment, chemical production, and metal processing industries.- pH adjustment in industrial processes: Muriatic acid, also known as hydrochloric acid, is commonly used for pH adjustment in various industrial processes. The acid's concentration and the desired pH level are crucial factors in determining the amount of acid to be added. Proper control of pH levels is essential for optimizing chemical reactions, maintaining equipment, and ensuring product quality in industries such as water treatment, metal processing, and chemical manufacturing.
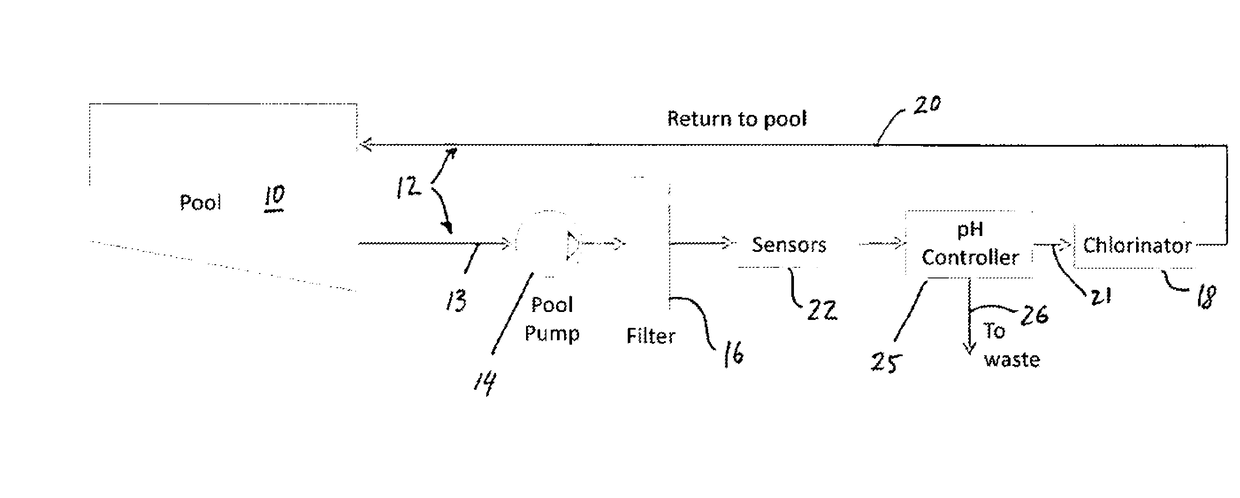
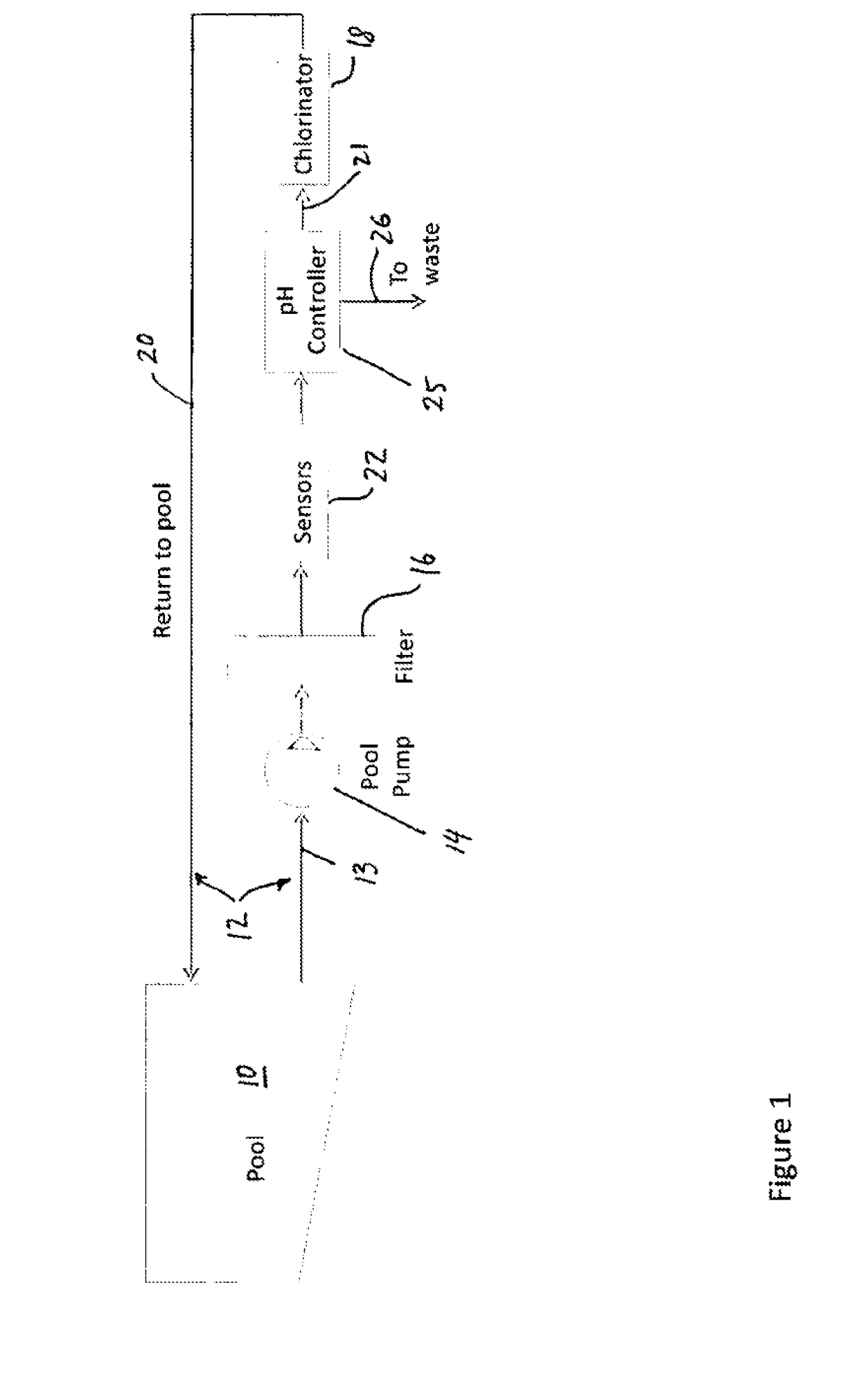
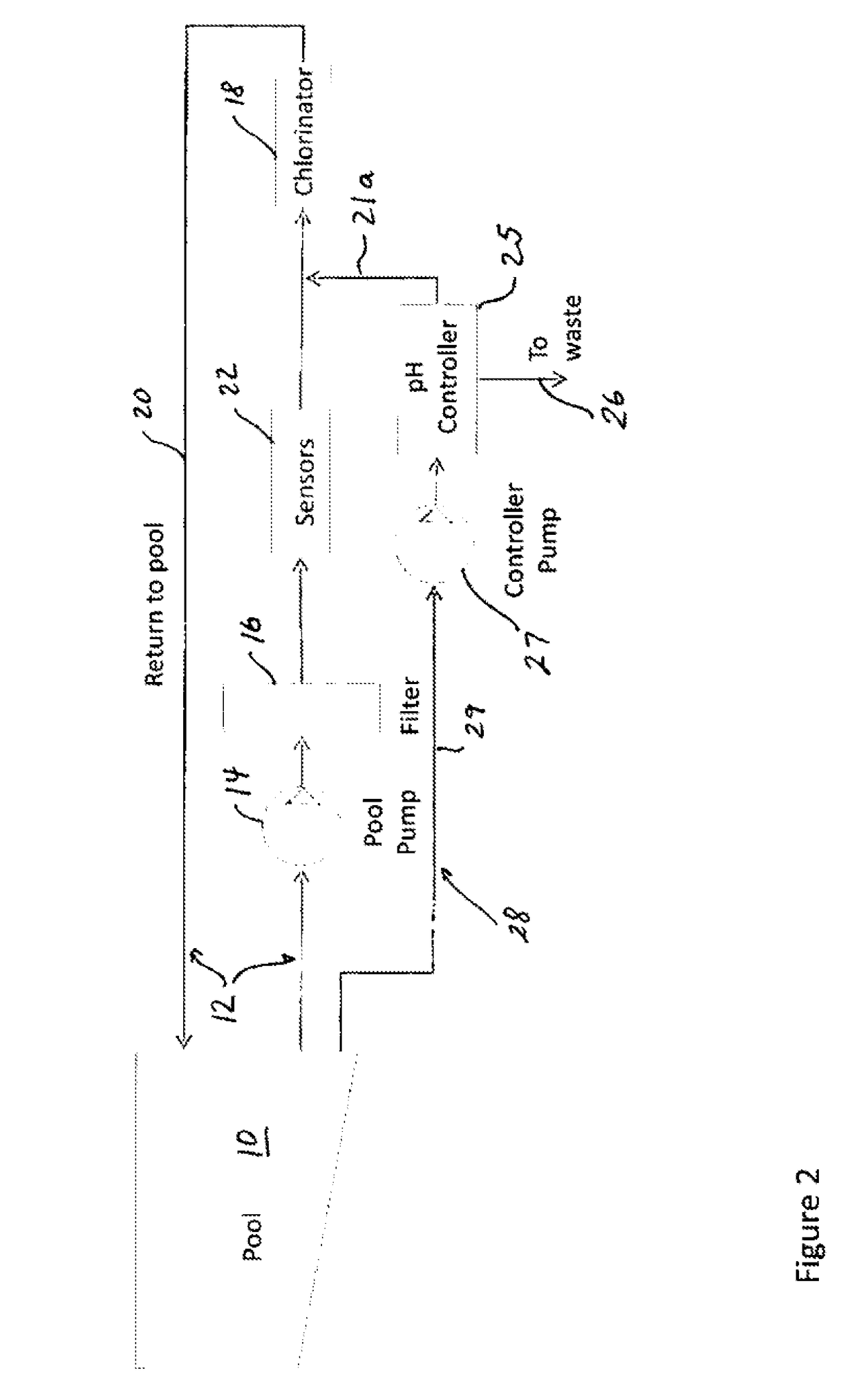
- Muriatic acid in swimming pool maintenance: Muriatic acid plays a significant role in swimming pool maintenance by helping to control pH levels. It is used to lower the pH of pool water when it becomes too alkaline. The ideal pH range for swimming pools is typically between 7.2 and 7.8. Proper application of muriatic acid ensures a safe and comfortable swimming environment while also optimizing the effectiveness of chlorine and other pool chemicals.
- pH control in biological and biochemical applications: Muriatic acid is utilized in various biological and biochemical applications for pH control. In laboratory settings, it is used to adjust the pH of buffers, culture media, and reaction mixtures. The precise control of pH levels is crucial for enzyme activity, protein stability, and cellular processes. Careful consideration of acid concentration and target pH is necessary to maintain optimal conditions for biological experiments and industrial bioprocesses.
- Corrosion prevention and surface treatment: Muriatic acid is employed in corrosion prevention and surface treatment applications. By carefully controlling the pH levels, it can be used to remove rust, scale, and other contaminants from metal surfaces. The acid's ability to etch and clean surfaces makes it valuable in industries such as metalworking, construction, and automotive manufacturing. Proper pH control is essential to achieve the desired surface treatment while minimizing damage to the underlying material.
- Environmental and waste treatment applications: Muriatic acid is used in environmental and waste treatment applications to control pH levels. In wastewater treatment, it helps neutralize alkaline effluents and adjust pH to optimal levels for biological treatment processes. The acid is also used in air pollution control systems to neutralize alkaline emissions. Careful monitoring and control of pH levels are crucial to ensure compliance with environmental regulations and to optimize treatment efficiency.
02 Muriatic acid in cleaning and etching
Muriatic acid is commonly used in cleaning and etching applications due to its ability to dissolve mineral deposits and remove rust. The pH level of the acid solution is crucial for effective cleaning without damaging the underlying material. Proper dilution and handling are essential for safety and optimal results.Expand Specific Solutions03 pH monitoring in biological systems
In biological and biochemical applications, monitoring and controlling pH levels using muriatic acid is critical. This includes maintaining optimal conditions for enzymatic reactions, cell cultures, and fermentation processes. Precise pH control can significantly impact the efficiency and yield of biological processes.Expand Specific Solutions04 Environmental impact of muriatic acid
The use of muriatic acid and its impact on environmental pH levels is an important consideration. Proper handling, neutralization, and disposal of muriatic acid are necessary to prevent adverse effects on aquatic ecosystems and soil chemistry. Regulations and guidelines exist to manage the environmental impact of acid use in various industries.Expand Specific Solutions05 Safety measures for handling muriatic acid
Due to its corrosive nature and potential to alter pH levels dramatically, specific safety measures are required when handling muriatic acid. This includes proper storage, personal protective equipment, neutralization procedures, and emergency response protocols. Training and adherence to safety guidelines are crucial to prevent accidents and ensure safe usage in various applications.Expand Specific Solutions
Key Players in Pool Chemical Industry
The market for muriatic acid in swimming pool chemistry is in a mature stage, with a stable global market size estimated at several hundred million dollars annually. The technology for pH balancing in pools is well-established, with major players like Fluidra, Pentair, and Zodiac Pool Care dominating the market. These companies offer comprehensive pool maintenance solutions, including pH control products. The competitive landscape is characterized by incremental innovations in product formulations and delivery systems, rather than disruptive technologies. Smaller specialized companies like Halogen Systems are focusing on niche areas such as chlorine sensing and monitoring, which complement pH balancing efforts. Overall, the market shows steady growth, driven by increasing pool ownership and growing awareness of water quality management.
Fluidra North America LLC
Technical Solution: Fluidra North America LLC has innovated in the field of swimming pool chemistry management with their advanced muriatic acid dispensing systems. Their technology focuses on precise acid injection methods that ensure even distribution throughout the pool. Fluidra's systems incorporate flow sensors and variable speed pumps to optimize acid distribution based on pool circulation patterns. They have also developed specialized acid-resistant materials for their dispensing equipment, enhancing durability and safety. Fluidra's approach includes the integration of pH management with other water quality parameters, such as chlorine levels and total alkalinity, to provide a holistic water balance solution. The company has invested in developing user-friendly interfaces that simplify the complex chemistry of pool maintenance for everyday pool owners[2][5].
Strengths: Holistic approach to water chemistry, durable equipment design, and user-friendly interfaces. Weaknesses: Potentially higher initial investment costs for comprehensive systems, and possible complexity in installation for existing pools.
Pentair Water Pool & Spa, Inc.
Technical Solution: Pentair Water Pool & Spa, Inc. has developed advanced pH control systems for swimming pools that utilize muriatic acid. Their technology includes automated dosing systems that precisely monitor and adjust pH levels. These systems employ sophisticated sensors and algorithms to detect pH fluctuations and dispense the appropriate amount of muriatic acid to maintain optimal water chemistry. Pentair's approach integrates with their broader pool management ecosystems, allowing for remote monitoring and control via smartphone apps. The company has also invested in research to develop more environmentally friendly alternatives to traditional muriatic acid, exploring the use of CO2 injection systems as a complementary or alternative pH lowering method[1][3].
Strengths: Precise automated control, integration with smart pool systems, and research into eco-friendly alternatives. Weaknesses: Reliance on proprietary systems may increase costs for pool owners, and potential over-reliance on automation could lead to less hands-on understanding of pool chemistry by users.
Innovations in pH Control Technologies
Chemical management for swimming pools
PatentInactiveUS20170203974A1
Innovation
- An electrolytic pH control system that selectively removes alkaline or acidic species from the electrolysis process, allowing for controlled pH adjustment without bulk acid addition, using a cell with separate compartments and a controller to manage electric potential and drainage of chemical species.
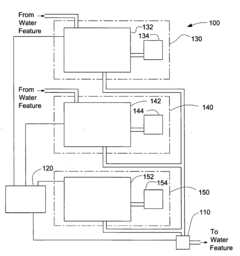
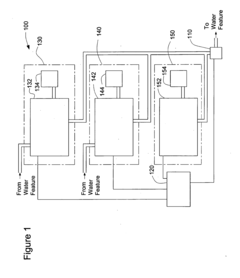
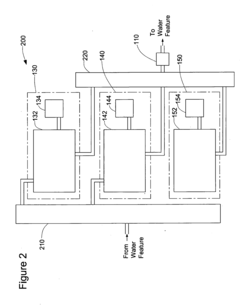
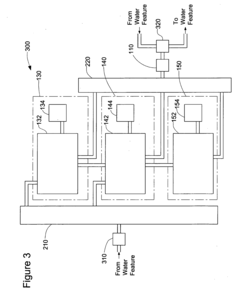
System for Maintaining pH and Sanitizing Agent Levels of Water in a Water Feature
PatentInactiveUS20090212782A1
Innovation
- An automated system with sensors and valves that monitor and adjust pH and sanitizing agent levels, using a controller to manage the flow of pH-modifying and sanitizing agent materials, ensuring preset levels are maintained and calibrating as needed.
Environmental Impact of Pool Chemicals
The use of muriatic acid and other pool chemicals for maintaining swimming pool water quality has significant environmental implications. These chemicals, while essential for ensuring safe and hygienic swimming conditions, can have adverse effects on the surrounding ecosystem when not properly managed.
Muriatic acid, also known as hydrochloric acid, is commonly used to lower the pH levels in swimming pools. When this acid is introduced into the pool water, it can alter the chemical composition of the water that is eventually discharged into the environment. If the pool water is not properly neutralized before disposal, it can lead to acidification of local water bodies, potentially harming aquatic life and disrupting ecosystems.
The impact extends beyond just the acid itself. The chemical reactions that occur in the pool water can result in the formation of disinfection by-products (DBPs), such as chloramines and trihalomethanes. These compounds can be harmful to both human health and the environment. When pool water containing these substances is released into the environment, it can contaminate groundwater and surface water sources.
Furthermore, the production and transportation of pool chemicals contribute to carbon emissions and energy consumption. The manufacturing processes for these chemicals often involve energy-intensive procedures and the use of non-renewable resources. The packaging and distribution of these products also add to their overall environmental footprint.
The overuse or misuse of pool chemicals can lead to chemical runoff, which can affect soil quality and vegetation in the surrounding areas. Excessive amounts of chlorine and other disinfectants can seep into the ground, potentially altering soil pH and affecting plant growth. This can have cascading effects on local biodiversity and ecosystem balance.
There are also concerns about the long-term accumulation of chemical residues in the environment. While individual pool owners may use relatively small amounts of chemicals, the cumulative effect of numerous pools in an area can be substantial. Over time, this can lead to the buildup of chemical compounds in soil and water systems, potentially affecting wildlife and human health.
To mitigate these environmental impacts, there is a growing trend towards more sustainable pool maintenance practices. This includes the use of alternative sanitization methods such as saltwater systems, UV treatment, and ozone generators, which can reduce the reliance on harsh chemicals. Additionally, proper pool water management techniques, such as regular testing and precise chemical dosing, can help minimize the amount of chemicals needed and reduce environmental discharge.
Muriatic acid, also known as hydrochloric acid, is commonly used to lower the pH levels in swimming pools. When this acid is introduced into the pool water, it can alter the chemical composition of the water that is eventually discharged into the environment. If the pool water is not properly neutralized before disposal, it can lead to acidification of local water bodies, potentially harming aquatic life and disrupting ecosystems.
The impact extends beyond just the acid itself. The chemical reactions that occur in the pool water can result in the formation of disinfection by-products (DBPs), such as chloramines and trihalomethanes. These compounds can be harmful to both human health and the environment. When pool water containing these substances is released into the environment, it can contaminate groundwater and surface water sources.
Furthermore, the production and transportation of pool chemicals contribute to carbon emissions and energy consumption. The manufacturing processes for these chemicals often involve energy-intensive procedures and the use of non-renewable resources. The packaging and distribution of these products also add to their overall environmental footprint.
The overuse or misuse of pool chemicals can lead to chemical runoff, which can affect soil quality and vegetation in the surrounding areas. Excessive amounts of chlorine and other disinfectants can seep into the ground, potentially altering soil pH and affecting plant growth. This can have cascading effects on local biodiversity and ecosystem balance.
There are also concerns about the long-term accumulation of chemical residues in the environment. While individual pool owners may use relatively small amounts of chemicals, the cumulative effect of numerous pools in an area can be substantial. Over time, this can lead to the buildup of chemical compounds in soil and water systems, potentially affecting wildlife and human health.
To mitigate these environmental impacts, there is a growing trend towards more sustainable pool maintenance practices. This includes the use of alternative sanitization methods such as saltwater systems, UV treatment, and ozone generators, which can reduce the reliance on harsh chemicals. Additionally, proper pool water management techniques, such as regular testing and precise chemical dosing, can help minimize the amount of chemicals needed and reduce environmental discharge.
Safety Protocols for Chemical Handling
Safety protocols for chemical handling in swimming pool maintenance, particularly concerning muriatic acid, are of paramount importance. The proper use and storage of muriatic acid require strict adherence to established guidelines to ensure the safety of pool operators and swimmers alike.
Personal protective equipment (PPE) is essential when handling muriatic acid. This includes chemical-resistant gloves, safety goggles, and protective clothing. A face shield may also be necessary to protect against splashes. Proper respiratory protection, such as a respirator with acid gas cartridges, should be worn when working in poorly ventilated areas or when there is a risk of inhaling acid fumes.
Storage of muriatic acid demands careful consideration. The acid should be kept in a cool, dry, well-ventilated area, away from direct sunlight and heat sources. Containers must be tightly sealed and stored in a locked cabinet or storage room, accessible only to authorized personnel. It is crucial to keep muriatic acid separate from other chemicals, especially chlorine-based products, as their interaction can produce dangerous chlorine gas.
When adding muriatic acid to a swimming pool, it should always be diluted before application. The correct procedure is to add acid to water, never water to acid, as this can cause a violent reaction. The dilution process should be performed in a well-ventilated area, preferably outdoors, to minimize exposure to fumes.
Proper disposal of muriatic acid and its containers is equally important. Empty containers should be thoroughly rinsed and disposed of according to local regulations. Any unused acid should be neutralized before disposal, typically by adding a base such as sodium bicarbonate under controlled conditions.
Emergency protocols must be in place and clearly communicated to all staff. This includes the location of emergency showers and eyewash stations, which should be easily accessible and regularly tested. A spill kit specifically designed for acid spills should be readily available, containing materials such as sodium bicarbonate or soda ash for neutralization.
Training is a critical component of safety protocols. All personnel involved in pool maintenance should receive comprehensive training on the proper handling, use, and storage of muriatic acid. This training should cover the potential hazards, proper PPE use, emergency procedures, and first aid measures in case of exposure.
Regular safety audits and inspections should be conducted to ensure compliance with safety protocols. This includes checking the integrity of storage containers, verifying the availability and condition of PPE, and reviewing emergency response procedures.
By implementing and strictly adhering to these safety protocols, the risks associated with handling muriatic acid in swimming pool chemistry can be significantly mitigated, ensuring a safer environment for both pool maintenance staff and swimmers.
Personal protective equipment (PPE) is essential when handling muriatic acid. This includes chemical-resistant gloves, safety goggles, and protective clothing. A face shield may also be necessary to protect against splashes. Proper respiratory protection, such as a respirator with acid gas cartridges, should be worn when working in poorly ventilated areas or when there is a risk of inhaling acid fumes.
Storage of muriatic acid demands careful consideration. The acid should be kept in a cool, dry, well-ventilated area, away from direct sunlight and heat sources. Containers must be tightly sealed and stored in a locked cabinet or storage room, accessible only to authorized personnel. It is crucial to keep muriatic acid separate from other chemicals, especially chlorine-based products, as their interaction can produce dangerous chlorine gas.
When adding muriatic acid to a swimming pool, it should always be diluted before application. The correct procedure is to add acid to water, never water to acid, as this can cause a violent reaction. The dilution process should be performed in a well-ventilated area, preferably outdoors, to minimize exposure to fumes.
Proper disposal of muriatic acid and its containers is equally important. Empty containers should be thoroughly rinsed and disposed of according to local regulations. Any unused acid should be neutralized before disposal, typically by adding a base such as sodium bicarbonate under controlled conditions.
Emergency protocols must be in place and clearly communicated to all staff. This includes the location of emergency showers and eyewash stations, which should be easily accessible and regularly tested. A spill kit specifically designed for acid spills should be readily available, containing materials such as sodium bicarbonate or soda ash for neutralization.
Training is a critical component of safety protocols. All personnel involved in pool maintenance should receive comprehensive training on the proper handling, use, and storage of muriatic acid. This training should cover the potential hazards, proper PPE use, emergency procedures, and first aid measures in case of exposure.
Regular safety audits and inspections should be conducted to ensure compliance with safety protocols. This includes checking the integrity of storage containers, verifying the availability and condition of PPE, and reviewing emergency response procedures.
By implementing and strictly adhering to these safety protocols, the risks associated with handling muriatic acid in swimming pool chemistry can be significantly mitigated, ensuring a safer environment for both pool maintenance staff and swimmers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!