Closed-loop Brain-Computer Interfaces for adaptive neurostimulation therapies
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Neurostimulation Background and Objectives
Brain-Computer Interfaces (BCIs) have evolved significantly since their inception in the 1970s, transitioning from rudimentary systems capable of basic signal detection to sophisticated platforms enabling bidirectional communication between the brain and external devices. The closed-loop BCI paradigm represents a revolutionary advancement in neurotechnology, integrating real-time neural signal acquisition, processing, and adaptive stimulation delivery to create responsive therapeutic systems.
The evolution of closed-loop BCIs for neurostimulation has been accelerated by parallel developments in microelectronics, machine learning algorithms, and neuroscience. Early open-loop neurostimulation systems, such as those used in deep brain stimulation for Parkinson's disease, delivered constant stimulation regardless of the patient's neural state. In contrast, closed-loop systems continuously monitor neural activity and dynamically adjust stimulation parameters based on detected biomarkers, offering personalized and efficient therapeutic interventions.
Recent technological breakthroughs in miniaturized implantable devices, wireless data transmission, and long-lasting power sources have overcome significant barriers to practical implementation. Additionally, advances in signal processing techniques have enhanced the ability to extract meaningful information from complex neural recordings in noisy environments, improving the precision of intervention timing and parameters.
The primary objective of closed-loop BCI neurostimulation research is to develop adaptive therapeutic systems capable of detecting pathological neural states and delivering precisely calibrated stimulation to normalize brain function. This approach aims to maximize therapeutic efficacy while minimizing side effects and energy consumption, addressing limitations of conventional pharmaceutical and open-loop neurostimulation approaches.
Specific technical goals include developing robust algorithms for real-time biomarker detection, optimizing stimulation parameters through machine learning approaches, creating minimally invasive recording and stimulation interfaces, and extending device longevity. Clinical objectives focus on expanding applications beyond movement disorders to conditions such as epilepsy, depression, anxiety disorders, and cognitive impairments.
The field is trending toward increasingly sophisticated closed-loop systems with multiple input channels, adaptive learning capabilities, and personalized stimulation protocols. Future developments are expected to incorporate advanced AI for predictive intervention, enhanced spatial and temporal precision of stimulation, and seamless integration with other therapeutic modalities.
As computational capabilities continue to advance and our understanding of neural circuit dynamics deepens, closed-loop BCIs are positioned to transform neurological and psychiatric care by offering unprecedented precision in treatment delivery and the potential for neural circuit restoration rather than mere symptom management.
The evolution of closed-loop BCIs for neurostimulation has been accelerated by parallel developments in microelectronics, machine learning algorithms, and neuroscience. Early open-loop neurostimulation systems, such as those used in deep brain stimulation for Parkinson's disease, delivered constant stimulation regardless of the patient's neural state. In contrast, closed-loop systems continuously monitor neural activity and dynamically adjust stimulation parameters based on detected biomarkers, offering personalized and efficient therapeutic interventions.
Recent technological breakthroughs in miniaturized implantable devices, wireless data transmission, and long-lasting power sources have overcome significant barriers to practical implementation. Additionally, advances in signal processing techniques have enhanced the ability to extract meaningful information from complex neural recordings in noisy environments, improving the precision of intervention timing and parameters.
The primary objective of closed-loop BCI neurostimulation research is to develop adaptive therapeutic systems capable of detecting pathological neural states and delivering precisely calibrated stimulation to normalize brain function. This approach aims to maximize therapeutic efficacy while minimizing side effects and energy consumption, addressing limitations of conventional pharmaceutical and open-loop neurostimulation approaches.
Specific technical goals include developing robust algorithms for real-time biomarker detection, optimizing stimulation parameters through machine learning approaches, creating minimally invasive recording and stimulation interfaces, and extending device longevity. Clinical objectives focus on expanding applications beyond movement disorders to conditions such as epilepsy, depression, anxiety disorders, and cognitive impairments.
The field is trending toward increasingly sophisticated closed-loop systems with multiple input channels, adaptive learning capabilities, and personalized stimulation protocols. Future developments are expected to incorporate advanced AI for predictive intervention, enhanced spatial and temporal precision of stimulation, and seamless integration with other therapeutic modalities.
As computational capabilities continue to advance and our understanding of neural circuit dynamics deepens, closed-loop BCIs are positioned to transform neurological and psychiatric care by offering unprecedented precision in treatment delivery and the potential for neural circuit restoration rather than mere symptom management.
Market Analysis for Adaptive Neural Therapies
The adaptive neurostimulation therapy market is experiencing significant growth, driven by increasing prevalence of neurological disorders and limitations of conventional pharmaceutical treatments. The global neurostimulation devices market was valued at approximately $6.8 billion in 2021 and is projected to reach $13.3 billion by 2028, growing at a CAGR of 9.5% during the forecast period. Closed-loop brain-computer interfaces (BCIs) represent a particularly promising segment within this broader market.
Neurological disorders affect over 1 billion people worldwide, with conditions like Parkinson's disease, epilepsy, and treatment-resistant depression creating substantial demand for innovative therapeutic approaches. Traditional medications often provide inadequate symptom control and produce significant side effects, creating a market opportunity for adaptive neurostimulation technologies that can deliver personalized, responsive treatment.
The market for adaptive neural therapies can be segmented by application, with movement disorders (particularly Parkinson's disease) currently representing the largest share at approximately 45% of the market. Epilepsy management applications are growing rapidly at 12% annually, while psychiatric applications (depression, OCD) represent the fastest-growing segment with 15% annual growth as clinical evidence accumulates.
Geographically, North America dominates the market with 42% share due to favorable reimbursement policies and high healthcare expenditure. Europe follows at 28%, while Asia-Pacific represents the fastest-growing region with 14% annual growth driven by improving healthcare infrastructure and increasing awareness of neurostimulation therapies.
Key market drivers include technological advancements in sensing technologies, miniaturization of implantable devices, and improvements in battery longevity. The integration of artificial intelligence algorithms that can predict symptom onset and optimize stimulation parameters represents a particularly valuable market differentiator, with companies investing heavily in proprietary algorithms.
Barriers to market expansion include high device costs ($15,000-$35,000 per implant), limited reimbursement coverage in many regions, and the invasive nature of current implantable systems. Regulatory approval pathways remain complex, with clinical trials for closed-loop systems requiring extensive safety validation.
Patient acceptance represents another critical market factor, with surveys indicating that 65% of potential candidates express concerns about device implantation. However, this resistance decreases significantly when patients are educated about the potential benefits of adaptive therapies compared to conventional treatments.
Neurological disorders affect over 1 billion people worldwide, with conditions like Parkinson's disease, epilepsy, and treatment-resistant depression creating substantial demand for innovative therapeutic approaches. Traditional medications often provide inadequate symptom control and produce significant side effects, creating a market opportunity for adaptive neurostimulation technologies that can deliver personalized, responsive treatment.
The market for adaptive neural therapies can be segmented by application, with movement disorders (particularly Parkinson's disease) currently representing the largest share at approximately 45% of the market. Epilepsy management applications are growing rapidly at 12% annually, while psychiatric applications (depression, OCD) represent the fastest-growing segment with 15% annual growth as clinical evidence accumulates.
Geographically, North America dominates the market with 42% share due to favorable reimbursement policies and high healthcare expenditure. Europe follows at 28%, while Asia-Pacific represents the fastest-growing region with 14% annual growth driven by improving healthcare infrastructure and increasing awareness of neurostimulation therapies.
Key market drivers include technological advancements in sensing technologies, miniaturization of implantable devices, and improvements in battery longevity. The integration of artificial intelligence algorithms that can predict symptom onset and optimize stimulation parameters represents a particularly valuable market differentiator, with companies investing heavily in proprietary algorithms.
Barriers to market expansion include high device costs ($15,000-$35,000 per implant), limited reimbursement coverage in many regions, and the invasive nature of current implantable systems. Regulatory approval pathways remain complex, with clinical trials for closed-loop systems requiring extensive safety validation.
Patient acceptance represents another critical market factor, with surveys indicating that 65% of potential candidates express concerns about device implantation. However, this resistance decreases significantly when patients are educated about the potential benefits of adaptive therapies compared to conventional treatments.
Closed-loop BCI Technical Challenges
Despite significant advancements in closed-loop Brain-Computer Interface (BCI) systems for adaptive neurostimulation therapies, several critical technical challenges persist that impede widespread clinical adoption and optimal therapeutic outcomes. These challenges span multiple domains including signal acquisition, processing, interpretation, and stimulation delivery.
Signal acquisition remains a fundamental challenge, with current technologies struggling to maintain stable, high-quality neural recordings over extended periods. Electrode degradation and tissue reactions at the neural interface often lead to signal deterioration over time, compromising the reliability of long-term implantable systems. Micromotion between electrodes and target neural tissue further exacerbates this issue, creating inconsistent recording conditions that complicate signal interpretation.
The miniaturization of closed-loop BCI systems presents another significant hurdle. Current systems typically require substantial external hardware for processing and power, limiting patient mobility and real-world applicability. The development of fully implantable systems with sufficient computational capacity while maintaining minimal power consumption and heat generation remains technically challenging.
Real-time processing capabilities represent a critical bottleneck in closed-loop systems. The detection of relevant biomarkers and neural signatures must occur with minimal latency to enable truly adaptive stimulation. This requires sophisticated algorithms capable of rapidly extracting meaningful information from complex, noisy neural signals while operating within the power and computational constraints of implantable devices.
Stimulation parameter optimization poses another complex challenge. Determining the optimal stimulation parameters (amplitude, frequency, pulse width, timing, and spatial targeting) for individual patients and specific neurological conditions requires sophisticated adaptive algorithms. Current approaches often rely on empirical testing rather than principled, patient-specific modeling.
Biological variability further complicates closed-loop BCI implementation. Neural responses to stimulation vary significantly between individuals and can change within the same individual over time due to neuroplasticity, disease progression, or environmental factors. Developing systems that can adapt to this variability while maintaining therapeutic efficacy remains challenging.
Security and safety considerations present additional technical hurdles. Closed-loop systems must incorporate robust safeguards against malfunction, unauthorized access, or inappropriate stimulation parameters that could harm patients. Implementing these safeguards while maintaining system performance and usability requires careful engineering and validation.
Regulatory pathways for adaptive neurostimulation systems remain complex, with limited precedent for systems that continuously modify their operation based on neural feedback. Demonstrating safety and efficacy for such dynamic systems requires novel testing paradigms and validation approaches beyond those used for conventional medical devices.
Signal acquisition remains a fundamental challenge, with current technologies struggling to maintain stable, high-quality neural recordings over extended periods. Electrode degradation and tissue reactions at the neural interface often lead to signal deterioration over time, compromising the reliability of long-term implantable systems. Micromotion between electrodes and target neural tissue further exacerbates this issue, creating inconsistent recording conditions that complicate signal interpretation.
The miniaturization of closed-loop BCI systems presents another significant hurdle. Current systems typically require substantial external hardware for processing and power, limiting patient mobility and real-world applicability. The development of fully implantable systems with sufficient computational capacity while maintaining minimal power consumption and heat generation remains technically challenging.
Real-time processing capabilities represent a critical bottleneck in closed-loop systems. The detection of relevant biomarkers and neural signatures must occur with minimal latency to enable truly adaptive stimulation. This requires sophisticated algorithms capable of rapidly extracting meaningful information from complex, noisy neural signals while operating within the power and computational constraints of implantable devices.
Stimulation parameter optimization poses another complex challenge. Determining the optimal stimulation parameters (amplitude, frequency, pulse width, timing, and spatial targeting) for individual patients and specific neurological conditions requires sophisticated adaptive algorithms. Current approaches often rely on empirical testing rather than principled, patient-specific modeling.
Biological variability further complicates closed-loop BCI implementation. Neural responses to stimulation vary significantly between individuals and can change within the same individual over time due to neuroplasticity, disease progression, or environmental factors. Developing systems that can adapt to this variability while maintaining therapeutic efficacy remains challenging.
Security and safety considerations present additional technical hurdles. Closed-loop systems must incorporate robust safeguards against malfunction, unauthorized access, or inappropriate stimulation parameters that could harm patients. Implementing these safeguards while maintaining system performance and usability requires careful engineering and validation.
Regulatory pathways for adaptive neurostimulation systems remain complex, with limited precedent for systems that continuously modify their operation based on neural feedback. Demonstrating safety and efficacy for such dynamic systems requires novel testing paradigms and validation approaches beyond those used for conventional medical devices.
Current Closed-loop BCI Solutions
01 Closed-loop BCI systems for adaptive neurostimulation
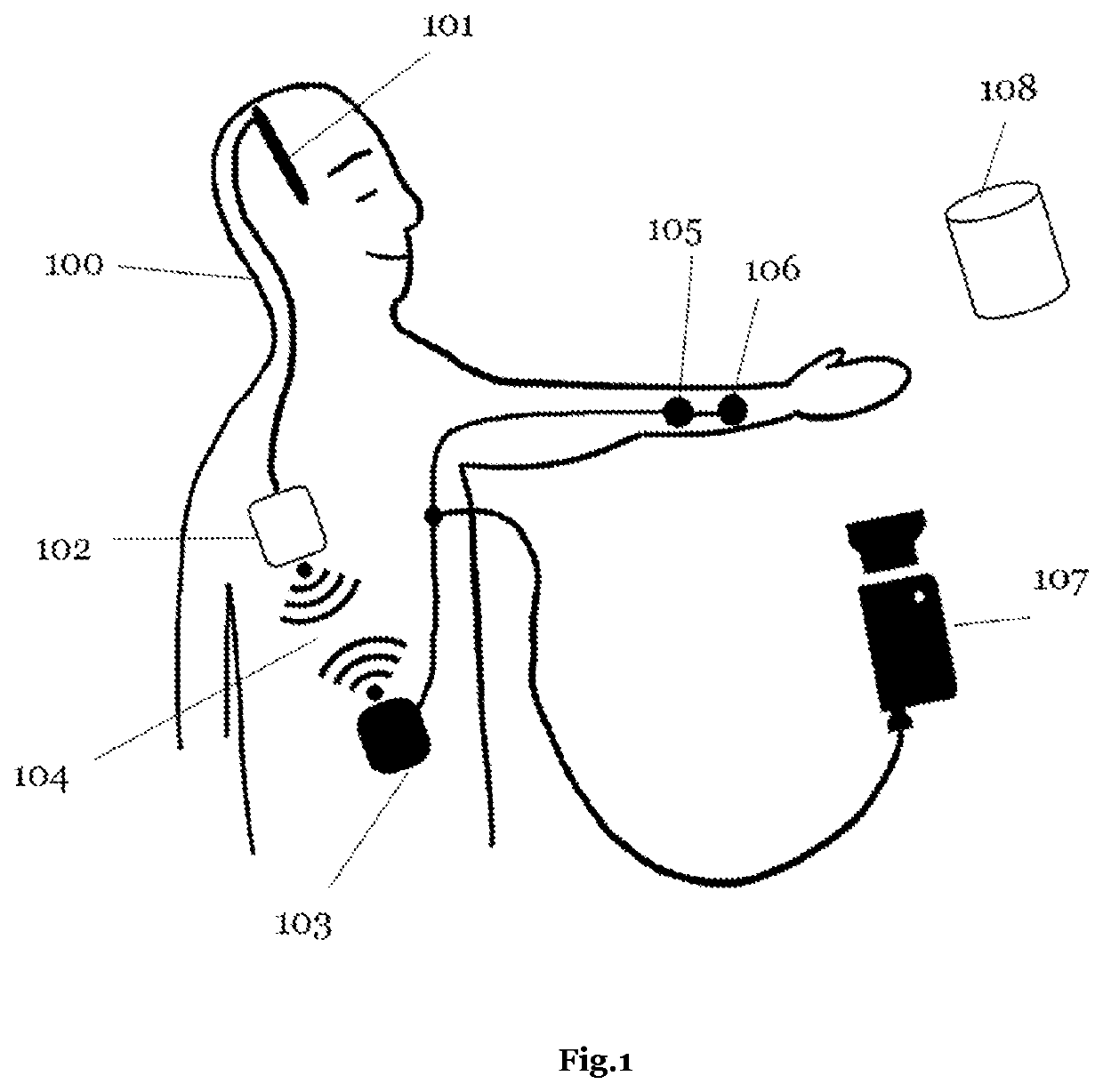
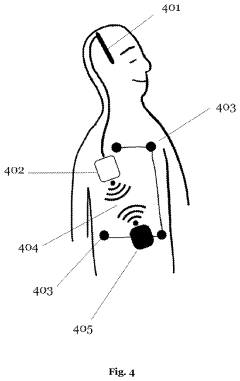
Closed-loop brain-computer interface systems that continuously monitor neural activity and automatically adjust stimulation parameters in real-time. These systems use feedback mechanisms to detect specific brain states or biomarkers and deliver appropriate neurostimulation, creating a self-regulating therapeutic approach. The adaptive algorithms can optimize stimulation parameters based on the patient's neural response, improving treatment efficacy while reducing side effects.- Closed-loop BCI systems for adaptive neurostimulation: Closed-loop brain-computer interface systems continuously monitor neural activity and automatically adjust stimulation parameters in real-time based on feedback. These systems detect specific neural patterns or biomarkers and deliver targeted stimulation only when needed, improving therapeutic efficacy while reducing side effects. The adaptive algorithms can learn from patient responses over time, personalizing treatment for conditions like epilepsy, Parkinson's disease, and other neurological disorders.
- Neural signal processing and biomarker detection: Advanced signal processing techniques are essential for extracting meaningful biomarkers from complex neural data in closed-loop BCI systems. These methods include machine learning algorithms, spectral analysis, and pattern recognition to identify specific neural signatures associated with disease states or cognitive functions. Real-time processing of EEG, ECoG, or deep brain signals allows for prompt detection of abnormal activity patterns, enabling timely intervention through adaptive neurostimulation.
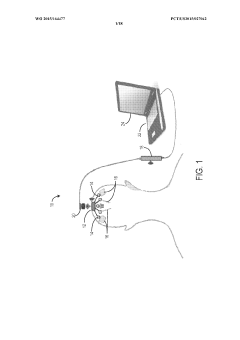
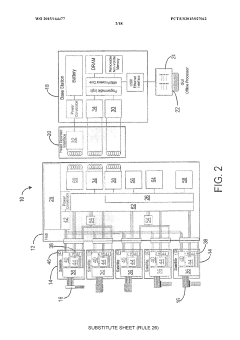
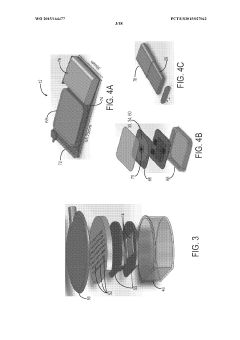
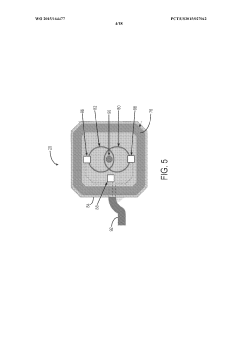
- Implantable devices and hardware innovations: Miniaturized implantable devices for closed-loop BCI systems incorporate sensing electrodes, processing units, and stimulation capabilities in compact form factors. These innovations include wireless power transmission, long-lasting batteries, and biocompatible materials to ensure durability and safety for chronic implantation. Advanced microelectronics enable simultaneous neural recording and stimulation with minimal interference, while wireless communication allows for external monitoring and parameter adjustments without additional surgical procedures.
- Machine learning and adaptive algorithms: Machine learning algorithms play a crucial role in optimizing closed-loop BCI systems by continuously adapting stimulation parameters based on neural feedback. These algorithms can identify patient-specific patterns and predict therapeutic needs, improving over time as they gather more data. Reinforcement learning approaches help determine optimal stimulation timing and intensity, while unsupervised learning methods can detect novel patterns in neural activity that may indicate changing disease states or treatment responses.
- Clinical applications and therapeutic targets: Closed-loop BCI systems with adaptive neurostimulation are being developed for various neurological and psychiatric conditions. Applications include seizure suppression in epilepsy, tremor control in movement disorders, mood regulation in psychiatric conditions, and cognitive enhancement in neurodegenerative diseases. These systems can be tailored to specific brain regions and neural circuits relevant to each condition, providing personalized therapy that responds to the dynamic nature of neurological disorders and minimizes disruption to normal brain function.
02 Neural signal processing and biomarker detection
Advanced signal processing techniques for extracting meaningful biomarkers from complex neural data. These methods involve filtering, feature extraction, and pattern recognition algorithms to identify specific neural signatures associated with neurological conditions. Real-time processing of EEG, LFP, or spike data enables the detection of pathological activity patterns that can trigger therapeutic interventions, forming the sensing component of closed-loop systems.Expand Specific Solutions03 Personalized stimulation parameter optimization
Machine learning and artificial intelligence approaches to customize neurostimulation parameters for individual patients. These systems analyze patient-specific neural responses to different stimulation settings and automatically determine optimal parameters. The algorithms can adapt over time as the patient's condition changes, providing continuously optimized therapy without requiring manual adjustments by clinicians.Expand Specific Solutions04 Implantable closed-loop neurostimulation devices
Miniaturized, fully implantable hardware systems that integrate sensing electrodes, signal processing, and stimulation capabilities. These devices are designed for long-term use with minimal maintenance, featuring power-efficient operation and wireless communication capabilities. The implantable systems can operate autonomously to deliver adaptive neurostimulation for conditions such as epilepsy, Parkinson's disease, and chronic pain.Expand Specific Solutions05 Therapeutic applications of adaptive neurostimulation
Specific clinical applications of closed-loop BCI systems for treating neurological and psychiatric disorders. These include seizure detection and prevention in epilepsy, tremor suppression in movement disorders, mood regulation in psychiatric conditions, and cognitive enhancement. The adaptive stimulation approaches are tailored to the pathophysiology of each condition, targeting specific neural circuits and adjusting stimulation based on real-time biomarkers of disease activity.Expand Specific Solutions
Leading BCI Industry Players
The closed-loop Brain-Computer Interface (BCI) market for adaptive neurostimulation therapies is in its early growth phase, characterized by significant research momentum and emerging commercial applications. The global market is projected to reach $3-5 billion by 2028, driven by increasing neurological disorder prevalence and technological advancements. Leading medical device companies like Medtronic and Boston Scientific are advancing commercial applications, while academic institutions (University of California, Tsinghua University, MIT) drive fundamental research. Emerging players such as PINS Medical, Inbrain Neuroelectronics, and CereGate are introducing innovative technologies. The field is transitioning from research to clinical implementation, with closed-loop systems showing promising results in treating conditions like Parkinson's disease, epilepsy, and chronic pain.
Boston Scientific Neuromodulation Corp.
Technical Solution: Boston Scientific has pioneered the Vercise Neural Navigator with STIMVIEW XT, an advanced closed-loop BCI system for adaptive neurostimulation. Their technology utilizes directional leads with multiple independent current control (MICC) that enables precise steering of electrical fields to target specific neural structures while avoiding adjacent areas that might cause side effects. The system incorporates proprietary sensing technology that can detect and analyze neural oscillations across different frequency bands, particularly beta band activity (13-30 Hz) which serves as a biomarker for motor symptoms in movement disorders. Boston Scientific's closed-loop architecture includes sophisticated machine learning algorithms that continuously analyze recorded neural signals and correlate them with clinical symptoms, automatically adjusting stimulation parameters to optimize therapeutic outcomes. Their platform features wireless connectivity for remote monitoring and adjustment capabilities, allowing clinicians to track neural responses and therapy effectiveness without requiring in-person visits[3][4].
Strengths: Boston Scientific's directional lead technology offers superior spatial precision in stimulation delivery, potentially reducing side effects while maximizing therapeutic benefit. Their system's ability to perform spectral analysis of neural signals enables more sophisticated biomarker detection. Weaknesses: The increased complexity of directional stimulation requires more complex programming and may present a steeper learning curve for clinicians. The system's closed-loop capabilities are still somewhat limited by the challenges of long-term reliable neural signal acquisition in chronic implants.
Medtronic, Inc.
Technical Solution: Medtronic has developed the Percept PC neurostimulator with BrainSense technology, representing a significant advancement in closed-loop brain-computer interfaces for adaptive neurostimulation. This system can simultaneously deliver stimulation therapy while recording and analyzing brain signals in real-time, allowing for personalized therapy adjustments based on neural feedback. The technology incorporates sophisticated sensing capabilities that can detect local field potentials (LFPs) and correlate them with patient symptoms and activities, creating a comprehensive closed-loop system. Medtronic's platform includes advanced algorithms that process neural data to automatically adjust stimulation parameters, addressing fluctuating symptoms in conditions like Parkinson's disease, essential tremor, and epilepsy. Their system architecture includes implantable leads with multiple electrode contacts, an implantable pulse generator with sensing capabilities, and external programming devices that allow clinicians to visualize neural data and customize therapy parameters[1][2].
Strengths: Medtronic's system offers real-time neural sensing during stimulation, allowing for dynamic therapy adjustments based on patient-specific biomarkers. Their extensive clinical experience and established market presence provide significant advantages in implementation and adoption. Weaknesses: The system still requires periodic clinical visits for major parameter adjustments, and the closed-loop capabilities are somewhat limited by battery life considerations and computational constraints of implantable devices.
Key Neural Interface Patents
Hybrid system for treating mental and emotional disorders with responsive brain stimulation
PatentWO2015164477A1
Innovation
- A closed-loop brain computer interface (BCI) system that uses affective decoding to monitor neural activity and deliver targeted stimulation based on patient intention, incorporating plasticity and volition components to adjust stimulation parameters in real-time, allowing patients to control the therapy and reducing side effects and improving battery life.
Closed loop computer-brain interface device
PatentActiveUS20220054830A1
Innovation
- A closed-loop computer brain interface (CLCBI) device that provides direct neurostimulation to afferent sensory axons, using a feedback loop to support behavioral tasks with tailored sensory cues, enhancing motor, sensory, and cognitive learning through a processing and transmission system that adjusts neuronal feedback signals based on sensor data.
Clinical Trial Requirements
Clinical trials for closed-loop Brain-Computer Interfaces (BCIs) in adaptive neurostimulation therapies require rigorous protocols that exceed standard medical device testing frameworks. These trials must adhere to both FDA regulations for implantable neural devices and specific BCI requirements that address the unique challenges of brain-machine interaction systems.
The design of clinical trials for closed-loop BCIs necessitates a phased approach. Phase I trials typically involve 10-20 patients and focus primarily on safety assessments, including surgical complications, device biocompatibility, and initial stimulation parameter tolerability. Phase II trials expand to 50-100 participants to evaluate preliminary efficacy and refine adaptive algorithms, while Phase III trials require 200-500 patients across multiple centers to establish definitive therapeutic benefits.
Patient selection criteria represent a critical component of trial design. Inclusion criteria must specify the neurological condition's severity, duration, and previous treatment failures. Exclusion criteria should address comorbidities that might interfere with BCI function or increase surgical risks. For conditions like treatment-resistant epilepsy or advanced Parkinson's disease, standardized assessment tools such as the Unified Parkinson's Disease Rating Scale (UPDRS) or seizure frequency logs must be incorporated.
Outcome measures for closed-loop BCI trials require multidimensional assessment frameworks. Primary endpoints typically include disease-specific metrics (seizure reduction, tremor improvement), while secondary endpoints must evaluate cognitive function, quality of life, and psychological adaptation to the neural interface. Long-term follow-up protocols extending 2-5 years post-implantation are essential to assess device durability and neural tissue responses.
Safety monitoring presents unique challenges in closed-loop systems. Continuous monitoring protocols must detect adverse events related to both hardware (electrode migration, infection) and software (stimulation algorithm malfunctions). Independent Data Safety Monitoring Boards with neurology, neurosurgery, and biomedical engineering expertise are required to evaluate emerging safety signals.
Regulatory considerations for these trials are particularly complex. The FDA's Investigational Device Exemption (IDE) pathway requires comprehensive preclinical testing data, including evidence of algorithm stability across varied physiological states. European trials must comply with the Medical Device Regulation (MDR) and specific neural interface provisions. Both jurisdictions require detailed plans for algorithm validation, stimulation parameter boundaries, and fail-safe mechanisms.
Ethical frameworks for closed-loop BCI trials must address informed consent challenges, particularly regarding algorithm modifications during the trial period. Protocols must include provisions for device explantation, continued access post-trial, and management of psychological dependence on neurostimulation therapies.
The design of clinical trials for closed-loop BCIs necessitates a phased approach. Phase I trials typically involve 10-20 patients and focus primarily on safety assessments, including surgical complications, device biocompatibility, and initial stimulation parameter tolerability. Phase II trials expand to 50-100 participants to evaluate preliminary efficacy and refine adaptive algorithms, while Phase III trials require 200-500 patients across multiple centers to establish definitive therapeutic benefits.
Patient selection criteria represent a critical component of trial design. Inclusion criteria must specify the neurological condition's severity, duration, and previous treatment failures. Exclusion criteria should address comorbidities that might interfere with BCI function or increase surgical risks. For conditions like treatment-resistant epilepsy or advanced Parkinson's disease, standardized assessment tools such as the Unified Parkinson's Disease Rating Scale (UPDRS) or seizure frequency logs must be incorporated.
Outcome measures for closed-loop BCI trials require multidimensional assessment frameworks. Primary endpoints typically include disease-specific metrics (seizure reduction, tremor improvement), while secondary endpoints must evaluate cognitive function, quality of life, and psychological adaptation to the neural interface. Long-term follow-up protocols extending 2-5 years post-implantation are essential to assess device durability and neural tissue responses.
Safety monitoring presents unique challenges in closed-loop systems. Continuous monitoring protocols must detect adverse events related to both hardware (electrode migration, infection) and software (stimulation algorithm malfunctions). Independent Data Safety Monitoring Boards with neurology, neurosurgery, and biomedical engineering expertise are required to evaluate emerging safety signals.
Regulatory considerations for these trials are particularly complex. The FDA's Investigational Device Exemption (IDE) pathway requires comprehensive preclinical testing data, including evidence of algorithm stability across varied physiological states. European trials must comply with the Medical Device Regulation (MDR) and specific neural interface provisions. Both jurisdictions require detailed plans for algorithm validation, stimulation parameter boundaries, and fail-safe mechanisms.
Ethical frameworks for closed-loop BCI trials must address informed consent challenges, particularly regarding algorithm modifications during the trial period. Protocols must include provisions for device explantation, continued access post-trial, and management of psychological dependence on neurostimulation therapies.
Neuroethical Considerations
The ethical implications of closed-loop Brain-Computer Interfaces (BCIs) for adaptive neurostimulation therapies represent a critical dimension requiring thorough examination. As these technologies advance toward clinical implementation, they raise profound questions about autonomy, identity, and the boundaries of human cognition. The direct interface between technology and neural processes introduces unprecedented ethical challenges that extend beyond traditional medical ethics frameworks.
Privacy concerns are paramount in BCI systems that continuously monitor neural activity. These devices collect vast amounts of brain data that may reveal sensitive information about cognitive processes, emotional states, and even thoughts. The storage, ownership, and potential commercialization of this neural data demand robust governance structures that currently lag behind technological capabilities. Questions of who controls this data—patients, clinicians, device manufacturers, or third parties—remain largely unresolved.
Informed consent takes on new complexity in adaptive neurostimulation contexts. Patients must understand not only the immediate therapeutic benefits but also the long-term implications of having a system that actively modifies neural activity. The dynamic nature of closed-loop systems, which evolve and adapt their stimulation parameters over time, creates scenarios where patients consent to interventions whose specific nature cannot be fully predicted at the outset.
The potential for neural adaptation to BCI systems raises questions about cognitive liberty and mental integrity. As neural circuits reorganize in response to ongoing stimulation, patients may experience subtle shifts in cognition, emotion, or personality. These changes may be therapeutic but simultaneously alter aspects of selfhood in ways that challenge conventional understandings of identity and authenticity.
Access and equity considerations cannot be overlooked. The sophisticated nature of closed-loop BCI technologies suggests they will initially be available only to privileged populations, potentially exacerbating existing healthcare disparities. Ensuring equitable access while maintaining safety and efficacy standards presents a significant ethical challenge for healthcare systems globally.
The risk of unauthorized access or "neural hacking" introduces security vulnerabilities with unprecedented consequences. Unlike conventional cybersecurity breaches, compromised BCIs could potentially influence neural function directly, raising concerns about cognitive security and mental sovereignty that have no historical parallel.
Regulatory frameworks must evolve to address these multifaceted ethical challenges. Current approaches to medical device regulation may be insufficient for technologies that actively modify neural function based on real-time processing of brain activity. Developing appropriate oversight mechanisms requires collaboration between neuroscientists, ethicists, policymakers, and patient advocates to ensure that innovation proceeds responsibly.
Privacy concerns are paramount in BCI systems that continuously monitor neural activity. These devices collect vast amounts of brain data that may reveal sensitive information about cognitive processes, emotional states, and even thoughts. The storage, ownership, and potential commercialization of this neural data demand robust governance structures that currently lag behind technological capabilities. Questions of who controls this data—patients, clinicians, device manufacturers, or third parties—remain largely unresolved.
Informed consent takes on new complexity in adaptive neurostimulation contexts. Patients must understand not only the immediate therapeutic benefits but also the long-term implications of having a system that actively modifies neural activity. The dynamic nature of closed-loop systems, which evolve and adapt their stimulation parameters over time, creates scenarios where patients consent to interventions whose specific nature cannot be fully predicted at the outset.
The potential for neural adaptation to BCI systems raises questions about cognitive liberty and mental integrity. As neural circuits reorganize in response to ongoing stimulation, patients may experience subtle shifts in cognition, emotion, or personality. These changes may be therapeutic but simultaneously alter aspects of selfhood in ways that challenge conventional understandings of identity and authenticity.
Access and equity considerations cannot be overlooked. The sophisticated nature of closed-loop BCI technologies suggests they will initially be available only to privileged populations, potentially exacerbating existing healthcare disparities. Ensuring equitable access while maintaining safety and efficacy standards presents a significant ethical challenge for healthcare systems globally.
The risk of unauthorized access or "neural hacking" introduces security vulnerabilities with unprecedented consequences. Unlike conventional cybersecurity breaches, compromised BCIs could potentially influence neural function directly, raising concerns about cognitive security and mental sovereignty that have no historical parallel.
Regulatory frameworks must evolve to address these multifaceted ethical challenges. Current approaches to medical device regulation may be insufficient for technologies that actively modify neural function based on real-time processing of brain activity. Developing appropriate oversight mechanisms requires collaboration between neuroscientists, ethicists, policymakers, and patient advocates to ensure that innovation proceeds responsibly.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!