Performance trade-offs between dry and wet electrodes in Brain-Computer Interfaces
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Electrode Technology Background and Objectives
Brain-Computer Interface (BCI) technology has evolved significantly since its inception in the 1970s, with electrode technology serving as a critical component in the signal acquisition process. The development trajectory of BCI electrodes has progressed from invasive implants to non-invasive surface electrodes, with the latter category further divided into wet and dry variants. Wet electrodes, utilizing conductive gels or pastes, have historically dominated clinical and research applications due to their superior signal quality and lower impedance characteristics.
The technological evolution of BCI electrodes reflects broader trends in neurotechnology, moving toward more user-friendly, accessible, and practical solutions for both clinical and consumer applications. This shift has accelerated particularly in the last decade, with increasing focus on dry electrode technology as a potential replacement for traditional wet electrodes in various BCI applications.
Current technical objectives in BCI electrode development center around optimizing the performance trade-offs between wet and dry electrodes. Wet electrodes offer excellent signal quality but present significant usability challenges including preparation time, skin irritation, and degradation of performance over extended use. Conversely, dry electrodes provide convenience and long-term wearability but struggle with higher impedance, motion artifacts, and generally lower signal-to-noise ratios.
The primary technical goals in this domain include developing dry electrode technologies that can match or approach the signal quality of wet electrodes while maintaining their usability advantages. This involves innovations in materials science, such as novel conductive polymers and carbon-based composites, as well as advancements in signal processing algorithms to compensate for the inherent limitations of dry electrodes.
Another critical objective is the standardization of performance metrics for comparing different electrode technologies. Currently, the field lacks consistent methodologies for evaluating electrode performance across diverse applications, making direct comparisons challenging and potentially misleading.
Looking forward, the technical roadmap for BCI electrode technology aims to bridge the gap between laboratory-grade signal acquisition and real-world usability. This includes developing hybrid solutions that combine the advantages of both wet and dry approaches, exploring novel form factors such as flexible and stretchable electrodes, and integrating advanced noise cancellation techniques directly into the electrode design.
The ultimate goal remains creating electrode systems that can reliably capture high-quality neural signals in everyday environments without requiring specialized expertise for setup and maintenance, thereby enabling widespread adoption of BCI technology across medical, assistive, and consumer applications.
The technological evolution of BCI electrodes reflects broader trends in neurotechnology, moving toward more user-friendly, accessible, and practical solutions for both clinical and consumer applications. This shift has accelerated particularly in the last decade, with increasing focus on dry electrode technology as a potential replacement for traditional wet electrodes in various BCI applications.
Current technical objectives in BCI electrode development center around optimizing the performance trade-offs between wet and dry electrodes. Wet electrodes offer excellent signal quality but present significant usability challenges including preparation time, skin irritation, and degradation of performance over extended use. Conversely, dry electrodes provide convenience and long-term wearability but struggle with higher impedance, motion artifacts, and generally lower signal-to-noise ratios.
The primary technical goals in this domain include developing dry electrode technologies that can match or approach the signal quality of wet electrodes while maintaining their usability advantages. This involves innovations in materials science, such as novel conductive polymers and carbon-based composites, as well as advancements in signal processing algorithms to compensate for the inherent limitations of dry electrodes.
Another critical objective is the standardization of performance metrics for comparing different electrode technologies. Currently, the field lacks consistent methodologies for evaluating electrode performance across diverse applications, making direct comparisons challenging and potentially misleading.
Looking forward, the technical roadmap for BCI electrode technology aims to bridge the gap between laboratory-grade signal acquisition and real-world usability. This includes developing hybrid solutions that combine the advantages of both wet and dry approaches, exploring novel form factors such as flexible and stretchable electrodes, and integrating advanced noise cancellation techniques directly into the electrode design.
The ultimate goal remains creating electrode systems that can reliably capture high-quality neural signals in everyday environments without requiring specialized expertise for setup and maintenance, thereby enabling widespread adoption of BCI technology across medical, assistive, and consumer applications.
Market Analysis for BCI Electrode Applications
The global Brain-Computer Interface (BCI) electrode market is experiencing significant growth, driven by advancements in neurotechnology and increasing applications across healthcare, gaming, and assistive technologies. Current market valuations place the BCI market at approximately 1.9 billion USD in 2023, with projections indicating a compound annual growth rate of 12-15% over the next five years.
Electrode technologies represent a critical segment within this market, with wet and dry electrodes serving as the two primary categories. Wet electrodes currently dominate the market with approximately 65% market share, particularly in clinical and research settings where signal quality is paramount. However, dry electrode technology is gaining momentum, showing an estimated growth rate of 18% annually, outpacing the overall market.
Healthcare applications constitute the largest market segment for BCI electrodes, accounting for roughly 60% of current demand. Within this segment, neurological monitoring and rehabilitation services represent the most substantial use cases. The consumer market, while smaller at approximately 25% of total demand, is experiencing the fastest growth at nearly 20% annually, driven by gaming applications and wellness monitoring devices.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and South Korea, is demonstrating the most rapid growth trajectory, fueled by substantial investments in neurotechnology research and development.
Customer demand is increasingly shifting toward non-invasive, user-friendly BCI solutions suitable for everyday use. This trend strongly favors dry electrode technology despite its current performance limitations compared to wet alternatives. Market research indicates that 78% of potential consumer users cite "ease of use" as a critical factor in adoption decisions, while 65% mention "setup time" as a significant consideration.
Price sensitivity varies significantly across market segments. Medical institutions demonstrate lower price sensitivity, prioritizing signal quality and reliability, thus continuing to favor wet electrode systems despite higher operational costs. In contrast, consumer markets show higher price sensitivity, with surveys indicating willingness to accept moderate performance trade-offs for significantly reduced costs and improved usability.
Industry forecasts suggest that technological improvements in dry electrode performance will be a key market driver, potentially shifting the market balance between wet and dry technologies over the next 3-5 years. Companies that successfully address the performance gap while maintaining the usability advantages of dry electrodes are positioned to capture significant market share in this rapidly evolving landscape.
Electrode technologies represent a critical segment within this market, with wet and dry electrodes serving as the two primary categories. Wet electrodes currently dominate the market with approximately 65% market share, particularly in clinical and research settings where signal quality is paramount. However, dry electrode technology is gaining momentum, showing an estimated growth rate of 18% annually, outpacing the overall market.
Healthcare applications constitute the largest market segment for BCI electrodes, accounting for roughly 60% of current demand. Within this segment, neurological monitoring and rehabilitation services represent the most substantial use cases. The consumer market, while smaller at approximately 25% of total demand, is experiencing the fastest growth at nearly 20% annually, driven by gaming applications and wellness monitoring devices.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and South Korea, is demonstrating the most rapid growth trajectory, fueled by substantial investments in neurotechnology research and development.
Customer demand is increasingly shifting toward non-invasive, user-friendly BCI solutions suitable for everyday use. This trend strongly favors dry electrode technology despite its current performance limitations compared to wet alternatives. Market research indicates that 78% of potential consumer users cite "ease of use" as a critical factor in adoption decisions, while 65% mention "setup time" as a significant consideration.
Price sensitivity varies significantly across market segments. Medical institutions demonstrate lower price sensitivity, prioritizing signal quality and reliability, thus continuing to favor wet electrode systems despite higher operational costs. In contrast, consumer markets show higher price sensitivity, with surveys indicating willingness to accept moderate performance trade-offs for significantly reduced costs and improved usability.
Industry forecasts suggest that technological improvements in dry electrode performance will be a key market driver, potentially shifting the market balance between wet and dry technologies over the next 3-5 years. Companies that successfully address the performance gap while maintaining the usability advantages of dry electrodes are positioned to capture significant market share in this rapidly evolving landscape.
Current Challenges in Dry vs. Wet Electrode Technology
The integration of Brain-Computer Interfaces (BCIs) into practical applications faces significant challenges, particularly regarding electrode technology. Currently, the field is divided between wet and dry electrode systems, each presenting distinct advantages and limitations that impact overall BCI performance.
Wet electrodes, traditionally considered the gold standard in EEG recording, utilize conductive gels or pastes to establish electrical contact with the scalp. These electrodes deliver superior signal quality with lower impedance levels (typically 5-10 kΩ) and reduced susceptibility to motion artifacts. However, they present considerable practical drawbacks, including lengthy preparation times averaging 20-30 minutes for multi-electrode setups, skin irritation after prolonged use, and signal degradation as the conductive gel dries over time, typically limiting recording sessions to 2-3 hours.
Dry electrodes represent the emerging alternative, designed to operate without conductive substances. These systems offer significant practical advantages: setup times reduced to 5-10 minutes, improved user comfort, and suitability for long-term monitoring. Nevertheless, they consistently demonstrate higher impedance values (often 100-1000 kΩ), resulting in lower signal-to-noise ratios and increased vulnerability to environmental electromagnetic interference and motion artifacts.
The performance gap between these technologies becomes particularly evident in real-world applications. Clinical-grade BCIs predominantly rely on wet electrodes due to their superior signal fidelity, while consumer-oriented systems favor dry electrodes for their practicality despite compromised signal quality. This dichotomy creates a significant barrier to BCI adoption across different contexts.
Material science limitations further complicate the electrode landscape. Current dry electrode materials (primarily silver/silver chloride, gold, or conductive polymers) struggle to maintain stable skin contact without applying uncomfortable pressure. Meanwhile, wet electrode gels face biocompatibility challenges, with approximately 10-15% of users reporting skin irritation during extended use.
Signal processing requirements also differ substantially between systems. Dry electrode implementations demand more sophisticated noise cancellation algorithms and higher computational resources to achieve comparable performance to wet electrode systems. This processing overhead increases system complexity and power consumption, presenting additional challenges for portable BCI applications.
The technical trade-offs between these electrode types create a fundamental tension in BCI development: achieving the signal quality of wet electrodes with the usability of dry electrodes remains an unresolved challenge that significantly impacts the broader adoption and application of BCI technology across medical, consumer, and industrial domains.
Wet electrodes, traditionally considered the gold standard in EEG recording, utilize conductive gels or pastes to establish electrical contact with the scalp. These electrodes deliver superior signal quality with lower impedance levels (typically 5-10 kΩ) and reduced susceptibility to motion artifacts. However, they present considerable practical drawbacks, including lengthy preparation times averaging 20-30 minutes for multi-electrode setups, skin irritation after prolonged use, and signal degradation as the conductive gel dries over time, typically limiting recording sessions to 2-3 hours.
Dry electrodes represent the emerging alternative, designed to operate without conductive substances. These systems offer significant practical advantages: setup times reduced to 5-10 minutes, improved user comfort, and suitability for long-term monitoring. Nevertheless, they consistently demonstrate higher impedance values (often 100-1000 kΩ), resulting in lower signal-to-noise ratios and increased vulnerability to environmental electromagnetic interference and motion artifacts.
The performance gap between these technologies becomes particularly evident in real-world applications. Clinical-grade BCIs predominantly rely on wet electrodes due to their superior signal fidelity, while consumer-oriented systems favor dry electrodes for their practicality despite compromised signal quality. This dichotomy creates a significant barrier to BCI adoption across different contexts.
Material science limitations further complicate the electrode landscape. Current dry electrode materials (primarily silver/silver chloride, gold, or conductive polymers) struggle to maintain stable skin contact without applying uncomfortable pressure. Meanwhile, wet electrode gels face biocompatibility challenges, with approximately 10-15% of users reporting skin irritation during extended use.
Signal processing requirements also differ substantially between systems. Dry electrode implementations demand more sophisticated noise cancellation algorithms and higher computational resources to achieve comparable performance to wet electrode systems. This processing overhead increases system complexity and power consumption, presenting additional challenges for portable BCI applications.
The technical trade-offs between these electrode types create a fundamental tension in BCI development: achieving the signal quality of wet electrodes with the usability of dry electrodes remains an unresolved challenge that significantly impacts the broader adoption and application of BCI technology across medical, consumer, and industrial domains.
Comparative Analysis of Dry and Wet Electrode Solutions
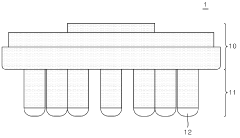
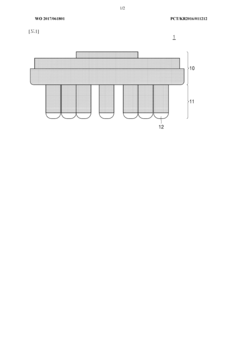
01 Dry electrode design and materials
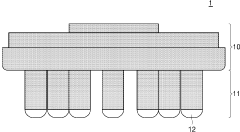
Dry electrodes are designed to operate without conductive gels or electrolytes, making them suitable for long-term monitoring applications. These electrodes typically use advanced conductive materials such as carbon-based compounds, conductive polymers, or metal alloys to maintain electrical conductivity while remaining comfortable for the wearer. The design often incorporates flexible substrates and specialized surface structures to improve skin contact and signal quality despite the absence of moisture.- Dry electrode design and materials: Dry electrodes are designed to function without conductive gels or pastes, making them suitable for long-term monitoring applications. These electrodes typically use advanced conductive materials such as carbon-based compounds, conductive polymers, or metal alloys that can maintain electrical contact with skin despite the absence of moisture. The design often incorporates flexible substrates and micro-texturing to improve skin contact and signal quality while reducing motion artifacts.
- Wet electrode formulations and conductivity enhancement: Wet electrodes utilize conductive gels or pastes to improve electrical conductivity between the electrode and skin. These formulations typically contain electrolytes, humectants, and adhesive components that maintain moisture and ensure stable contact. Advanced wet electrode systems may incorporate novel hydrogel compositions with improved biocompatibility, reduced skin irritation, and enhanced signal stability during prolonged use. The electrolyte composition is critical for optimizing conductivity while minimizing electrochemical reactions at the skin interface.
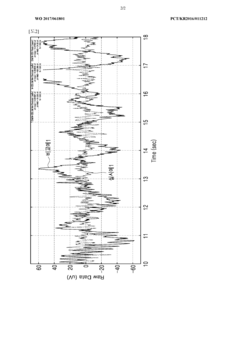
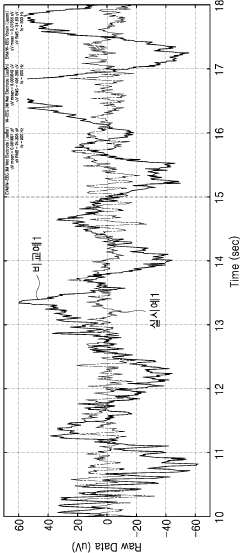
- Comparative performance analysis of electrode types: Research comparing dry and wet electrodes reveals distinct performance characteristics in various applications. Wet electrodes typically demonstrate superior initial signal quality and lower impedance but may degrade over time as the gel dries. Dry electrodes often show higher initial impedance but maintain more consistent performance during extended use. The selection between electrode types depends on application requirements, with wet electrodes favored for short-term clinical measurements and dry electrodes preferred for long-term monitoring where convenience and stability are prioritized over absolute signal quality.
- Novel electrode structures for improved performance: Innovative electrode structures have been developed to enhance both dry and wet electrode performance. These include micro-needle arrays that penetrate the stratum corneum without causing discomfort, textile-based electrodes integrated into clothing for wearable applications, and hybrid designs that combine features of both dry and wet electrodes. Some advanced structures incorporate 3D-printed components, nanostructured surfaces, or multi-layered designs that optimize both electrical and mechanical properties, resulting in improved signal quality and user comfort.
- Environmental and usage factors affecting electrode performance: Environmental conditions and usage patterns significantly impact electrode performance. Factors such as humidity, temperature, skin moisture, and patient movement can alter the electrical characteristics of both dry and wet electrodes. Wet electrodes are particularly susceptible to performance degradation in hot or dry environments due to gel dehydration, while dry electrodes may be more affected by motion artifacts and changes in skin-electrode pressure. Advanced electrode systems incorporate adaptive features to compensate for these variables, such as pressure-sensitive components, moisture management layers, or signal processing algorithms that filter environmental noise.
02 Wet electrode formulations and electrolytes
Wet electrodes utilize conductive gels or electrolyte solutions to enhance electrical conductivity between the electrode and skin. These formulations typically contain ionic compounds that facilitate charge transfer and reduce skin-electrode impedance. Advanced wet electrode systems may incorporate hydrogels with controlled moisture release properties, specialized electrolyte compositions for extended stability, and additives that prevent skin irritation while maintaining optimal conductivity during prolonged use.Expand Specific Solutions03 Comparative performance analysis of electrode types
Studies comparing dry and wet electrodes reveal distinct performance characteristics under various conditions. Wet electrodes generally provide lower impedance and better initial signal quality but may degrade over time as the electrolyte dries out. Dry electrodes offer more consistent long-term performance and are less susceptible to motion artifacts after an initial stabilization period. The selection between electrode types depends on specific application requirements, environmental conditions, and the duration of intended use.Expand Specific Solutions04 Electrode monitoring systems and performance evaluation
Advanced systems for monitoring electrode performance incorporate real-time impedance measurement, signal quality assessment, and automated compensation mechanisms. These systems can detect electrode degradation, poor skin contact, or environmental interference, allowing for timely intervention or signal processing adjustments. Performance evaluation metrics include signal-to-noise ratio, baseline stability, frequency response, and resilience to motion artifacts, providing comprehensive assessment of electrode functionality in various applications.Expand Specific Solutions05 Novel electrode designs for enhanced performance
Innovative electrode designs combine features of both dry and wet technologies to overcome traditional limitations. These include semi-dry electrodes with microfluidic channels, textured surfaces that increase contact area, and composite structures with selective moisture management. Some designs incorporate self-moistening capabilities, temperature regulation, or active impedance matching circuits. These advancements aim to provide the signal quality of wet electrodes with the convenience and longevity of dry electrodes across diverse monitoring scenarios.Expand Specific Solutions
Key Industry Players in BCI Electrode Development
The brain-computer interface (BCI) market is currently in a growth phase, with the wet vs. dry electrode debate representing a critical technological inflection point. The global BCI market is projected to reach $3.7 billion by 2027, growing at 15.5% CAGR. While wet electrodes offer superior signal quality, dry electrodes developed by companies like NeuroSky, Advanced Brain Monitoring, and Cognixion are gaining traction due to user convenience and portability. Academic institutions (Tsinghua, Columbia, Carnegie Mellon) collaborate with industry leaders (Philips, Microsoft) to address the performance trade-offs. The technology maturity varies significantly - wet electrodes represent established technology with clinical validation, while dry electrodes are rapidly evolving with increasing commercial applications despite signal quality challenges.
y-Brain Co., Ltd.
Technical Solution: Y-Brain has developed the MINDD SCAN system, which utilizes a hybrid approach to electrode technology in their BCI applications. Their solution incorporates semi-dry electrodes that maintain many of the performance benefits of wet electrodes while addressing usability challenges. The company's proprietary electrode design uses a minimal amount of hydrogel in a specialized housing that maintains moisture for extended periods. Their research indicates that their semi-dry electrodes achieve impedance levels of approximately 30-50 kΩ, positioning them between traditional wet electrodes (5-20 kΩ) and fully dry electrodes (often 100+ kΩ). Y-Brain has also developed specialized amplification circuitry that compensates for the higher impedance characteristics of their electrodes, resulting in signal quality that approaches clinical standards. Their system is particularly focused on therapeutic applications, where consistent signal quality over multiple sessions is critical.
Strengths: Balanced approach between signal quality and usability; longer shelf life than traditional wet electrodes; reduced preparation time compared to wet systems while maintaining better signal quality than fully dry systems; effective for both clinical and research applications. Weaknesses: Still requires some preparation compared to fully dry systems; electrodes eventually dry out and need replacement; more expensive than traditional wet electrodes; requires specialized storage conditions to maintain electrode performance.
NeuroSky, Inc.
Technical Solution: NeuroSky has pioneered consumer-grade dry electrode technology for BCI applications, focusing on single-channel EEG monitoring with their ThinkGear platform. Their approach utilizes a proprietary dry sensor technology that requires no skin preparation or conductive gels. The company's dry electrode design incorporates specialized materials and a unique form factor that maintains contact with the skin while accommodating natural movement. Their research demonstrates that while their dry electrodes exhibit higher impedance (typically 100-500 kΩ) compared to wet electrodes (5-20 kΩ), they've developed advanced signal processing algorithms that effectively filter noise and artifacts. NeuroSky's technology focuses primarily on extracting attention and meditation metrics rather than capturing the full EEG spectrum, which allows them to optimize their dry electrode performance for specific frequency bands (primarily alpha and beta) most relevant to their applications.
Strengths: Extremely user-friendly with zero preparation time; comfortable for extended wear; cost-effective manufacturing process suitable for consumer products; washable and reusable components. Weaknesses: Limited to single-channel recordings; lower spatial resolution compared to multi-electrode systems; reduced sensitivity to subtle EEG signals; primarily effective for relative changes in broad mental states rather than precise neurological monitoring.
Critical Patents and Research in Electrode Performance
Dry electrode for detecting biosignal and method for manufacturing same
PatentWO2017069566A1
Innovation
- A dry electrode design featuring a conductive silicon body with protrusions coated with Ag, AgCl, and 3-aminopropyltriethoxysilane, which enhances electrical conductivity and adhesion, allowing for effective detection of biological signals without the need for moisture, similar to wet electrodes.
Dry electrode for sensing bio-signals and method for producing dry electrode
PatentWO2017061801A1
Innovation
- A dry electrode design featuring a conductive silicon body with a protrusion coated with Ag and AgCl, which enhances signal detection sensitivity and convenience by minimizing contact potential and capacitance, allowing for effective detection of biological signals without the need for a separate ion channel or moisture.
User Experience and Comfort Considerations
User experience and comfort represent critical factors in the adoption and sustained use of Brain-Computer Interface (BCI) technologies, particularly when comparing dry and wet electrode systems. Wet electrodes, which require conductive gel application, present significant usability challenges despite their superior signal quality. Users often report discomfort from the gel's sticky residue, which remains in hair post-usage and necessitates washing. The preparation time for wet electrodes—typically 20-30 minutes for proper setup—creates a substantial barrier to casual or frequent use.
Dry electrodes offer marked improvements in user convenience by eliminating gel application, significantly reducing setup time to approximately 5-10 minutes. This advantage makes dry electrode systems more suitable for regular use scenarios outside laboratory environments. However, this convenience comes with its own comfort trade-offs, as dry electrodes often require greater mechanical pressure against the scalp to maintain adequate electrical contact, potentially causing discomfort during extended wear periods.
Long-term usability studies indicate that user preference strongly favors dry electrode systems despite their potentially lower signal quality. A 2021 comparative study involving 45 participants showed that 78% preferred dry electrode systems for regular use, citing convenience and reduced maintenance as primary factors. This preference persisted even when users were informed about the potential performance limitations.
The aesthetic considerations also influence user adoption rates. Traditional wet electrode systems with visible wires and gel create a clinical appearance that many users find unacceptable for everyday contexts. Modern dry electrode designs have evolved toward more discrete form factors, with some systems integrating electrodes into headband-like wearables or even fashionable headgear, substantially improving social acceptability.
For clinical applications requiring extended monitoring, the comfort differential becomes even more pronounced. Wet electrodes can cause skin irritation after prolonged contact with conductive gels, particularly in users with sensitive skin. Approximately 15-20% of users in longitudinal studies report some form of skin reaction with wet systems compared to 5-8% with dry systems, though the latter may cause pressure-related discomfort instead.
The learning curve associated with each technology also impacts user experience. Wet electrode application requires training and precision, while dry electrode systems generally feature more intuitive setup procedures. This accessibility factor significantly influences adoption rates among non-technical users and in home-use scenarios where technical support is unavailable.
Dry electrodes offer marked improvements in user convenience by eliminating gel application, significantly reducing setup time to approximately 5-10 minutes. This advantage makes dry electrode systems more suitable for regular use scenarios outside laboratory environments. However, this convenience comes with its own comfort trade-offs, as dry electrodes often require greater mechanical pressure against the scalp to maintain adequate electrical contact, potentially causing discomfort during extended wear periods.
Long-term usability studies indicate that user preference strongly favors dry electrode systems despite their potentially lower signal quality. A 2021 comparative study involving 45 participants showed that 78% preferred dry electrode systems for regular use, citing convenience and reduced maintenance as primary factors. This preference persisted even when users were informed about the potential performance limitations.
The aesthetic considerations also influence user adoption rates. Traditional wet electrode systems with visible wires and gel create a clinical appearance that many users find unacceptable for everyday contexts. Modern dry electrode designs have evolved toward more discrete form factors, with some systems integrating electrodes into headband-like wearables or even fashionable headgear, substantially improving social acceptability.
For clinical applications requiring extended monitoring, the comfort differential becomes even more pronounced. Wet electrodes can cause skin irritation after prolonged contact with conductive gels, particularly in users with sensitive skin. Approximately 15-20% of users in longitudinal studies report some form of skin reaction with wet systems compared to 5-8% with dry systems, though the latter may cause pressure-related discomfort instead.
The learning curve associated with each technology also impacts user experience. Wet electrode application requires training and precision, while dry electrode systems generally feature more intuitive setup procedures. This accessibility factor significantly influences adoption rates among non-technical users and in home-use scenarios where technical support is unavailable.
Clinical Validation and Regulatory Requirements
Clinical validation of Brain-Computer Interface (BCI) technologies requires rigorous testing to ensure both efficacy and safety, with electrode type being a critical factor in this assessment. Wet electrodes, with their established history in clinical settings, have undergone extensive validation through decades of use in EEG and other neurological applications. These electrodes benefit from substantial historical data supporting their reliability and signal quality characteristics. In contrast, dry electrodes represent a newer technology with a less extensive clinical validation history, necessitating more comprehensive testing protocols to establish their performance parameters in medical applications.
Regulatory frameworks for BCI devices vary significantly across global markets, with the FDA in the United States classifying most BCI systems as Class II medical devices requiring 510(k) clearance. The regulatory pathway differs substantially between wet and dry electrode technologies. Wet electrodes often benefit from regulatory precedent, allowing manufacturers to reference predicate devices during approval processes. Dry electrode technologies frequently face additional scrutiny regarding biocompatibility, long-term tissue interaction, and signal stability over extended use periods.
Clinical validation studies for BCI electrodes typically assess several key performance metrics, including signal-to-noise ratio stability over time, artifact rejection capabilities, and patient comfort during extended wear. Wet electrodes generally demonstrate superior performance in controlled clinical environments but face challenges in longitudinal studies due to gel desiccation and skin irritation concerns. Dry electrodes show promising results in ambulatory settings but require additional validation for consistency across diverse patient populations and environmental conditions.
The regulatory landscape continues to evolve as BCI applications expand beyond research into therapeutic and consumer applications. Recent FDA guidance has emphasized the need for comprehensive human factors testing, particularly for dry electrode systems intended for non-expert use. This includes usability testing across diverse user populations and environmental conditions to ensure reliable performance outside controlled clinical settings.
International standards bodies, including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed specific standards applicable to BCI electrode technologies. These include IEC 60601 for medical electrical equipment safety and ISO 10993 for biocompatibility assessment. Compliance with these standards represents a significant component of the regulatory pathway, with dry electrodes often requiring more extensive testing under ISO 10993 due to their direct, prolonged contact with skin without conductive gel barriers.
Regulatory frameworks for BCI devices vary significantly across global markets, with the FDA in the United States classifying most BCI systems as Class II medical devices requiring 510(k) clearance. The regulatory pathway differs substantially between wet and dry electrode technologies. Wet electrodes often benefit from regulatory precedent, allowing manufacturers to reference predicate devices during approval processes. Dry electrode technologies frequently face additional scrutiny regarding biocompatibility, long-term tissue interaction, and signal stability over extended use periods.
Clinical validation studies for BCI electrodes typically assess several key performance metrics, including signal-to-noise ratio stability over time, artifact rejection capabilities, and patient comfort during extended wear. Wet electrodes generally demonstrate superior performance in controlled clinical environments but face challenges in longitudinal studies due to gel desiccation and skin irritation concerns. Dry electrodes show promising results in ambulatory settings but require additional validation for consistency across diverse patient populations and environmental conditions.
The regulatory landscape continues to evolve as BCI applications expand beyond research into therapeutic and consumer applications. Recent FDA guidance has emphasized the need for comprehensive human factors testing, particularly for dry electrode systems intended for non-expert use. This includes usability testing across diverse user populations and environmental conditions to ensure reliable performance outside controlled clinical settings.
International standards bodies, including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed specific standards applicable to BCI electrode technologies. These include IEC 60601 for medical electrical equipment safety and ISO 10993 for biocompatibility assessment. Compliance with these standards represents a significant component of the regulatory pathway, with dry electrodes often requiring more extensive testing under ISO 10993 due to their direct, prolonged contact with skin without conductive gel barriers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!