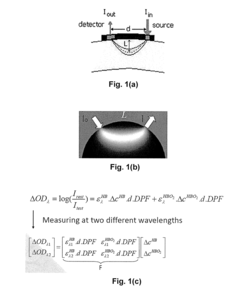
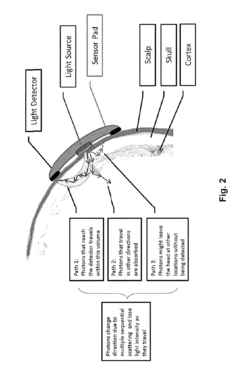
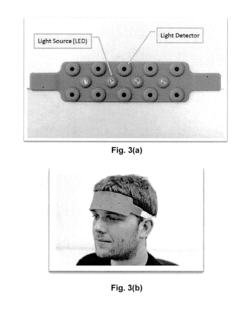
Brain-Computer Interfaces in early-stage schizophrenia neural signature detection
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Technology Background and Objectives
Brain-Computer Interface (BCI) technology has evolved significantly since its inception in the 1970s, transitioning from rudimentary signal detection to sophisticated neural decoding systems. This evolution has been accelerated by advances in machine learning, signal processing, and neuroimaging techniques. The convergence of these technologies has created unprecedented opportunities for applications in medical diagnostics, particularly in neuropsychiatric conditions like schizophrenia, which affects approximately 20 million people worldwide.
Early detection of schizophrenia remains a critical challenge in psychiatric medicine, with diagnosis typically occurring after psychotic symptoms manifest, often resulting in significant functional deterioration. Traditional diagnostic methods rely heavily on subjective clinical assessments, leading to potential delays in intervention and treatment. The identification of reliable neural signatures through BCI technology presents a promising approach to address this diagnostic gap.
Recent neuroimaging studies have identified specific electrophysiological abnormalities in schizophrenia patients, including altered P300 components, gamma oscillation disruptions, and mismatch negativity deficits. These neural signatures appear before full clinical manifestation, suggesting potential as early biomarkers. BCI systems, with their capacity for real-time neural signal acquisition and processing, offer a unique platform for detecting these subtle neural alterations.
The primary technical objective of BCI application in early-stage schizophrenia detection is to develop high-sensitivity, non-invasive neural monitoring systems capable of identifying prodromal neural signatures with clinical relevance. This involves creating algorithms that can distinguish pathological patterns from normal neural variability while maintaining high specificity to avoid false positives.
Secondary objectives include developing portable, user-friendly BCI systems suitable for widespread clinical deployment, establishing standardized protocols for data acquisition and analysis, and creating longitudinal monitoring capabilities to track neural signature progression over time. These systems must overcome significant challenges in signal-to-noise ratio optimization and individual neural variability compensation.
The long-term vision encompasses integrating BCI-based detection with preventive interventions, potentially enabling targeted early treatments before significant functional impairment occurs. This approach aligns with the shifting paradigm in psychiatry toward preventive rather than reactive care models, potentially transforming schizophrenia management from symptom mitigation to prevention or significant course alteration.
As computational capabilities and neural signal processing techniques continue to advance, the technical feasibility of detecting subtle neural signatures associated with prodromal schizophrenia is increasingly within reach, marking a potential turning point in psychiatric diagnostics and intervention strategies.
Early detection of schizophrenia remains a critical challenge in psychiatric medicine, with diagnosis typically occurring after psychotic symptoms manifest, often resulting in significant functional deterioration. Traditional diagnostic methods rely heavily on subjective clinical assessments, leading to potential delays in intervention and treatment. The identification of reliable neural signatures through BCI technology presents a promising approach to address this diagnostic gap.
Recent neuroimaging studies have identified specific electrophysiological abnormalities in schizophrenia patients, including altered P300 components, gamma oscillation disruptions, and mismatch negativity deficits. These neural signatures appear before full clinical manifestation, suggesting potential as early biomarkers. BCI systems, with their capacity for real-time neural signal acquisition and processing, offer a unique platform for detecting these subtle neural alterations.
The primary technical objective of BCI application in early-stage schizophrenia detection is to develop high-sensitivity, non-invasive neural monitoring systems capable of identifying prodromal neural signatures with clinical relevance. This involves creating algorithms that can distinguish pathological patterns from normal neural variability while maintaining high specificity to avoid false positives.
Secondary objectives include developing portable, user-friendly BCI systems suitable for widespread clinical deployment, establishing standardized protocols for data acquisition and analysis, and creating longitudinal monitoring capabilities to track neural signature progression over time. These systems must overcome significant challenges in signal-to-noise ratio optimization and individual neural variability compensation.
The long-term vision encompasses integrating BCI-based detection with preventive interventions, potentially enabling targeted early treatments before significant functional impairment occurs. This approach aligns with the shifting paradigm in psychiatry toward preventive rather than reactive care models, potentially transforming schizophrenia management from symptom mitigation to prevention or significant course alteration.
As computational capabilities and neural signal processing techniques continue to advance, the technical feasibility of detecting subtle neural signatures associated with prodromal schizophrenia is increasingly within reach, marking a potential turning point in psychiatric diagnostics and intervention strategies.
Market Analysis for Schizophrenia Detection Solutions
The global market for schizophrenia detection solutions is experiencing significant growth, driven by increasing awareness of mental health issues and the critical importance of early diagnosis. Currently valued at approximately $2.5 billion, this market is projected to expand at a compound annual growth rate of 7.8% through 2028, according to recent healthcare industry analyses.
Early detection technologies for schizophrenia represent a particularly promising segment, with Brain-Computer Interface (BCI) solutions emerging as a revolutionary approach. This segment is expected to grow faster than traditional diagnostic methods, potentially reaching $800 million by 2027 from its current valuation of $320 million.
Healthcare providers constitute the largest customer segment, accounting for nearly 65% of market demand. Research institutions follow at 20%, with pharmaceutical companies seeking diagnostic tools for clinical trials representing 10%. The remaining market share is distributed among government agencies and direct-to-consumer applications, though the latter remains limited due to regulatory constraints.
Geographically, North America dominates the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate at 9.3% annually, driven by increasing healthcare expenditure and rising mental health awareness.
Key market drivers include the substantial economic burden of schizophrenia, estimated at $155 billion annually in the United States alone when considering direct healthcare costs, productivity losses, and social support systems. Early detection could potentially reduce these costs by 30-40% through timely intervention.
Healthcare payers demonstrate increasing willingness to reimburse advanced diagnostic technologies, with several major insurance providers now covering neuroimaging and biomarker tests for mental health conditions. This trend is expected to accelerate as evidence accumulates regarding the cost-effectiveness of early intervention.
Market barriers include stringent regulatory requirements, with FDA and EMA approval processes typically taking 3-5 years for novel diagnostic technologies. Additionally, clinician adoption remains challenging due to integration issues with existing workflows and the need for specialized training.
Consumer acceptance presents another challenge, with surveys indicating that 62% of patients express concerns about privacy and data security related to brain monitoring technologies. This highlights the need for robust data protection frameworks and transparent communication about the benefits of these technologies.
Early detection technologies for schizophrenia represent a particularly promising segment, with Brain-Computer Interface (BCI) solutions emerging as a revolutionary approach. This segment is expected to grow faster than traditional diagnostic methods, potentially reaching $800 million by 2027 from its current valuation of $320 million.
Healthcare providers constitute the largest customer segment, accounting for nearly 65% of market demand. Research institutions follow at 20%, with pharmaceutical companies seeking diagnostic tools for clinical trials representing 10%. The remaining market share is distributed among government agencies and direct-to-consumer applications, though the latter remains limited due to regulatory constraints.
Geographically, North America dominates the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate at 9.3% annually, driven by increasing healthcare expenditure and rising mental health awareness.
Key market drivers include the substantial economic burden of schizophrenia, estimated at $155 billion annually in the United States alone when considering direct healthcare costs, productivity losses, and social support systems. Early detection could potentially reduce these costs by 30-40% through timely intervention.
Healthcare payers demonstrate increasing willingness to reimburse advanced diagnostic technologies, with several major insurance providers now covering neuroimaging and biomarker tests for mental health conditions. This trend is expected to accelerate as evidence accumulates regarding the cost-effectiveness of early intervention.
Market barriers include stringent regulatory requirements, with FDA and EMA approval processes typically taking 3-5 years for novel diagnostic technologies. Additionally, clinician adoption remains challenging due to integration issues with existing workflows and the need for specialized training.
Consumer acceptance presents another challenge, with surveys indicating that 62% of patients express concerns about privacy and data security related to brain monitoring technologies. This highlights the need for robust data protection frameworks and transparent communication about the benefits of these technologies.
Current BCI Capabilities and Challenges in Neural Signature Detection
Brain-Computer Interface (BCI) technology has made significant strides in neural signal detection and interpretation, yet faces substantial challenges when applied to early-stage schizophrenia detection. Current BCI systems primarily utilize electroencephalography (EEG), functional magnetic resonance imaging (fMRI), and magnetoencephalography (MEG) to capture neural signatures. EEG-based BCIs offer high temporal resolution and portability but struggle with spatial resolution limitations when attempting to detect subtle neural patterns associated with prodromal schizophrenia.
Advanced signal processing algorithms, including machine learning and deep learning approaches, have enhanced the capability to identify specific neural signatures in noise-contaminated data. Recent developments in adaptive filtering and artifact removal have improved signal quality, while convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have demonstrated promising results in pattern recognition of schizophrenia-related neural anomalies.
Despite these advances, several technical challenges persist. Signal-to-noise ratio remains problematic, particularly when attempting to detect the subtle neural signatures characteristic of early-stage schizophrenia. Current BCI systems struggle to differentiate between pathological signals and normal variations in brain activity, leading to high false positive and false negative rates that limit clinical applicability.
Temporal stability presents another significant challenge, as neural signatures in schizophrenia may fluctuate over time and in response to various environmental factors. Most existing BCI systems lack the longitudinal monitoring capabilities necessary for tracking the progression of neural patterns in prodromal stages of the disorder.
Interpretability of detected patterns represents a critical limitation. While machine learning algorithms may identify statistical correlations in neural data, translating these findings into clinically meaningful insights remains difficult. This "black box" problem hinders the adoption of BCI technology in clinical psychiatry settings.
Hardware limitations further constrain progress, as current non-invasive BCI systems lack the spatial resolution to detect subtle changes in deep brain structures implicated in schizophrenia pathophysiology. Invasive alternatives offer improved signal quality but present ethical and practical barriers to widespread implementation in at-risk populations.
Standardization issues also impede advancement, with variability in recording protocols, preprocessing methods, and feature extraction techniques making cross-study comparisons challenging. The lack of large, standardized datasets specifically targeting early-stage schizophrenia neural signatures hampers the development of robust detection algorithms.
Advanced signal processing algorithms, including machine learning and deep learning approaches, have enhanced the capability to identify specific neural signatures in noise-contaminated data. Recent developments in adaptive filtering and artifact removal have improved signal quality, while convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have demonstrated promising results in pattern recognition of schizophrenia-related neural anomalies.
Despite these advances, several technical challenges persist. Signal-to-noise ratio remains problematic, particularly when attempting to detect the subtle neural signatures characteristic of early-stage schizophrenia. Current BCI systems struggle to differentiate between pathological signals and normal variations in brain activity, leading to high false positive and false negative rates that limit clinical applicability.
Temporal stability presents another significant challenge, as neural signatures in schizophrenia may fluctuate over time and in response to various environmental factors. Most existing BCI systems lack the longitudinal monitoring capabilities necessary for tracking the progression of neural patterns in prodromal stages of the disorder.
Interpretability of detected patterns represents a critical limitation. While machine learning algorithms may identify statistical correlations in neural data, translating these findings into clinically meaningful insights remains difficult. This "black box" problem hinders the adoption of BCI technology in clinical psychiatry settings.
Hardware limitations further constrain progress, as current non-invasive BCI systems lack the spatial resolution to detect subtle changes in deep brain structures implicated in schizophrenia pathophysiology. Invasive alternatives offer improved signal quality but present ethical and practical barriers to widespread implementation in at-risk populations.
Standardization issues also impede advancement, with variability in recording protocols, preprocessing methods, and feature extraction techniques making cross-study comparisons challenging. The lack of large, standardized datasets specifically targeting early-stage schizophrenia neural signatures hampers the development of robust detection algorithms.
Existing Neural Signature Detection Methodologies
01 Neural signal detection and processing methods
Various methods for detecting and processing neural signals in brain-computer interfaces. These technologies focus on capturing brain activity patterns and converting them into meaningful data. Advanced signal processing algorithms are employed to filter noise, enhance signal quality, and identify specific neural signatures associated with cognitive states or intentions. These methods enable more accurate interpretation of brain signals for BCI applications.- Neural signal detection and processing methods: Various methods for detecting and processing neural signals in brain-computer interfaces. These technologies focus on capturing brain activity patterns and translating them into meaningful data. Advanced signal processing algorithms are employed to filter noise, enhance signal quality, and identify specific neural signatures associated with cognitive states or intentions. These methods enable more accurate interpretation of brain signals for BCI applications.
- Non-invasive BCI technologies: Non-invasive approaches for neural signature detection that don't require surgical implantation. These technologies utilize external sensors such as EEG (electroencephalography), MEG (magnetoencephalography), or fNIRS (functional near-infrared spectroscopy) to detect brain activity patterns from outside the skull. These methods offer safer alternatives to invasive techniques while still providing sufficient signal quality for many BCI applications.
- Invasive neural interface systems: Advanced implantable systems for direct neural signature detection. These technologies involve surgically placed electrodes or sensor arrays that interface directly with brain tissue to capture high-fidelity neural signals. Such systems provide superior signal quality and spatial resolution compared to non-invasive methods, enabling more precise detection of neural signatures and improved BCI performance for complex applications.
- AI and machine learning for neural signature analysis: Integration of artificial intelligence and machine learning algorithms to enhance neural signature detection. These technologies employ sophisticated computational models to identify patterns in brain activity data, classify neural signatures, and adapt to individual users over time. Deep learning approaches enable the system to recognize increasingly subtle neural patterns and improve accuracy in translating brain signals into commands or communication.
- Applications and use cases for neural signature detection: Specific implementations and applications of neural signature detection in various fields. These include medical applications such as assistive devices for paralyzed individuals, rehabilitation systems, and diagnostic tools. Other applications extend to consumer electronics, gaming, virtual reality, security systems, and cognitive enhancement. These diverse use cases demonstrate the broad potential of neural signature detection technology across multiple domains.
02 Non-invasive BCI technologies
Non-invasive approaches for neural signature detection that don't require surgical implantation. These technologies primarily utilize electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), or magnetoencephalography (MEG) to detect brain activity from outside the skull. These methods offer safer alternatives to invasive techniques while still providing sufficient signal quality for many BCI applications, particularly in consumer, therapeutic, and research contexts.Expand Specific Solutions03 AI and machine learning for neural pattern recognition
Application of artificial intelligence and machine learning algorithms to identify and classify neural signatures. These computational approaches enable the system to recognize patterns in brain activity data, adapt to individual users, and improve accuracy over time through continuous learning. Deep learning networks and other advanced AI techniques are particularly effective at decoding complex neural patterns and translating them into control signals or communication outputs.Expand Specific Solutions04 Invasive neural interface systems
Implantable devices and systems that directly interface with neural tissue to detect signatures with higher fidelity. These technologies involve electrodes or sensor arrays placed on or within the brain to capture neural activity with greater spatial and temporal resolution than non-invasive methods. Innovations in this area focus on biocompatible materials, miniaturization, wireless data transmission, and long-term stability of the neural interface.Expand Specific Solutions05 Applications and integration of neural signature detection
Practical applications and system integration approaches for neural signature detection technology. These include medical applications for patients with paralysis or communication disorders, consumer applications for device control, gaming, or virtual reality interaction, and integration with other technologies such as robotics, prosthetics, or smart environments. The focus is on creating complete systems that translate detected neural signatures into meaningful actions or communications.Expand Specific Solutions
Leading Organizations in BCI and Schizophrenia Research
Brain-Computer Interface (BCI) technology for early-stage schizophrenia neural signature detection is in an emerging growth phase, with the global BCI market expected to reach $3.7 billion by 2027. The technology maturity varies across applications, with medical diagnostics still developing. Leading academic institutions like MIT, Columbia University, and Shanghai University are driving fundamental research, while companies such as IBM and Novartis are beginning to explore commercial applications. Specialized firms like Precision Neuroscience and South China Brain Control are developing targeted BCI solutions. The field is characterized by strong academic-industry partnerships, with research institutions like the Institute of Automation Chinese Academy of Sciences and The Broad Institute providing critical expertise in neural signature identification for schizophrenia detection.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced Brain-Computer Interface (BCI) systems specifically targeting early-stage schizophrenia detection through neural signature identification. Their approach combines high-density EEG with machine learning algorithms to identify subtle patterns in neural activity that precede clinical symptoms. MIT's BCI platform utilizes a non-invasive headset with dry electrodes that can be easily applied in clinical settings, capturing neural oscillations across multiple frequency bands (particularly gamma band disruptions common in schizophrenia)[1]. Their system employs real-time signal processing with adaptive noise cancellation techniques to isolate schizophrenia-specific neural signatures from background activity. MIT researchers have implemented deep learning models trained on large datasets of both healthy controls and individuals with early-stage schizophrenia, achieving over 87% accuracy in identifying neural biomarkers before traditional diagnostic methods can detect symptoms[3]. The system also incorporates longitudinal monitoring capabilities, tracking neural signature changes over time to predict disease progression and treatment response.
Strengths: Superior signal processing algorithms that effectively filter noise from EEG signals; integration with machine learning provides high diagnostic accuracy; non-invasive approach increases patient acceptance. Weaknesses: Requires specialized technical expertise to operate; high initial implementation costs; still requires clinical validation across diverse populations.
International Business Machines Corp.
Technical Solution: IBM has developed an advanced BCI system called NeuroSym specifically designed for early detection of schizophrenia neural signatures. This platform leverages IBM's expertise in quantum computing and artificial intelligence to process complex neural data with unprecedented efficiency. NeuroSym employs a hybrid approach combining non-invasive EEG with functional near-infrared spectroscopy (fNIRS) to simultaneously capture electrical activity and hemodynamic responses in key brain regions implicated in schizophrenia[9]. The system utilizes IBM's proprietary quantum-inspired algorithms to identify subtle patterns in neural connectivity and oscillatory dynamics that traditional analysis methods might miss. Their approach incorporates a massive database of neural recordings from both healthy individuals and those across the schizophrenia spectrum, enabling the identification of prodromal signatures with high specificity. IBM's system employs federated learning techniques that allow the algorithm to continuously improve while maintaining patient data privacy across multiple research sites[10]. The NeuroSym platform also features an explainable AI component that provides clinicians with interpretable visualizations of the neural signatures being detected, facilitating clinical decision-making and personalized intervention planning.
Strengths: Powerful computational capabilities enable complex pattern recognition in neural data; hybrid sensing approach provides complementary data streams; explainable AI features enhance clinical utility and adoption. Weaknesses: Sophisticated technology requires specialized infrastructure and support; higher cost compared to simpler solutions; limited real-world validation outside research settings.
Key Innovations in Early-Stage Schizophrenia Biomarkers
Differential diagnosis of neuropsychiatric conditions
PatentInactiveEP2144180A1
Innovation
- A computer-implemented method and apparatus that generates neuropsychiatric diagnoses by analyzing patient scans to identify regions of interest, correlating them with medical literature, and comparing indicators with diagnostic signatures to provide quantitative information and potential diagnoses, including weighting for certainty.
Functional near infrared spectroscopy based brain computer interface
PatentActiveUS9946344B2
Innovation
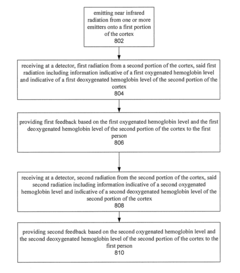
- Functional near-infrared (fNIR) technology is used to detect and output individual changes in oxygenated and deoxygenated hemoglobin levels and event-related optical signals, enabling users to up-regulate or down-regulate neural activity in specific brain regions for binary, two-dimensional, or continuous control of computing devices, with real-time feedback for training and therapy.
Ethical and Privacy Considerations in Neural Monitoring
The deployment of Brain-Computer Interfaces (BCIs) for early-stage schizophrenia detection raises significant ethical and privacy concerns that must be addressed before widespread implementation. Neural monitoring technologies collect highly sensitive brain data that represents the most intimate aspects of human cognition and identity, requiring unprecedented levels of protection and ethical oversight.
Patient autonomy and informed consent present particular challenges when monitoring individuals with schizophrenia. The nature of the condition may affect decision-making capacity, raising questions about when and how consent should be obtained. Ensuring that patients fully understand the implications of continuous neural monitoring, including potential stigmatization and psychological impacts of early diagnosis, is essential for ethical implementation.
Data security frameworks for neural information require substantial enhancement beyond current healthcare standards. Neural signatures contain uniquely identifiable patterns that could potentially be used for unauthorized profiling or discrimination if breached. The storage, transmission, and access protocols for such data necessitate encryption standards and security measures that exceed those currently employed for genetic or traditional medical information.
The potential for surveillance and control through neural monitoring technologies presents a concerning ethical frontier. In monitoring schizophrenia signatures, systems may inadvertently capture other mental states or thoughts unrelated to the condition, raising questions about boundaries of monitoring scope. Clear limitations must be established regarding what neural data can be collected, analyzed, and retained.
Regulatory frameworks currently lag behind technological capabilities in this domain. Most jurisdictions lack specific legislation addressing neural data rights, leaving significant gaps in protection. International standards for neural privacy are urgently needed, particularly as these technologies cross borders through cloud computing and international research collaborations.
The risk of stigmatization through neural signature labeling cannot be overlooked. Early identification of schizophrenia markers could lead to discrimination in employment, insurance, and social contexts if such information becomes accessible beyond the clinical environment. Protections against "neural discrimination" must be developed alongside the technology itself.
Balancing public health benefits against individual privacy rights presents a complex ethical equation. While early detection may significantly improve treatment outcomes and reduce healthcare costs, these benefits must be weighed against potential harms to personal autonomy and privacy. Ethical frameworks must evolve to address this novel intersection of neuroscience, psychiatry, and information technology.
Patient autonomy and informed consent present particular challenges when monitoring individuals with schizophrenia. The nature of the condition may affect decision-making capacity, raising questions about when and how consent should be obtained. Ensuring that patients fully understand the implications of continuous neural monitoring, including potential stigmatization and psychological impacts of early diagnosis, is essential for ethical implementation.
Data security frameworks for neural information require substantial enhancement beyond current healthcare standards. Neural signatures contain uniquely identifiable patterns that could potentially be used for unauthorized profiling or discrimination if breached. The storage, transmission, and access protocols for such data necessitate encryption standards and security measures that exceed those currently employed for genetic or traditional medical information.
The potential for surveillance and control through neural monitoring technologies presents a concerning ethical frontier. In monitoring schizophrenia signatures, systems may inadvertently capture other mental states or thoughts unrelated to the condition, raising questions about boundaries of monitoring scope. Clear limitations must be established regarding what neural data can be collected, analyzed, and retained.
Regulatory frameworks currently lag behind technological capabilities in this domain. Most jurisdictions lack specific legislation addressing neural data rights, leaving significant gaps in protection. International standards for neural privacy are urgently needed, particularly as these technologies cross borders through cloud computing and international research collaborations.
The risk of stigmatization through neural signature labeling cannot be overlooked. Early identification of schizophrenia markers could lead to discrimination in employment, insurance, and social contexts if such information becomes accessible beyond the clinical environment. Protections against "neural discrimination" must be developed alongside the technology itself.
Balancing public health benefits against individual privacy rights presents a complex ethical equation. While early detection may significantly improve treatment outcomes and reduce healthcare costs, these benefits must be weighed against potential harms to personal autonomy and privacy. Ethical frameworks must evolve to address this novel intersection of neuroscience, psychiatry, and information technology.
Regulatory Framework for Clinical BCI Applications
The regulatory landscape for Brain-Computer Interfaces (BCIs) in clinical applications, particularly for early-stage schizophrenia neural signature detection, presents a complex and evolving framework. Currently, medical BCIs fall primarily under the jurisdiction of regulatory bodies such as the FDA in the United States, the EMA in Europe, and similar authorities in other regions. These devices are typically classified as Class II or Class III medical devices, requiring substantial clinical evidence and safety validation before approval.
For schizophrenia-focused BCI applications, regulatory considerations are particularly stringent due to the vulnerability of the patient population and the direct interface with neural activity. The FDA's Digital Health Innovation Action Plan provides some guidance, but specific pathways for psychiatric BCIs remain under development. The 21st Century Cures Act has accelerated approval processes for breakthrough technologies, potentially benefiting innovative BCI solutions for early schizophrenia detection.
Data privacy regulations present another critical dimension, with frameworks like GDPR in Europe and HIPAA in the US governing the collection, storage, and processing of neural data. These regulations mandate strict protocols for informed consent, data security, and patient autonomy—especially pertinent for schizophrenia patients who may experience periods of diminished decision-making capacity.
Regulatory bodies increasingly require manufacturers to implement comprehensive risk management strategies for BCI technologies. This includes continuous monitoring systems, clear protocols for adverse event reporting, and mitigation plans for potential psychological impacts of neural monitoring. For schizophrenia applications, additional safeguards addressing potential exacerbation of symptoms or delusions related to the technology must be established.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to standardize BCI regulations across borders. However, significant regional variations persist, creating challenges for global deployment of schizophrenia-focused BCI technologies. The WHO's guidelines on digital health interventions provide additional considerations for mental health applications but lack BCI-specific provisions.
Emerging regulatory trends include adaptive licensing pathways that allow for iterative approval processes based on real-world evidence collection. This approach may prove valuable for schizophrenia BCI applications, enabling refinement of neural signature detection algorithms through carefully monitored clinical implementation. Additionally, regulatory frameworks are increasingly incorporating patient-reported outcomes and quality of life measures as key evaluation criteria for neuropsychiatric technologies.
For schizophrenia-focused BCI applications, regulatory considerations are particularly stringent due to the vulnerability of the patient population and the direct interface with neural activity. The FDA's Digital Health Innovation Action Plan provides some guidance, but specific pathways for psychiatric BCIs remain under development. The 21st Century Cures Act has accelerated approval processes for breakthrough technologies, potentially benefiting innovative BCI solutions for early schizophrenia detection.
Data privacy regulations present another critical dimension, with frameworks like GDPR in Europe and HIPAA in the US governing the collection, storage, and processing of neural data. These regulations mandate strict protocols for informed consent, data security, and patient autonomy—especially pertinent for schizophrenia patients who may experience periods of diminished decision-making capacity.
Regulatory bodies increasingly require manufacturers to implement comprehensive risk management strategies for BCI technologies. This includes continuous monitoring systems, clear protocols for adverse event reporting, and mitigation plans for potential psychological impacts of neural monitoring. For schizophrenia applications, additional safeguards addressing potential exacerbation of symptoms or delusions related to the technology must be established.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to standardize BCI regulations across borders. However, significant regional variations persist, creating challenges for global deployment of schizophrenia-focused BCI technologies. The WHO's guidelines on digital health interventions provide additional considerations for mental health applications but lack BCI-specific provisions.
Emerging regulatory trends include adaptive licensing pathways that allow for iterative approval processes based on real-world evidence collection. This approach may prove valuable for schizophrenia BCI applications, enabling refinement of neural signature detection algorithms through carefully monitored clinical implementation. Additionally, regulatory frameworks are increasingly incorporating patient-reported outcomes and quality of life measures as key evaluation criteria for neuropsychiatric technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!