Integration of Brain-Computer Interfaces with bioelectronic medicine platforms
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BCI-Bioelectronics Integration Background and Objectives
Brain-Computer Interfaces (BCIs) have evolved significantly since their inception in the 1970s, transitioning from rudimentary systems capable of basic signal detection to sophisticated platforms that can interpret complex neural patterns. This technological progression has been accelerated by advancements in computational power, machine learning algorithms, and miniaturization of electronic components. Concurrently, bioelectronic medicine has emerged as a revolutionary approach to treating diseases by targeting the nervous system with precision electrical impulses, offering alternatives to traditional pharmaceutical interventions.
The convergence of these two fields represents a frontier with transformative potential for healthcare. BCIs provide the means to decode neural signals, while bioelectronic medicine platforms offer mechanisms to deliver therapeutic interventions directly to the nervous system. This integration aims to create closed-loop systems capable of real-time monitoring and response, potentially revolutionizing treatment paradigms for neurological disorders, movement disabilities, and chronic pain conditions.
Historical developments in both fields have been marked by significant milestones. Early BCI research focused primarily on assistive technologies for severely disabled individuals, while bioelectronic medicine evolved from traditional neuromodulation techniques such as deep brain stimulation. The last decade has witnessed accelerated progress in both domains, driven by interdisciplinary collaboration between neuroscientists, engineers, clinicians, and data scientists.
The technical objectives of this integration are multifaceted: to develop bidirectional interfaces that can both record neural activity and deliver precise stimulation; to create adaptive algorithms capable of learning from neural feedback; to design biocompatible materials that minimize tissue reaction and maximize longevity; and to establish wireless communication protocols that ensure reliable data transmission while maintaining patient mobility.
Beyond technical goals, this integration aims to address critical healthcare challenges, including the management of treatment-resistant conditions, reduction of pharmaceutical side effects, and provision of personalized therapeutic approaches. The ultimate vision encompasses a new paradigm of precision medicine where interventions are tailored to individual neural signatures and adjusted in real-time based on physiological feedback.
As we examine the trajectory of these technologies, it becomes evident that their integration represents not merely an incremental advancement but potentially a paradigm shift in how we conceptualize the treatment of neurological and systemic disorders, moving toward truly personalized, adaptive therapeutic systems that work in harmony with the body's own neural mechanisms.
The convergence of these two fields represents a frontier with transformative potential for healthcare. BCIs provide the means to decode neural signals, while bioelectronic medicine platforms offer mechanisms to deliver therapeutic interventions directly to the nervous system. This integration aims to create closed-loop systems capable of real-time monitoring and response, potentially revolutionizing treatment paradigms for neurological disorders, movement disabilities, and chronic pain conditions.
Historical developments in both fields have been marked by significant milestones. Early BCI research focused primarily on assistive technologies for severely disabled individuals, while bioelectronic medicine evolved from traditional neuromodulation techniques such as deep brain stimulation. The last decade has witnessed accelerated progress in both domains, driven by interdisciplinary collaboration between neuroscientists, engineers, clinicians, and data scientists.
The technical objectives of this integration are multifaceted: to develop bidirectional interfaces that can both record neural activity and deliver precise stimulation; to create adaptive algorithms capable of learning from neural feedback; to design biocompatible materials that minimize tissue reaction and maximize longevity; and to establish wireless communication protocols that ensure reliable data transmission while maintaining patient mobility.
Beyond technical goals, this integration aims to address critical healthcare challenges, including the management of treatment-resistant conditions, reduction of pharmaceutical side effects, and provision of personalized therapeutic approaches. The ultimate vision encompasses a new paradigm of precision medicine where interventions are tailored to individual neural signatures and adjusted in real-time based on physiological feedback.
As we examine the trajectory of these technologies, it becomes evident that their integration represents not merely an incremental advancement but potentially a paradigm shift in how we conceptualize the treatment of neurological and systemic disorders, moving toward truly personalized, adaptive therapeutic systems that work in harmony with the body's own neural mechanisms.
Market Analysis for Neural Interface Medical Applications
The global market for neural interface medical applications is experiencing robust growth, driven by advancements in Brain-Computer Interface (BCI) technologies and their integration with bioelectronic medicine platforms. Current market valuations place this sector at approximately $2.4 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 15.7% through 2030, potentially reaching $7.6 billion by the end of the decade.
The demand landscape for neural interface applications spans multiple medical domains. Neurodegenerative disease management represents the largest market segment, accounting for roughly 38% of current applications. This includes treatments for Parkinson's disease, epilepsy, and essential tremor, where neural interfaces provide symptom monitoring and intervention capabilities.
Rehabilitation medicine constitutes the second-largest segment at 27% of the market, with applications in stroke recovery, spinal cord injury treatment, and mobility restoration. The integration of BCIs with bioelectronic medicine has shown particular promise in this area, enabling more precise neuromodulation therapies and improved functional outcomes.
Mental health applications are emerging as the fastest-growing segment, currently representing 18% of the market but expanding at a CAGR of 21.3%. These applications include treatment-resistant depression, anxiety disorders, and PTSD, where neural interfaces offer novel therapeutic approaches through targeted neuromodulation.
Geographically, North America dominates the market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is demonstrating the highest growth rate at 18.2% annually, driven by increasing healthcare expenditure and research investments in countries like China, Japan, and South Korea.
Key market drivers include the rising prevalence of neurological disorders, growing acceptance of implantable medical devices, and increasing research funding from both public and private sectors. The convergence of artificial intelligence with neural interface technologies is accelerating development cycles and expanding potential applications.
Market barriers remain significant, including high development and implementation costs, regulatory hurdles, and concerns regarding data privacy and security. The average cost of bringing a neural interface medical application to market exceeds $75 million, with regulatory approval timelines averaging 4-6 years in major markets.
Reimbursement landscapes vary considerably across regions, with more favorable coverage emerging in North America and parts of Europe. This variability significantly impacts market penetration rates and commercial viability of new technologies, particularly for novel applications without established reimbursement codes.
The demand landscape for neural interface applications spans multiple medical domains. Neurodegenerative disease management represents the largest market segment, accounting for roughly 38% of current applications. This includes treatments for Parkinson's disease, epilepsy, and essential tremor, where neural interfaces provide symptom monitoring and intervention capabilities.
Rehabilitation medicine constitutes the second-largest segment at 27% of the market, with applications in stroke recovery, spinal cord injury treatment, and mobility restoration. The integration of BCIs with bioelectronic medicine has shown particular promise in this area, enabling more precise neuromodulation therapies and improved functional outcomes.
Mental health applications are emerging as the fastest-growing segment, currently representing 18% of the market but expanding at a CAGR of 21.3%. These applications include treatment-resistant depression, anxiety disorders, and PTSD, where neural interfaces offer novel therapeutic approaches through targeted neuromodulation.
Geographically, North America dominates the market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is demonstrating the highest growth rate at 18.2% annually, driven by increasing healthcare expenditure and research investments in countries like China, Japan, and South Korea.
Key market drivers include the rising prevalence of neurological disorders, growing acceptance of implantable medical devices, and increasing research funding from both public and private sectors. The convergence of artificial intelligence with neural interface technologies is accelerating development cycles and expanding potential applications.
Market barriers remain significant, including high development and implementation costs, regulatory hurdles, and concerns regarding data privacy and security. The average cost of bringing a neural interface medical application to market exceeds $75 million, with regulatory approval timelines averaging 4-6 years in major markets.
Reimbursement landscapes vary considerably across regions, with more favorable coverage emerging in North America and parts of Europe. This variability significantly impacts market penetration rates and commercial viability of new technologies, particularly for novel applications without established reimbursement codes.
Technical Challenges in BCI-Bioelectronic Medicine Integration
The integration of Brain-Computer Interfaces (BCIs) with bioelectronic medicine platforms presents numerous technical challenges that must be addressed for successful implementation. Signal acquisition remains a fundamental obstacle, as neural signals are inherently weak (typically in the microvolt range) and susceptible to various forms of interference. This necessitates sophisticated amplification and filtering techniques to achieve adequate signal-to-noise ratios without distorting the underlying neural information.
Biocompatibility issues represent another significant hurdle. Long-term implantation of BCI devices often triggers foreign body responses, leading to electrode encapsulation by glial scarring, which degrades signal quality over time. Current materials science approaches are exploring novel electrode coatings and flexible substrates to mitigate these tissue reactions, but achieving truly long-term stable interfaces remains elusive.
Power management presents complex trade-offs between functionality and safety. Implantable BCI-bioelectronic systems require minimal power consumption to prevent tissue heating while maintaining sufficient processing capabilities. Wireless power transmission technologies show promise but face efficiency limitations and potential tissue damage from electromagnetic radiation.
Data processing challenges are equally formidable. Neural signals exhibit high dimensionality and variability both within and between individuals. Extracting meaningful information requires advanced signal processing algorithms capable of real-time operation within the power and computational constraints of implantable devices. Machine learning approaches show promise but must be optimized for on-device implementation.
Bidirectional communication between neural tissue and bioelectronic medicine platforms introduces additional complexity. While sensing neural activity has progressed significantly, precisely modulating neural circuits through electrical, optical, or chemical means without unintended effects remains challenging. Closed-loop systems that can both record and stimulate require sophisticated control algorithms to maintain physiological homeostasis.
Miniaturization represents another critical challenge. Integrating sensing, processing, power, and communication components into devices small enough for practical implantation, particularly in sensitive neural tissues, demands advanced fabrication techniques. Current systems often compromise on functionality to achieve acceptable form factors.
Security and privacy concerns cannot be overlooked. BCI-bioelectronic medicine systems potentially access and manipulate highly sensitive neural data, raising unprecedented cybersecurity challenges. Developing robust encryption and authentication protocols that can operate within the severe resource constraints of implantable devices remains an open research question.
Biocompatibility issues represent another significant hurdle. Long-term implantation of BCI devices often triggers foreign body responses, leading to electrode encapsulation by glial scarring, which degrades signal quality over time. Current materials science approaches are exploring novel electrode coatings and flexible substrates to mitigate these tissue reactions, but achieving truly long-term stable interfaces remains elusive.
Power management presents complex trade-offs between functionality and safety. Implantable BCI-bioelectronic systems require minimal power consumption to prevent tissue heating while maintaining sufficient processing capabilities. Wireless power transmission technologies show promise but face efficiency limitations and potential tissue damage from electromagnetic radiation.
Data processing challenges are equally formidable. Neural signals exhibit high dimensionality and variability both within and between individuals. Extracting meaningful information requires advanced signal processing algorithms capable of real-time operation within the power and computational constraints of implantable devices. Machine learning approaches show promise but must be optimized for on-device implementation.
Bidirectional communication between neural tissue and bioelectronic medicine platforms introduces additional complexity. While sensing neural activity has progressed significantly, precisely modulating neural circuits through electrical, optical, or chemical means without unintended effects remains challenging. Closed-loop systems that can both record and stimulate require sophisticated control algorithms to maintain physiological homeostasis.
Miniaturization represents another critical challenge. Integrating sensing, processing, power, and communication components into devices small enough for practical implantation, particularly in sensitive neural tissues, demands advanced fabrication techniques. Current systems often compromise on functionality to achieve acceptable form factors.
Security and privacy concerns cannot be overlooked. BCI-bioelectronic medicine systems potentially access and manipulate highly sensitive neural data, raising unprecedented cybersecurity challenges. Developing robust encryption and authentication protocols that can operate within the severe resource constraints of implantable devices remains an open research question.
Current BCI-Bioelectronic Integration Approaches
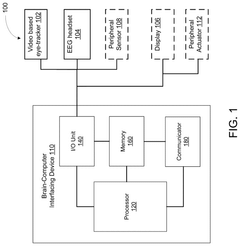
01 Neural interface systems for bioelectronic medicine
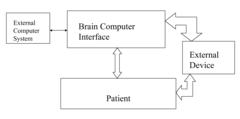
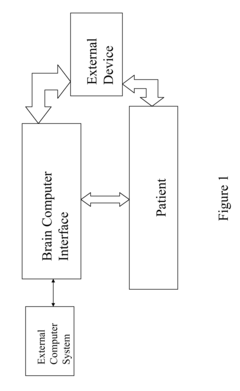
Neural interface systems integrate brain-computer interfaces with bioelectronic medicine platforms to monitor and modulate neural activity. These systems use implantable electrodes and sensors to record brain signals and deliver therapeutic stimulation. The integration enables closed-loop systems that can detect abnormal neural patterns and automatically adjust treatment parameters, providing personalized therapy for neurological disorders.- Neural interfaces for bioelectronic medicine: Neural interfaces serve as the foundation for integrating brain-computer interfaces with bioelectronic medicine platforms. These systems establish direct communication pathways between neural tissues and electronic devices, enabling both monitoring of neural activity and delivery of therapeutic stimulation. Advanced neural interfaces incorporate biocompatible materials and miniaturized electronics to minimize tissue damage while maximizing signal quality. These technologies facilitate closed-loop systems that can detect abnormal neural patterns and deliver targeted interventions in real-time.
- Implantable bioelectronic devices for therapeutic applications: Implantable bioelectronic devices represent a critical component in the integration of brain-computer interfaces with bioelectronic medicine. These devices are designed to be placed within the body to directly interface with neural tissues for therapeutic purposes. They incorporate miniaturized electronics, power management systems, and wireless communication capabilities to enable long-term operation within the body. Advanced implantable systems can target specific neural circuits to treat neurological disorders, chronic pain, and other medical conditions through precise electrical stimulation protocols.
- Data processing and AI algorithms for neural signal interpretation: Sophisticated data processing and artificial intelligence algorithms are essential for interpreting the complex neural signals acquired through brain-computer interfaces. These computational approaches enable real-time analysis of neural activity patterns, identification of biomarkers associated with specific conditions, and translation of neural signals into control commands for external devices. Machine learning techniques improve signal classification accuracy and adapt to individual neural patterns over time, enhancing the performance and usability of integrated bioelectronic medicine platforms.
- Wireless communication and power systems for BCI devices: Wireless communication and power systems are crucial for practical implementation of brain-computer interfaces in bioelectronic medicine. These technologies eliminate the need for transcutaneous wires, reducing infection risk and improving patient mobility. Advanced wireless systems enable bidirectional data transfer between implanted devices and external processors while maintaining low power consumption. Innovative power solutions, including electromagnetic induction, ultrasonic energy transfer, and biofuel cells, support long-term operation of implanted components without requiring battery replacement procedures.
- Closed-loop neuromodulation systems: Closed-loop neuromodulation systems represent the integration of sensing and stimulation capabilities in brain-computer interfaces for bioelectronic medicine. These systems continuously monitor neural activity, physiological parameters, or behavioral markers to detect specific patterns that trigger therapeutic interventions. The closed-loop approach enables personalized, responsive treatment that adjusts in real-time to the patient's changing condition. This adaptive capability improves treatment efficacy while minimizing side effects by delivering stimulation only when needed and at optimized parameters based on the current neural state.
02 Wireless communication and data processing for BCI platforms
Advanced wireless communication technologies enable seamless data transmission between brain-computer interfaces and bioelectronic medicine systems. These platforms incorporate sophisticated signal processing algorithms and machine learning techniques to analyze neural data in real-time. The wireless capabilities allow for continuous monitoring and therapy adjustment without physical connections, improving patient mobility and comfort while maintaining therapeutic efficacy.Expand Specific Solutions03 Miniaturized implantable BCI devices for bioelectronic medicine
Miniaturized implantable devices combine brain-computer interface technology with bioelectronic medicine capabilities in compact form factors. These devices utilize advanced materials and microfabrication techniques to create minimally invasive neural interfaces. The miniaturization enables precise placement near target neural structures, reducing surgical complexity and improving biocompatibility while maintaining therapeutic effectiveness for various neurological and physiological conditions.Expand Specific Solutions04 Therapeutic applications of integrated BCI-bioelectronic platforms
Integrated brain-computer interfaces and bioelectronic medicine platforms offer novel therapeutic approaches for various medical conditions. These systems can treat neurological disorders like epilepsy and Parkinson's disease by monitoring brain activity and delivering targeted stimulation. They also address psychiatric conditions, chronic pain, and autonomic disorders by modulating specific neural circuits. The integration enables more precise and responsive therapies compared to traditional pharmaceutical approaches.Expand Specific Solutions05 User interfaces and control systems for BCI-bioelectronic platforms
Advanced user interfaces and control systems facilitate the operation of integrated brain-computer interfaces and bioelectronic medicine platforms. These systems incorporate intuitive software interfaces that allow healthcare providers to program and adjust therapeutic parameters. They also enable patients to monitor their therapy and provide feedback. The control systems include safety mechanisms to prevent adverse effects and optimize therapeutic outcomes through adaptive algorithms that learn from patient responses.Expand Specific Solutions
Key Industry Players and Research Institutions
The integration of Brain-Computer Interfaces (BCIs) with bioelectronic medicine platforms is currently in an early growth phase, with the market expected to expand significantly as technologies mature. The competitive landscape features established academic institutions (Tianjin University, Washington University, Zhejiang University) conducting foundational research alongside emerging commercial players (Neuralink, Neurolutions, Precision Neuroscience) developing practical applications. While academic research focuses on expanding theoretical frameworks, companies like Neuralink and Neurable are advancing minimally invasive neural interfaces for clinical applications. The technology remains in early-to-mid maturity stages, with significant challenges in biocompatibility, signal processing, and regulatory approval still being addressed through collaborative efforts between research institutions and commercial entities.
South China Brain Control Guangdong Intelligent Tech Co Ltd.
Technical Solution: South China Brain Control has developed an integrated BCI-bioelectronic medicine platform that combines non-invasive and semi-invasive neural recording techniques with targeted electrical stimulation therapies. Their system utilizes a hybrid approach that incorporates both EEG and subdermal microelectrodes to achieve a balance between signal quality and invasiveness. The company has created a comprehensive closed-loop system that can detect abnormal brain activity patterns and deliver precisely calibrated electrical stimulation to normalize neural function. Their platform includes proprietary AI algorithms that continuously learn from collected neural data to improve both detection accuracy and stimulation parameters over time. South China Brain Control has focused particularly on applications for movement disorders, epilepsy, and chronic pain management, with their technology enabling personalized bioelectronic medicine interventions based on individual neural signatures. The system features wireless connectivity and cloud-based processing capabilities, allowing for remote monitoring and adjustment of therapeutic parameters by healthcare providers.
Strengths: Hybrid recording approach balances signal quality with minimal invasiveness; integrated closed-loop stimulation capabilities; AI-driven personalization of therapy; focus on practical clinical applications. Weaknesses: Less established in international markets; limited published clinical validation data; potential regulatory challenges in Western markets; questions about long-term reliability of hybrid recording approach.
Neuralink Corp.
Technical Solution: Neuralink has developed an advanced brain-computer interface system called the N1 Link, which consists of ultra-thin flexible threads (less than 1/10 the width of a human hair) containing electrodes that can be inserted into the brain to record neural activity. Their proprietary robot performs the precision surgery to implant these threads with minimal tissue damage. The system wirelessly transmits brain signals to external devices via a small, behind-the-ear implant. Neuralink's BCI platform integrates with bioelectronic medicine by creating a direct communication pathway between the brain and external medical devices. Their technology aims to treat various neurological conditions by reading brain signals and delivering precise therapeutic electrical stimulation. The company has demonstrated successful implantation in animals and received FDA approval for human clinical trials in 2023, focusing initially on helping paralyzed patients control digital devices.
Strengths: Cutting-edge miniaturization of electrodes; proprietary surgical robot for precise implantation; wireless data transmission capabilities; strong funding and high-profile leadership. Weaknesses: Limited clinical data in humans; potential long-term biocompatibility concerns; regulatory hurdles for invasive brain technology; questions about long-term stability of implanted electrodes.
Critical Patents and Breakthroughs in Neural Interfaces
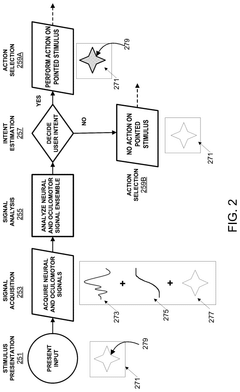
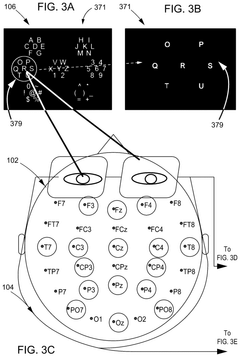
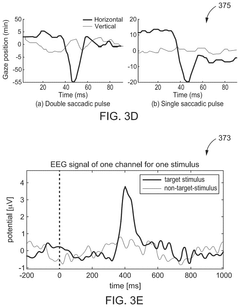
Brain-computer interface with adaptations for high-speed, accurate, and intuitive user interactions
PatentPendingUS20250093951A1
Innovation
- A hardware-agnostic integrated oculomotor-neural hybrid BCI platform that combines real-time eye-movement tracking with brain activity tracking to mediate user interactions, using a hybrid BCI system with pointing and action control features to enhance user interface design for high-speed and accurate interactions.
Patient controlled brain repair system and method of use
PatentActiveUS20110106206A1
Innovation
- A BCI system that includes sensors to detect neuronal activity in arousal regulation regions, a state monitoring module to process variables, and a processing module to stimulate these regions to adjust information flow, ensuring optimal communication and control by maintaining basal arousal levels through feedback mechanisms.
Regulatory Framework for Implantable Neural Technologies
The regulatory landscape for implantable neural technologies represents a complex and evolving framework that directly impacts the integration of Brain-Computer Interfaces (BCIs) with bioelectronic medicine platforms. Currently, these technologies fall under multiple regulatory jurisdictions worldwide, with the FDA in the United States classifying most implantable neural devices as Class III medical devices, requiring premarket approval (PMA) with extensive clinical trials demonstrating safety and efficacy.
The European Union's Medical Device Regulation (MDR) imposes similarly stringent requirements, with neural implants typically classified in the highest risk categories. These regulations mandate comprehensive technical documentation, clinical evaluation reports, and post-market surveillance systems. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specialized pathways for novel neurotechnologies.
A significant regulatory challenge lies in the convergence of multiple technological domains within BCI-bioelectronic medicine platforms. These systems often incorporate elements of traditional medical devices, software as a medical device (SaMD), artificial intelligence algorithms, and wireless communication technologies, creating regulatory complexity that crosses traditional boundaries.
Privacy and cybersecurity regulations present additional compliance requirements. The collection and transmission of neural data raise unique concerns under frameworks like GDPR in Europe and HIPAA in the United States. Regulatory bodies increasingly require manufacturers to implement robust cybersecurity measures to protect against unauthorized access to neural interfaces.
Regulatory adaptation is evident in the emergence of specialized guidance documents. The FDA's "Implanted Brain-Computer Interface Devices for Patients with Paralysis or Amputation" guidance (2021) represents an important step toward addressing the unique characteristics of these technologies. Similarly, the International Medical Device Regulators Forum (IMDRF) has begun developing harmonized approaches to neural technology regulation.
Ethical considerations are increasingly incorporated into regulatory frameworks, with requirements for informed consent processes that address the unique implications of neural interfaces. Several jurisdictions are developing specific ethical guidelines for neurotechnology that may eventually become regulatory requirements, particularly regarding cognitive liberty, mental privacy, and identity.
The regulatory pathway for BCI-bioelectronic medicine integration remains challenging, with timelines for approval typically extending 3-5 years. Companies pursuing these technologies must develop comprehensive regulatory strategies early in development, incorporating regulatory considerations into design processes rather than addressing them retrospectively.
The European Union's Medical Device Regulation (MDR) imposes similarly stringent requirements, with neural implants typically classified in the highest risk categories. These regulations mandate comprehensive technical documentation, clinical evaluation reports, and post-market surveillance systems. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specialized pathways for novel neurotechnologies.
A significant regulatory challenge lies in the convergence of multiple technological domains within BCI-bioelectronic medicine platforms. These systems often incorporate elements of traditional medical devices, software as a medical device (SaMD), artificial intelligence algorithms, and wireless communication technologies, creating regulatory complexity that crosses traditional boundaries.
Privacy and cybersecurity regulations present additional compliance requirements. The collection and transmission of neural data raise unique concerns under frameworks like GDPR in Europe and HIPAA in the United States. Regulatory bodies increasingly require manufacturers to implement robust cybersecurity measures to protect against unauthorized access to neural interfaces.
Regulatory adaptation is evident in the emergence of specialized guidance documents. The FDA's "Implanted Brain-Computer Interface Devices for Patients with Paralysis or Amputation" guidance (2021) represents an important step toward addressing the unique characteristics of these technologies. Similarly, the International Medical Device Regulators Forum (IMDRF) has begun developing harmonized approaches to neural technology regulation.
Ethical considerations are increasingly incorporated into regulatory frameworks, with requirements for informed consent processes that address the unique implications of neural interfaces. Several jurisdictions are developing specific ethical guidelines for neurotechnology that may eventually become regulatory requirements, particularly regarding cognitive liberty, mental privacy, and identity.
The regulatory pathway for BCI-bioelectronic medicine integration remains challenging, with timelines for approval typically extending 3-5 years. Companies pursuing these technologies must develop comprehensive regulatory strategies early in development, incorporating regulatory considerations into design processes rather than addressing them retrospectively.
Ethical and Privacy Considerations in Neural Integration
The integration of Brain-Computer Interfaces (BCIs) with bioelectronic medicine platforms raises significant ethical and privacy concerns that must be addressed before widespread adoption. Neural data represents perhaps the most intimate form of personal information, containing potential insights into thoughts, emotions, and intentions. This unprecedented access to cognitive processes demands robust ethical frameworks and privacy protections that currently outpace existing regulatory structures.
Privacy vulnerabilities in neural integration systems present unique challenges. Unlike conventional data breaches, neural data compromises could reveal cognitive patterns, emotional responses, and even subconscious processes. The potential for unauthorized access to neural signals raises concerns about "brain hacking" or neural surveillance. Current encryption methods for neural data transmission and storage require substantial enhancement to match the sensitivity of the information being protected.
Informed consent becomes particularly complex in the BCI domain. Patients may not fully comprehend the extent of data collection or potential future uses of their neural information. The dynamic nature of machine learning algorithms used in many BCI systems means that even developers cannot always predict what patterns might be detected or inferred from collected neural data, creating a "consent gap" that traditional frameworks struggle to address.
Autonomy and agency concerns emerge when considering the bidirectional nature of advanced BCI-bioelectronic medicine systems. As these technologies progress from merely reading neural signals to actively influencing neural activity, questions arise about where human agency ends and technological intervention begins. The potential for subtle manipulation of decision-making processes through neuromodulation requires careful ethical boundaries.
Equity and access considerations must also be examined. The high cost and specialized nature of integrated neural technologies risk creating a "neural divide" where only privileged populations benefit from these advances. This divide could exacerbate existing healthcare disparities and create new forms of cognitive inequality if not addressed through thoughtful policy and inclusive development practices.
Regulatory frameworks currently lag behind technological capabilities in this domain. While some regions have begun developing neurospecific data protection guidelines, most jurisdictions rely on adapting existing medical device and data privacy regulations that were not designed for the unique challenges of neural integration. International harmonization of these regulations remains minimal, creating potential for regulatory arbitrage and inconsistent protection standards across borders.
Privacy vulnerabilities in neural integration systems present unique challenges. Unlike conventional data breaches, neural data compromises could reveal cognitive patterns, emotional responses, and even subconscious processes. The potential for unauthorized access to neural signals raises concerns about "brain hacking" or neural surveillance. Current encryption methods for neural data transmission and storage require substantial enhancement to match the sensitivity of the information being protected.
Informed consent becomes particularly complex in the BCI domain. Patients may not fully comprehend the extent of data collection or potential future uses of their neural information. The dynamic nature of machine learning algorithms used in many BCI systems means that even developers cannot always predict what patterns might be detected or inferred from collected neural data, creating a "consent gap" that traditional frameworks struggle to address.
Autonomy and agency concerns emerge when considering the bidirectional nature of advanced BCI-bioelectronic medicine systems. As these technologies progress from merely reading neural signals to actively influencing neural activity, questions arise about where human agency ends and technological intervention begins. The potential for subtle manipulation of decision-making processes through neuromodulation requires careful ethical boundaries.
Equity and access considerations must also be examined. The high cost and specialized nature of integrated neural technologies risk creating a "neural divide" where only privileged populations benefit from these advances. This divide could exacerbate existing healthcare disparities and create new forms of cognitive inequality if not addressed through thoughtful policy and inclusive development practices.
Regulatory frameworks currently lag behind technological capabilities in this domain. While some regions have begun developing neurospecific data protection guidelines, most jurisdictions rely on adapting existing medical device and data privacy regulations that were not designed for the unique challenges of neural integration. International harmonization of these regulations remains minimal, creating potential for regulatory arbitrage and inconsistent protection standards across borders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!