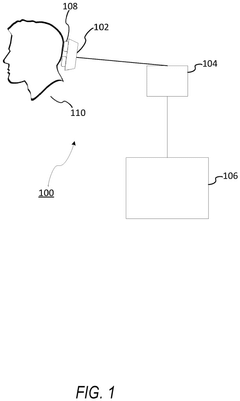
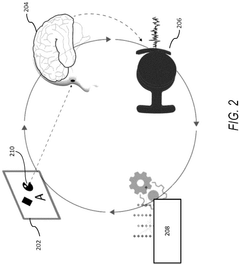
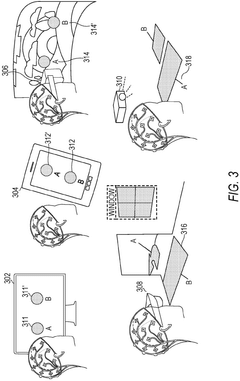
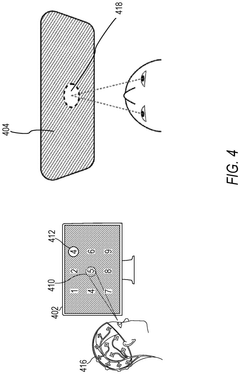
Role of Brain-Computer Interfaces in personalized neuromodulation therapies
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI Neuromodulation Background and Objectives
Brain-Computer Interfaces (BCIs) have evolved significantly since their inception in the 1970s, transitioning from rudimentary systems capable of basic signal detection to sophisticated platforms enabling complex neural decoding and bidirectional communication with the brain. This technological evolution has been accelerated by advances in computational power, machine learning algorithms, electrode materials, and neuroscientific understanding of brain function. The convergence of these developments has positioned BCIs as a promising frontier for personalized neuromodulation therapies.
Neuromodulation, the process of altering neural activity through targeted delivery of stimuli, has traditionally relied on predetermined protocols with limited adaptability to individual neural dynamics. The integration of BCIs introduces a paradigm shift by enabling real-time monitoring and responsive adjustment of neuromodulation parameters based on the individual's unique neural signatures and therapeutic needs.
The primary objective of BCI-enabled personalized neuromodulation is to establish closed-loop systems that continuously monitor neural activity, identify pathological patterns, and deliver precisely calibrated stimulation to normalize brain function. This approach aims to transcend the limitations of conventional "one-size-fits-all" neuromodulation by tailoring interventions to the specific neurophysiological characteristics of each patient.
Current technological trajectories suggest several key developments on the horizon, including miniaturization of implantable devices, enhanced biocompatibility of materials, improved spatial and temporal resolution of neural recordings, and more sophisticated algorithms for decoding neural intentions and emotional states. These advancements are expected to expand the therapeutic applications of BCI-guided neuromodulation beyond current use cases in epilepsy and movement disorders.
The field is witnessing a gradual shift from invasive to minimally invasive or non-invasive technologies, potentially broadening the accessibility of these therapies. Simultaneously, there is increasing emphasis on developing self-calibrating systems capable of adapting to the brain's neuroplastic changes over time, ensuring sustained therapeutic efficacy.
From a clinical perspective, the goal is to develop BCI-neuromodulation platforms that can address a spectrum of neurological and psychiatric conditions, including treatment-resistant depression, anxiety disorders, chronic pain syndromes, and neurodegenerative diseases. The ultimate vision encompasses personalized neural interfaces that can restore lost function, alleviate symptoms, and potentially enhance cognitive capabilities through precisely targeted neuromodulation.
As this technology continues to mature, interdisciplinary collaboration between neuroscientists, engineers, clinicians, and ethicists will be essential to navigate the complex challenges of translating laboratory innovations into viable therapeutic interventions while addressing important considerations regarding privacy, autonomy, and equitable access.
Neuromodulation, the process of altering neural activity through targeted delivery of stimuli, has traditionally relied on predetermined protocols with limited adaptability to individual neural dynamics. The integration of BCIs introduces a paradigm shift by enabling real-time monitoring and responsive adjustment of neuromodulation parameters based on the individual's unique neural signatures and therapeutic needs.
The primary objective of BCI-enabled personalized neuromodulation is to establish closed-loop systems that continuously monitor neural activity, identify pathological patterns, and deliver precisely calibrated stimulation to normalize brain function. This approach aims to transcend the limitations of conventional "one-size-fits-all" neuromodulation by tailoring interventions to the specific neurophysiological characteristics of each patient.
Current technological trajectories suggest several key developments on the horizon, including miniaturization of implantable devices, enhanced biocompatibility of materials, improved spatial and temporal resolution of neural recordings, and more sophisticated algorithms for decoding neural intentions and emotional states. These advancements are expected to expand the therapeutic applications of BCI-guided neuromodulation beyond current use cases in epilepsy and movement disorders.
The field is witnessing a gradual shift from invasive to minimally invasive or non-invasive technologies, potentially broadening the accessibility of these therapies. Simultaneously, there is increasing emphasis on developing self-calibrating systems capable of adapting to the brain's neuroplastic changes over time, ensuring sustained therapeutic efficacy.
From a clinical perspective, the goal is to develop BCI-neuromodulation platforms that can address a spectrum of neurological and psychiatric conditions, including treatment-resistant depression, anxiety disorders, chronic pain syndromes, and neurodegenerative diseases. The ultimate vision encompasses personalized neural interfaces that can restore lost function, alleviate symptoms, and potentially enhance cognitive capabilities through precisely targeted neuromodulation.
As this technology continues to mature, interdisciplinary collaboration between neuroscientists, engineers, clinicians, and ethicists will be essential to navigate the complex challenges of translating laboratory innovations into viable therapeutic interventions while addressing important considerations regarding privacy, autonomy, and equitable access.
Market Analysis for Personalized Neural Therapies
The personalized neuromodulation therapy market is experiencing significant growth, driven by advancements in Brain-Computer Interface (BCI) technologies and increasing prevalence of neurological disorders. Current market estimates value the global neuromodulation sector at approximately $6.8 billion, with projections suggesting a compound annual growth rate of 13.1% through 2028. The personalized segment, while currently representing about 15% of this market, is expected to grow at an accelerated rate of 17.5% annually as precision medicine approaches gain traction.
Demand for these therapies is primarily concentrated in regions with advanced healthcare infrastructure, with North America accounting for 42% of the global market share, followed by Europe at 28% and Asia-Pacific at 21%. The remaining regions collectively represent 9% of market demand, though emerging economies are showing increased adoption rates as healthcare spending rises.
Key market drivers include the aging global population, with neurological disorders affecting over 25% of individuals over 65 years of age. The economic burden of these conditions exceeds $800 billion annually in the United States alone, creating strong incentives for more effective treatment modalities. Additionally, patient preference for minimally invasive procedures and personalized medicine approaches is reshaping treatment paradigms across neurology and psychiatry.
Market segmentation reveals distinct therapeutic applications, with movement disorders (particularly Parkinson's disease) representing the largest current market segment at 38%, followed by psychiatric conditions (22%), pain management (19%), epilepsy (12%), and emerging applications (9%). The integration of BCI technologies is particularly transformative in the movement disorder segment, where closed-loop systems can provide real-time adaptive stimulation based on neural feedback.
Reimbursement landscapes significantly influence market penetration, with coverage policies varying substantially across regions. In the United States, Medicare now covers several neuromodulation therapies, though personalized approaches face additional hurdles in demonstrating cost-effectiveness. European markets benefit from more centralized assessment processes but face similar challenges in securing reimbursement for personalized therapeutic approaches.
Consumer willingness to pay remains strong for these therapies, with surveys indicating that patients with treatment-resistant conditions would accept significant out-of-pocket costs for effective personalized neuromodulation options. This price elasticity is particularly evident in movement disorders and chronic pain segments, where quality of life improvements can be substantial and immediately perceptible.
Demand for these therapies is primarily concentrated in regions with advanced healthcare infrastructure, with North America accounting for 42% of the global market share, followed by Europe at 28% and Asia-Pacific at 21%. The remaining regions collectively represent 9% of market demand, though emerging economies are showing increased adoption rates as healthcare spending rises.
Key market drivers include the aging global population, with neurological disorders affecting over 25% of individuals over 65 years of age. The economic burden of these conditions exceeds $800 billion annually in the United States alone, creating strong incentives for more effective treatment modalities. Additionally, patient preference for minimally invasive procedures and personalized medicine approaches is reshaping treatment paradigms across neurology and psychiatry.
Market segmentation reveals distinct therapeutic applications, with movement disorders (particularly Parkinson's disease) representing the largest current market segment at 38%, followed by psychiatric conditions (22%), pain management (19%), epilepsy (12%), and emerging applications (9%). The integration of BCI technologies is particularly transformative in the movement disorder segment, where closed-loop systems can provide real-time adaptive stimulation based on neural feedback.
Reimbursement landscapes significantly influence market penetration, with coverage policies varying substantially across regions. In the United States, Medicare now covers several neuromodulation therapies, though personalized approaches face additional hurdles in demonstrating cost-effectiveness. European markets benefit from more centralized assessment processes but face similar challenges in securing reimbursement for personalized therapeutic approaches.
Consumer willingness to pay remains strong for these therapies, with surveys indicating that patients with treatment-resistant conditions would accept significant out-of-pocket costs for effective personalized neuromodulation options. This price elasticity is particularly evident in movement disorders and chronic pain segments, where quality of life improvements can be substantial and immediately perceptible.
BCI Technology Landscape and Barriers
The current BCI technology landscape is characterized by a diverse array of approaches, ranging from invasive to non-invasive methodologies. Invasive BCIs, which involve surgical implantation of electrodes directly onto or into the brain tissue, offer superior signal quality and spatial resolution. These systems, exemplified by Utah arrays and ECoG grids, have demonstrated remarkable capabilities in decoding neural activity for controlling prosthetic limbs and facilitating communication for paralyzed individuals. However, their widespread adoption faces significant barriers including surgical risks, long-term biocompatibility issues, and the need for regular recalibration due to electrode degradation.
Non-invasive BCIs, primarily represented by EEG-based systems, have gained considerable traction in research and consumer applications due to their accessibility and minimal risk profile. Recent advancements in dry electrode technology and signal processing algorithms have improved their usability and performance. Nevertheless, these systems continue to struggle with limited spatial resolution, susceptibility to noise, and difficulty in detecting signals from deeper brain structures—critical limitations for personalized neuromodulation therapies.
The integration of BCIs with neuromodulation technologies presents additional technical challenges. Current closed-loop systems exhibit significant latency between signal detection and stimulation delivery, potentially reducing therapeutic efficacy. Furthermore, the heterogeneity of neurological conditions necessitates highly personalized approaches, requiring sophisticated algorithms capable of adapting to individual neural signatures and their evolution over time.
Regulatory barriers also significantly impact the BCI landscape. The FDA and similar international bodies have established stringent requirements for implantable neural devices, particularly those intended for therapeutic use. These regulations, while necessary for patient safety, often extend development timelines and increase costs, creating substantial hurdles for translating promising research into clinical applications.
Data security and privacy concerns represent another critical barrier, especially as BCIs become more sophisticated in their ability to decode cognitive and emotional states. The potential for unauthorized access to neural data raises profound ethical questions about mental privacy and cognitive liberty that remain largely unaddressed in current regulatory frameworks.
Scalability challenges further complicate the widespread implementation of BCI-based neuromodulation therapies. Current manufacturing processes for high-precision neural interfaces are costly and difficult to scale, while the expertise required for proper system calibration and maintenance remains scarce in clinical settings.
Non-invasive BCIs, primarily represented by EEG-based systems, have gained considerable traction in research and consumer applications due to their accessibility and minimal risk profile. Recent advancements in dry electrode technology and signal processing algorithms have improved their usability and performance. Nevertheless, these systems continue to struggle with limited spatial resolution, susceptibility to noise, and difficulty in detecting signals from deeper brain structures—critical limitations for personalized neuromodulation therapies.
The integration of BCIs with neuromodulation technologies presents additional technical challenges. Current closed-loop systems exhibit significant latency between signal detection and stimulation delivery, potentially reducing therapeutic efficacy. Furthermore, the heterogeneity of neurological conditions necessitates highly personalized approaches, requiring sophisticated algorithms capable of adapting to individual neural signatures and their evolution over time.
Regulatory barriers also significantly impact the BCI landscape. The FDA and similar international bodies have established stringent requirements for implantable neural devices, particularly those intended for therapeutic use. These regulations, while necessary for patient safety, often extend development timelines and increase costs, creating substantial hurdles for translating promising research into clinical applications.
Data security and privacy concerns represent another critical barrier, especially as BCIs become more sophisticated in their ability to decode cognitive and emotional states. The potential for unauthorized access to neural data raises profound ethical questions about mental privacy and cognitive liberty that remain largely unaddressed in current regulatory frameworks.
Scalability challenges further complicate the widespread implementation of BCI-based neuromodulation therapies. Current manufacturing processes for high-precision neural interfaces are costly and difficult to scale, while the expertise required for proper system calibration and maintenance remains scarce in clinical settings.
Current BCI-Driven Neuromodulation Approaches
01 Neural signal processing and interpretation
Advanced algorithms and methods for processing and interpreting neural signals captured from the brain. These technologies enable accurate translation of brain activity into meaningful commands or information, improving the precision and responsiveness of brain-computer interfaces. The systems typically involve signal acquisition, filtering, feature extraction, and classification components to decode neural patterns associated with specific intentions or cognitive states.- Neural signal processing and interpretation: Advanced algorithms and methods for processing neural signals captured from the brain to interpret user intent. These technologies focus on accurately translating brain activity into meaningful commands for external devices. Signal processing techniques include filtering, feature extraction, and pattern recognition to improve the accuracy and reliability of brain-computer interfaces. Machine learning approaches are employed to adapt to individual users' neural patterns and improve interpretation over time.
- Non-invasive BCI hardware designs: Development of non-invasive brain-computer interface hardware that can detect neural activity without requiring surgical implantation. These designs typically utilize electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), or other external sensing technologies to capture brain signals through the skull. Innovations focus on improving signal quality, reducing noise, enhancing comfort for extended wear, and miniaturizing components for practical everyday use.
- Invasive neural implant technologies: Implantable devices designed to directly interface with brain tissue for higher fidelity signal acquisition. These technologies include microelectrode arrays, flexible neural interfaces, and biocompatible materials that minimize tissue damage and immune response. Innovations focus on increasing electrode density, improving longevity of implants, reducing surgical risks, and enhancing the stability of neural recordings over extended periods.
- BCI applications for medical rehabilitation: Brain-computer interface systems specifically designed for medical rehabilitation and assistive technologies. These applications focus on restoring function for individuals with paralysis, movement disorders, or communication impairments. Systems include neural prosthetics, communication aids for locked-in patients, and rehabilitation tools that leverage neuroplasticity to help patients regain motor control through brain-directed therapy and neurofeedback.
- Consumer and commercial BCI applications: Brain-computer interface technologies developed for consumer markets and commercial applications beyond medical use. These include systems for gaming, virtual reality interaction, productivity enhancement, and emotional state monitoring. Innovations focus on user-friendly designs, simplified setup procedures, wireless connectivity, and integration with existing consumer electronics and software platforms to bring BCI technology to mainstream users.
02 Non-invasive BCI technologies
Non-invasive brain-computer interface technologies that capture brain signals without requiring surgical implantation. These approaches typically utilize electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), or other external sensing methods to detect neural activity through the skull. Non-invasive BCIs offer safer alternatives to invasive methods while still enabling direct brain-to-computer communication for various applications including assistive technologies and consumer products.Expand Specific Solutions03 Implantable neural interfaces
Implantable devices designed to establish direct connections with neural tissue for high-fidelity brain signal acquisition. These technologies include microelectrode arrays, flexible neural probes, and wireless implantable systems that can be surgically placed in or on the brain. The implants are engineered to minimize tissue damage while maximizing signal quality and longevity, enabling more precise control of external devices through direct neural recording and stimulation.Expand Specific Solutions04 BCI applications for medical rehabilitation
Brain-computer interface systems specifically designed for medical rehabilitation and assistive purposes. These technologies help individuals with motor impairments, communication disorders, or neurological conditions regain functionality or develop alternative means of interaction with their environment. Applications include controlling prosthetic limbs, operating wheelchairs, facilitating communication for locked-in patients, and supporting rehabilitation exercises for stroke recovery or spinal cord injury patients.Expand Specific Solutions05 Consumer and commercial BCI applications
Brain-computer interface technologies developed for consumer markets and commercial applications beyond medical use. These include systems for gaming, virtual reality interaction, productivity enhancement, emotional state monitoring, and other everyday applications. The technologies typically prioritize user-friendliness, portability, and non-invasiveness while providing novel ways to interact with digital environments through thought or mental states alone.Expand Specific Solutions
Key Industry Players and Research Institutions
Brain-Computer Interfaces (BCIs) in personalized neuromodulation therapies are evolving rapidly, with the market currently in an early growth phase. The global neuromodulation market is projected to reach $13.3 billion by 2028, growing at 13-15% CAGR. Technologically, the field shows varying maturity levels across applications. Industry leaders like Boston Scientific Neuromodulation and NeuroPace have established clinical applications, while innovative startups such as INBRAIN Neuroelectronics, Precision Neuroscience, and NextMind are advancing cutting-edge approaches using novel materials and AI integration. Academic institutions including Tianjin University, University of Washington, and Carnegie Mellon are driving fundamental research, while collaborations between research centers and companies like HRL Laboratories are accelerating translation to clinical applications. The competitive landscape reflects a blend of established medical device manufacturers and emerging neurotechnology innovators focused on increasing precision and personalization.
Boston Scientific Neuromodulation Corp.
Technical Solution: Boston Scientific has developed advanced closed-loop neuromodulation systems that integrate BCI technology for personalized therapy delivery. Their Vercise Neural Navigator system combines directional lead technology with sophisticated sensing algorithms to detect brain activity patterns and automatically adjust stimulation parameters in real-time. The platform incorporates machine learning algorithms that analyze neural signals to identify patient-specific biomarkers associated with symptom manifestation, enabling truly adaptive therapy. Their GUIDE DBS system utilizes a proprietary algorithm that processes electrophysiological data to create individualized stimulation maps, allowing clinicians to visualize neural responses and optimize electrode placement and stimulation settings based on each patient's unique neural architecture[1][3]. The company has also pioneered multiple-source current steering technology that enables precise control over the spatial distribution of electrical fields, creating highly customized stimulation patterns tailored to individual neural anatomy.
Strengths: Industry-leading precision in stimulation delivery through directional leads and current steering technology; robust clinical validation across multiple neurological conditions; sophisticated closed-loop capabilities with real-time adaptation. Weaknesses: Higher cost compared to conventional systems; requires specialized training for optimal programming; limited battery life in some implantable pulse generators when running advanced sensing algorithms.
NeuroPace, Inc.
Technical Solution: NeuroPace has pioneered responsive neurostimulation (RNS) technology that represents one of the most advanced implementations of BCI for personalized neuromodulation therapy. Their RNS System continuously monitors brain activity through implanted electrodes, detecting patient-specific abnormal patterns that precede seizures in epilepsy patients. Upon detection, the system delivers precisely timed electrical stimulation to normalize brain activity before clinical symptoms occur. The platform incorporates sophisticated machine learning algorithms that improve over time, adapting to each patient's unique seizure patterns and optimizing stimulation parameters accordingly[2]. NeuroPace's technology includes a proprietary ECoG signal processing pipeline that extracts meaningful biomarkers from complex brain activity, enabling personalized therapy delivery based on individual neurophysiological signatures. Their system stores long-term neural recordings that clinicians can analyze through a dedicated software interface, allowing for data-driven adjustments to therapy parameters based on each patient's response patterns and evolving condition.
Strengths: Highly personalized closed-loop therapy with demonstrated long-term efficacy in drug-resistant epilepsy; continuous adaptation through machine learning; comprehensive data capture enabling longitudinal analysis of neural activity. Weaknesses: Currently limited to epilepsy applications; requires surgical implantation with associated risks; significant computational demands that impact battery life requiring periodic replacement procedures.
Critical Patents and Breakthroughs in Neural Interfaces
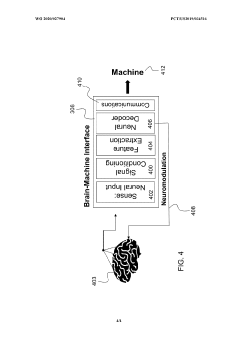
Enhanced brain-machine interfaces with neuromodulation
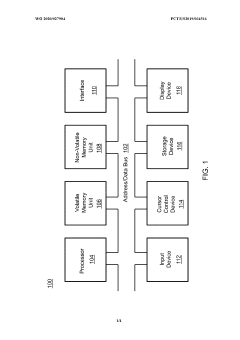
PatentWO2020027904A1
Innovation
- An enhanced brain-machine interface system that integrates neural sensors and processors to administer neuromodulation stimulation, such as unique spatiotemporal amplitude-modulated patterns (STAMPs), to condition and decode neural signals, providing direct control commands for devices like prosthetic limbs, and reinforcing operation through neuromodulation.
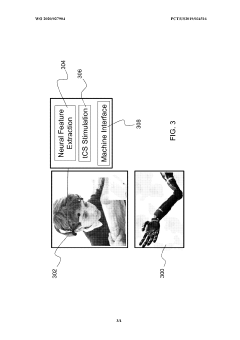
Brain-computer interface
PatentPendingEP4464250A2
Innovation
- The implementation of a compound visual stimulus with distinct characteristic modulations, such as overlapping 'ticks' and 'crosses' with different colors or frequencies, allows the BCI to capture neural responses and infer user intention by differentiating between exploration and selection through the relative strength of neural signals associated with each stimulus portion.
Regulatory Framework for Neural Implantable Devices
The regulatory landscape for neural implantable devices, particularly those used in Brain-Computer Interface (BCI) applications for personalized neuromodulation therapies, presents a complex and evolving framework that developers must navigate. Currently, the FDA classifies most neural implantable devices as Class III medical devices, requiring the most stringent regulatory oversight due to their invasive nature and direct interaction with brain tissue.
Premarket approval (PMA) represents the primary regulatory pathway for these devices, requiring comprehensive clinical trials to demonstrate safety and efficacy. This process typically takes 3-7 years and costs between $10-100 million, creating significant barriers to market entry for smaller companies and startups in the neuromodulation space.
The FDA has recently established the Digital Health Software Precertification Program, which may streamline approval for software components of BCI systems. Additionally, the FDA's Breakthrough Devices Program offers accelerated review for technologies addressing serious conditions with significant advantages over existing alternatives, which has benefited several neuromodulation device manufacturers.
Internationally, regulatory frameworks vary considerably. The European Union's Medical Device Regulation (MDR) implemented in 2021 has increased requirements for clinical evidence and post-market surveillance for neural implants. Japan has established the SAKIGAKE designation system for innovative medical technologies, while China has created a special approval pathway for innovative medical devices through its National Medical Products Administration.
Post-market surveillance requirements are particularly stringent for neural implantable devices, with manufacturers required to conduct long-term follow-up studies and maintain robust adverse event reporting systems. The FDA's Unique Device Identification system facilitates tracking of devices throughout their lifecycle.
Privacy and cybersecurity regulations present additional layers of complexity, with HIPAA in the US and GDPR in Europe establishing requirements for protecting neural data. The FDA has also issued guidance on cybersecurity for medical devices, emphasizing the need for security measures throughout the device lifecycle.
Emerging regulatory considerations include the development of standards for BCI data interoperability, ethical guidelines for neural data ownership and control, and frameworks for managing adaptive AI algorithms in neuromodulation devices that evolve with patient data. These evolving regulatory aspects will significantly impact the development timeline and commercialization strategy for personalized neuromodulation therapies.
Premarket approval (PMA) represents the primary regulatory pathway for these devices, requiring comprehensive clinical trials to demonstrate safety and efficacy. This process typically takes 3-7 years and costs between $10-100 million, creating significant barriers to market entry for smaller companies and startups in the neuromodulation space.
The FDA has recently established the Digital Health Software Precertification Program, which may streamline approval for software components of BCI systems. Additionally, the FDA's Breakthrough Devices Program offers accelerated review for technologies addressing serious conditions with significant advantages over existing alternatives, which has benefited several neuromodulation device manufacturers.
Internationally, regulatory frameworks vary considerably. The European Union's Medical Device Regulation (MDR) implemented in 2021 has increased requirements for clinical evidence and post-market surveillance for neural implants. Japan has established the SAKIGAKE designation system for innovative medical technologies, while China has created a special approval pathway for innovative medical devices through its National Medical Products Administration.
Post-market surveillance requirements are particularly stringent for neural implantable devices, with manufacturers required to conduct long-term follow-up studies and maintain robust adverse event reporting systems. The FDA's Unique Device Identification system facilitates tracking of devices throughout their lifecycle.
Privacy and cybersecurity regulations present additional layers of complexity, with HIPAA in the US and GDPR in Europe establishing requirements for protecting neural data. The FDA has also issued guidance on cybersecurity for medical devices, emphasizing the need for security measures throughout the device lifecycle.
Emerging regulatory considerations include the development of standards for BCI data interoperability, ethical guidelines for neural data ownership and control, and frameworks for managing adaptive AI algorithms in neuromodulation devices that evolve with patient data. These evolving regulatory aspects will significantly impact the development timeline and commercialization strategy for personalized neuromodulation therapies.
Ethical Implications of Direct Brain Intervention
The direct manipulation of brain function through Brain-Computer Interfaces (BCIs) in personalized neuromodulation therapies raises profound ethical questions that extend beyond technical feasibility. As these technologies advance from experimental to clinical applications, the ethical framework governing their use becomes increasingly critical.
Privacy concerns represent a primary ethical challenge, as BCIs collect unprecedented amounts of neural data that may reveal sensitive information about an individual's thoughts, emotions, and cognitive processes. This neural data exceeds traditional medical information in its intimacy, potentially exposing the very essence of human consciousness and identity. The question of who owns this data and how it should be protected remains largely unresolved in current regulatory frameworks.
Informed consent presents another significant ethical hurdle. The complexity of neuromodulation technologies makes it difficult for patients to fully comprehend the implications of these interventions. Traditional consent models may prove inadequate when dealing with therapies that could potentially alter personality, mood, or cognitive function. This is particularly problematic for vulnerable populations such as those with severe neurological disorders who may have compromised decision-making capacity.
The potential for unintended consequences looms large in direct brain interventions. Even targeted neuromodulation therapies may produce effects beyond their intended outcomes due to the brain's interconnected nature. These could include changes in personality, emotional responses, or cognitive abilities that were not anticipated, raising questions about responsibility and liability.
Autonomy and identity concerns emerge as BCIs become more sophisticated. Therapies that significantly alter brain function may challenge our understanding of personal identity and raise questions about whether changes in neural activity constitute changes in the "self." The boundary between therapeutic intervention and enhancement becomes increasingly blurred, potentially transforming our conception of normal brain function.
Access and equity issues also demand attention, as advanced neuromodulation therapies risk becoming available only to privileged populations. This could exacerbate existing healthcare disparities and create new forms of neurological inequality. The high cost of personalized BCI therapies may restrict access to those who could benefit most, particularly in regions with limited healthcare resources.
Regulatory frameworks currently lag behind technological developments in this field. The unique ethical challenges posed by direct brain intervention necessitate specialized governance structures that can balance innovation with protection of vulnerable individuals. International coordination will be essential to prevent regulatory arbitrage and ensure consistent ethical standards across different jurisdictions.
Privacy concerns represent a primary ethical challenge, as BCIs collect unprecedented amounts of neural data that may reveal sensitive information about an individual's thoughts, emotions, and cognitive processes. This neural data exceeds traditional medical information in its intimacy, potentially exposing the very essence of human consciousness and identity. The question of who owns this data and how it should be protected remains largely unresolved in current regulatory frameworks.
Informed consent presents another significant ethical hurdle. The complexity of neuromodulation technologies makes it difficult for patients to fully comprehend the implications of these interventions. Traditional consent models may prove inadequate when dealing with therapies that could potentially alter personality, mood, or cognitive function. This is particularly problematic for vulnerable populations such as those with severe neurological disorders who may have compromised decision-making capacity.
The potential for unintended consequences looms large in direct brain interventions. Even targeted neuromodulation therapies may produce effects beyond their intended outcomes due to the brain's interconnected nature. These could include changes in personality, emotional responses, or cognitive abilities that were not anticipated, raising questions about responsibility and liability.
Autonomy and identity concerns emerge as BCIs become more sophisticated. Therapies that significantly alter brain function may challenge our understanding of personal identity and raise questions about whether changes in neural activity constitute changes in the "self." The boundary between therapeutic intervention and enhancement becomes increasingly blurred, potentially transforming our conception of normal brain function.
Access and equity issues also demand attention, as advanced neuromodulation therapies risk becoming available only to privileged populations. This could exacerbate existing healthcare disparities and create new forms of neurological inequality. The high cost of personalized BCI therapies may restrict access to those who could benefit most, particularly in regions with limited healthcare resources.
Regulatory frameworks currently lag behind technological developments in this field. The unique ethical challenges posed by direct brain intervention necessitate specialized governance structures that can balance innovation with protection of vulnerable individuals. International coordination will be essential to prevent regulatory arbitrage and ensure consistent ethical standards across different jurisdictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!