Carbolic Acid Derivatives in Pharmaceutical Synthesis: A Green Approach
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Derivatives: Background and Objectives
Carbolic acid, also known as phenol, has been a cornerstone in organic chemistry and pharmaceutical synthesis for over a century. Its derivatives have played a crucial role in the development of numerous drugs and active pharmaceutical ingredients (APIs). The evolution of carbolic acid derivatives in pharmaceutical synthesis reflects the broader trends in medicinal chemistry, moving from serendipitous discoveries to rational drug design and, more recently, towards sustainable and environmentally friendly approaches.
The historical significance of carbolic acid in medicine dates back to the 19th century when Joseph Lister pioneered its use as an antiseptic. This discovery revolutionized surgical practices and laid the foundation for modern sterile techniques. As the field of organic chemistry advanced, the potential of carbolic acid derivatives in drug synthesis became increasingly apparent, leading to the development of various classes of pharmaceuticals, including analgesics, antiseptics, and antioxidants.
In recent decades, the pharmaceutical industry has faced growing pressure to adopt more sustainable practices. This shift has been driven by environmental concerns, regulatory requirements, and the need for cost-effective manufacturing processes. As a result, there has been a renewed focus on developing green approaches to pharmaceutical synthesis, with carbolic acid derivatives at the forefront of this movement.
The primary objective of exploring green approaches to carbolic acid derivatives in pharmaceutical synthesis is to develop more environmentally friendly and economically viable methods for drug production. This involves several key goals: reducing the use of hazardous reagents and solvents, minimizing waste generation, improving atom economy, and enhancing overall process efficiency. Additionally, there is a strong emphasis on developing catalytic processes that can operate under milder conditions and with higher selectivity.
Another critical objective is to expand the toolkit of synthetic methodologies available to medicinal chemists. By developing novel, green synthetic routes for carbolic acid derivatives, researchers aim to unlock new chemical space and potentially discover innovative drug candidates. This approach aligns with the broader trend in drug discovery towards exploring previously untapped areas of chemical diversity.
Furthermore, the green approach to carbolic acid derivatives seeks to address the challenges of scalability and industrial applicability. As promising drug candidates move from laboratory-scale synthesis to large-scale production, the environmental impact becomes increasingly significant. Therefore, a key goal is to develop synthetic methods that are not only green at the bench scale but also maintain their sustainability advantages when scaled up to industrial production levels.
In conclusion, the background and objectives of green approaches to carbolic acid derivatives in pharmaceutical synthesis reflect a convergence of historical significance, modern environmental concerns, and future-oriented drug discovery strategies. This field represents a critical area of research that has the potential to significantly impact the sustainability and efficiency of pharmaceutical manufacturing in the coming years.
The historical significance of carbolic acid in medicine dates back to the 19th century when Joseph Lister pioneered its use as an antiseptic. This discovery revolutionized surgical practices and laid the foundation for modern sterile techniques. As the field of organic chemistry advanced, the potential of carbolic acid derivatives in drug synthesis became increasingly apparent, leading to the development of various classes of pharmaceuticals, including analgesics, antiseptics, and antioxidants.
In recent decades, the pharmaceutical industry has faced growing pressure to adopt more sustainable practices. This shift has been driven by environmental concerns, regulatory requirements, and the need for cost-effective manufacturing processes. As a result, there has been a renewed focus on developing green approaches to pharmaceutical synthesis, with carbolic acid derivatives at the forefront of this movement.
The primary objective of exploring green approaches to carbolic acid derivatives in pharmaceutical synthesis is to develop more environmentally friendly and economically viable methods for drug production. This involves several key goals: reducing the use of hazardous reagents and solvents, minimizing waste generation, improving atom economy, and enhancing overall process efficiency. Additionally, there is a strong emphasis on developing catalytic processes that can operate under milder conditions and with higher selectivity.
Another critical objective is to expand the toolkit of synthetic methodologies available to medicinal chemists. By developing novel, green synthetic routes for carbolic acid derivatives, researchers aim to unlock new chemical space and potentially discover innovative drug candidates. This approach aligns with the broader trend in drug discovery towards exploring previously untapped areas of chemical diversity.
Furthermore, the green approach to carbolic acid derivatives seeks to address the challenges of scalability and industrial applicability. As promising drug candidates move from laboratory-scale synthesis to large-scale production, the environmental impact becomes increasingly significant. Therefore, a key goal is to develop synthetic methods that are not only green at the bench scale but also maintain their sustainability advantages when scaled up to industrial production levels.
In conclusion, the background and objectives of green approaches to carbolic acid derivatives in pharmaceutical synthesis reflect a convergence of historical significance, modern environmental concerns, and future-oriented drug discovery strategies. This field represents a critical area of research that has the potential to significantly impact the sustainability and efficiency of pharmaceutical manufacturing in the coming years.
Market Analysis for Green Pharmaceutical Synthesis
The global pharmaceutical industry is experiencing a significant shift towards green chemistry practices, driven by increasing environmental concerns and regulatory pressures. The market for green pharmaceutical synthesis, particularly focusing on carbolic acid derivatives, is showing promising growth trends. This segment is expected to expand rapidly due to the rising demand for sustainable and eco-friendly drug manufacturing processes.
The pharmaceutical industry's commitment to reducing its environmental footprint has led to a surge in research and development activities centered on green synthesis methods. Carbolic acid derivatives, known for their versatility in drug synthesis, are at the forefront of this green revolution. The market for these derivatives in pharmaceutical applications is witnessing a compound annual growth rate that outpaces traditional synthesis methods.
Key factors driving this market growth include stringent environmental regulations, increasing consumer awareness about sustainable products, and the pharmaceutical industry's push towards cost-effective and efficient manufacturing processes. The adoption of green chemistry principles in pharmaceutical synthesis not only addresses environmental concerns but also offers economic benefits through reduced waste generation and energy consumption.
Geographically, North America and Europe are leading the market for green pharmaceutical synthesis, with a strong focus on carbolic acid derivatives. These regions boast advanced research facilities and a robust regulatory framework supporting sustainable practices. However, emerging economies in Asia-Pacific, particularly China and India, are rapidly catching up, driven by their growing pharmaceutical manufacturing sectors and increasing environmental awareness.
The market is characterized by a mix of established pharmaceutical giants and innovative start-ups specializing in green chemistry solutions. Major pharmaceutical companies are investing heavily in developing and implementing green synthesis methods, recognizing the long-term benefits and competitive advantage they offer. This has led to a surge in partnerships and collaborations between industry players and research institutions to accelerate the development of novel green synthesis techniques.
Despite the positive outlook, challenges remain in the widespread adoption of green synthesis methods using carbolic acid derivatives. These include the need for substantial initial investments in new technologies and processes, as well as the time required for regulatory approvals of new synthesis routes. However, the long-term benefits, including reduced environmental impact, improved public perception, and potential cost savings, are expected to outweigh these initial hurdles.
As the pharmaceutical industry continues to evolve, the market for green synthesis methods, particularly those involving carbolic acid derivatives, is poised for substantial growth. This trend is likely to reshape the pharmaceutical manufacturing landscape, driving innovation and sustainability in drug production processes.
The pharmaceutical industry's commitment to reducing its environmental footprint has led to a surge in research and development activities centered on green synthesis methods. Carbolic acid derivatives, known for their versatility in drug synthesis, are at the forefront of this green revolution. The market for these derivatives in pharmaceutical applications is witnessing a compound annual growth rate that outpaces traditional synthesis methods.
Key factors driving this market growth include stringent environmental regulations, increasing consumer awareness about sustainable products, and the pharmaceutical industry's push towards cost-effective and efficient manufacturing processes. The adoption of green chemistry principles in pharmaceutical synthesis not only addresses environmental concerns but also offers economic benefits through reduced waste generation and energy consumption.
Geographically, North America and Europe are leading the market for green pharmaceutical synthesis, with a strong focus on carbolic acid derivatives. These regions boast advanced research facilities and a robust regulatory framework supporting sustainable practices. However, emerging economies in Asia-Pacific, particularly China and India, are rapidly catching up, driven by their growing pharmaceutical manufacturing sectors and increasing environmental awareness.
The market is characterized by a mix of established pharmaceutical giants and innovative start-ups specializing in green chemistry solutions. Major pharmaceutical companies are investing heavily in developing and implementing green synthesis methods, recognizing the long-term benefits and competitive advantage they offer. This has led to a surge in partnerships and collaborations between industry players and research institutions to accelerate the development of novel green synthesis techniques.
Despite the positive outlook, challenges remain in the widespread adoption of green synthesis methods using carbolic acid derivatives. These include the need for substantial initial investments in new technologies and processes, as well as the time required for regulatory approvals of new synthesis routes. However, the long-term benefits, including reduced environmental impact, improved public perception, and potential cost savings, are expected to outweigh these initial hurdles.
As the pharmaceutical industry continues to evolve, the market for green synthesis methods, particularly those involving carbolic acid derivatives, is poised for substantial growth. This trend is likely to reshape the pharmaceutical manufacturing landscape, driving innovation and sustainability in drug production processes.
Current Challenges in Carbolic Acid Derivative Synthesis
The synthesis of carbolic acid derivatives for pharmaceutical applications faces several significant challenges, particularly in the context of green chemistry approaches. One of the primary obstacles is the inherent toxicity of carbolic acid (phenol) and many of its derivatives. This toxicity poses risks not only to the environment but also to the personnel involved in the synthesis process, necessitating stringent safety measures and specialized handling procedures.
Another major challenge lies in the development of efficient and environmentally friendly reaction pathways. Traditional methods often rely on harsh reaction conditions, including high temperatures and pressures, which are energy-intensive and may lead to unwanted side products. The use of toxic or hazardous reagents and solvents further complicates the pursuit of green synthesis routes, as these substances can have detrimental effects on the environment and human health.
The selectivity of reactions in carbolic acid derivative synthesis presents an ongoing challenge. Achieving high regioselectivity and stereoselectivity, particularly in complex molecular structures, remains difficult. This issue is compounded when attempting to implement greener reaction conditions, as milder reagents and catalysts may not provide the same level of control over reaction outcomes.
Purification and isolation of carbolic acid derivatives pose additional challenges. Many conventional purification techniques, such as column chromatography, consume large volumes of organic solvents, which contradicts the principles of green chemistry. Developing more sustainable purification methods that maintain product quality while reducing environmental impact is a critical area of focus.
Scale-up of green synthesis processes for carbolic acid derivatives is another significant hurdle. Reactions that perform well at laboratory scale may encounter unforeseen difficulties when scaled up to industrial production levels. Maintaining reaction efficiency, product yield, and purity while adhering to green chemistry principles becomes increasingly challenging as production volumes increase.
The economic viability of green synthesis routes for carbolic acid derivatives remains a concern for pharmaceutical companies. While environmentally friendly processes are desirable, they must also be cost-effective to be adopted on a commercial scale. Balancing the often higher initial costs of green technologies with long-term sustainability benefits is an ongoing challenge in the industry.
Regulatory compliance adds another layer of complexity to the development of green synthesis methods for carbolic acid derivatives. As environmental regulations become more stringent, pharmaceutical companies must navigate a complex landscape of guidelines and restrictions while still meeting product quality and safety standards.
Another major challenge lies in the development of efficient and environmentally friendly reaction pathways. Traditional methods often rely on harsh reaction conditions, including high temperatures and pressures, which are energy-intensive and may lead to unwanted side products. The use of toxic or hazardous reagents and solvents further complicates the pursuit of green synthesis routes, as these substances can have detrimental effects on the environment and human health.
The selectivity of reactions in carbolic acid derivative synthesis presents an ongoing challenge. Achieving high regioselectivity and stereoselectivity, particularly in complex molecular structures, remains difficult. This issue is compounded when attempting to implement greener reaction conditions, as milder reagents and catalysts may not provide the same level of control over reaction outcomes.
Purification and isolation of carbolic acid derivatives pose additional challenges. Many conventional purification techniques, such as column chromatography, consume large volumes of organic solvents, which contradicts the principles of green chemistry. Developing more sustainable purification methods that maintain product quality while reducing environmental impact is a critical area of focus.
Scale-up of green synthesis processes for carbolic acid derivatives is another significant hurdle. Reactions that perform well at laboratory scale may encounter unforeseen difficulties when scaled up to industrial production levels. Maintaining reaction efficiency, product yield, and purity while adhering to green chemistry principles becomes increasingly challenging as production volumes increase.
The economic viability of green synthesis routes for carbolic acid derivatives remains a concern for pharmaceutical companies. While environmentally friendly processes are desirable, they must also be cost-effective to be adopted on a commercial scale. Balancing the often higher initial costs of green technologies with long-term sustainability benefits is an ongoing challenge in the industry.
Regulatory compliance adds another layer of complexity to the development of green synthesis methods for carbolic acid derivatives. As environmental regulations become more stringent, pharmaceutical companies must navigate a complex landscape of guidelines and restrictions while still meeting product quality and safety standards.
Existing Green Approaches for Carbolic Acid Derivatives
01 Synthesis of carbolic acid derivatives
Various methods for synthesizing carbolic acid derivatives are described, including chemical reactions and process improvements. These derivatives have applications in pharmaceuticals, agrochemicals, and industrial processes.- Synthesis of carbolic acid derivatives: Various methods for synthesizing carbolic acid derivatives are described, including chemical reactions and process improvements. These techniques aim to produce novel compounds with potential applications in pharmaceuticals, agrochemicals, and other industries.
- Applications in medical devices and treatments: Carbolic acid derivatives are utilized in medical devices and treatments, such as antimicrobial coatings for implants, disinfectants, and pharmaceutical formulations. These applications leverage the antiseptic and therapeutic properties of carbolic acid derivatives.
- Use in industrial processes and materials: Carbolic acid derivatives find applications in various industrial processes and materials, including polymer production, surface treatments, and as additives in lubricants and coatings. These compounds contribute to improved material properties and process efficiency.
- Environmental and waste treatment applications: Carbolic acid derivatives are employed in environmental and waste treatment applications, such as water purification, soil remediation, and industrial effluent treatment. These compounds help in removing contaminants and improving environmental quality.
- Analytical methods and detection techniques: Various analytical methods and detection techniques have been developed for carbolic acid derivatives, including spectroscopic analysis, chromatography, and sensor-based detection. These methods are crucial for quality control, environmental monitoring, and research purposes.
02 Carbolic acid derivatives in medical applications
Carbolic acid derivatives are utilized in medical and pharmaceutical formulations. These compounds exhibit antimicrobial, analgesic, and anti-inflammatory properties, making them valuable in various therapeutic applications.Expand Specific Solutions03 Industrial uses of carbolic acid derivatives
Carbolic acid derivatives find applications in industrial processes, including as intermediates in the production of plastics, resins, and other chemical compounds. They are also used in the manufacturing of dyes and fragrances.Expand Specific Solutions04 Environmental applications of carbolic acid derivatives
Carbolic acid derivatives are employed in environmental protection and remediation efforts. They are used in water treatment processes, air purification systems, and as components in eco-friendly products.Expand Specific Solutions05 Analytical methods for carbolic acid derivatives
Various analytical techniques and methods are developed for the detection, quantification, and characterization of carbolic acid derivatives. These methods are crucial for quality control, research, and regulatory compliance in industries using these compounds.Expand Specific Solutions
Key Players in Green Pharmaceutical Synthesis
The field of carbolic acid derivatives in pharmaceutical synthesis is experiencing significant growth, driven by the increasing demand for green chemistry approaches. The market is in a mature stage but continues to expand due to the push for more sustainable pharmaceutical processes. Key players like Takeda Pharmaceutical, Solvay SA, and Bayer AG are investing heavily in research and development to advance this technology. The market size is substantial, with pharmaceutical companies globally adopting greener synthesis methods. Technologically, the field is well-established but still evolving, with academic institutions like Zhejiang University of Technology and Rutgers University collaborating with industry leaders to develop novel, environmentally friendly synthesis techniques. This collaborative approach is accelerating the maturation of carbolic acid derivative technologies in pharmaceutical applications.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda Pharmaceutical has developed a green chemistry approach for the synthesis of carbolic acid derivatives used in their pharmaceutical products. Their method focuses on the use of biocatalysis, employing engineered enzymes to catalyze key transformations in the synthesis of phenol-based drug intermediates[7]. This approach significantly reduces the use of harsh reagents and solvents, while also improving stereoselectivity in complex molecule synthesis. Takeda has also implemented a solvent recycling system in their manufacturing processes, recovering and purifying organic solvents for reuse, thus minimizing waste generation[8]. Additionally, the company has developed a novel photocatalytic method for the functionalization of phenol rings, utilizing visible light to promote reactions under mild conditions[9].
Strengths: High selectivity in complex molecule synthesis, reduced use of harsh chemicals, and improved atom economy. Weaknesses: Potential challenges in enzyme stability and activity under industrial conditions, and higher costs associated with enzyme production and optimization.
Solvay SA
Technical Solution: Solvay SA has pioneered a green approach to carbolic acid derivative synthesis using their proprietary GreenSolv™ technology. This innovative process utilizes bio-based solvents derived from renewable resources, significantly reducing the carbon footprint of pharmaceutical synthesis[4]. The company has also developed a novel oxidation process for the production of phenol derivatives, employing hydrogen peroxide as an environmentally friendly oxidant. This method eliminates the need for traditional metal-based oxidants, resulting in cleaner reactions and easier product isolation[5]. Furthermore, Solvay has implemented a microreactor technology platform for the continuous production of carbolic acid derivatives, offering improved yield, selectivity, and process safety[6].
Strengths: Use of renewable resources, reduced waste generation, and improved process safety. Weaknesses: Potential limitations in scalability for some processes and higher production costs for certain bio-based solvents.
Innovative Green Synthesis Methods and Technologies
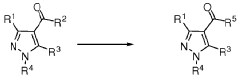
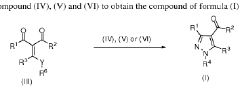
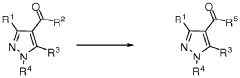
Process for the manufacture of carboxylic acids or carboxylic acid derivatives
PatentWO2018202677A1
Innovation
- A catalytic oxidation process using a catalyst and oxidant, such as oxygen or a metal salt, to convert compound (I) into compound (II), which includes specific steps and conditions to minimize salt waste and achieve high yields, allowing for the production of carboxylic acids or their derivatives on a large scale.
Environmental Impact Assessment of Synthesis Methods
The environmental impact assessment of synthesis methods for carbolic acid derivatives in pharmaceutical production is a critical aspect of developing green approaches. Traditional synthesis methods often involve the use of hazardous chemicals, generate significant waste, and consume substantial energy. These factors contribute to environmental pollution and resource depletion, making it imperative to evaluate and improve the sustainability of pharmaceutical synthesis processes.
One of the primary environmental concerns in carbolic acid derivative synthesis is the generation of toxic by-products and waste streams. Conventional methods frequently employ strong acids, bases, and organic solvents, which can lead to the formation of harmful effluents. These waste products require extensive treatment before disposal, increasing the overall environmental footprint of the process. Additionally, the use of volatile organic compounds (VOCs) as solvents contributes to air pollution and poses health risks to workers and surrounding communities.
Energy consumption is another significant factor in the environmental impact of synthesis methods. Many traditional processes require high temperatures and pressures, resulting in substantial energy usage and associated greenhouse gas emissions. The carbon footprint of these energy-intensive methods is a growing concern in the context of climate change and the need for more sustainable industrial practices.
Water usage and contamination are also important considerations in the environmental assessment of carbolic acid derivative synthesis. Some methods require large volumes of water for reactions, cooling, and purification steps. The resulting wastewater often contains trace amounts of pharmaceuticals and other chemicals, which can have detrimental effects on aquatic ecosystems if not properly treated.
To address these environmental challenges, green chemistry principles are being increasingly applied to the synthesis of carbolic acid derivatives. These approaches focus on reducing or eliminating the use of hazardous substances, improving atom economy, and minimizing waste generation. For example, the development of solvent-free reactions and the use of bio-based solvents can significantly reduce the environmental impact of synthesis processes.
The implementation of catalytic systems is another promising strategy for enhancing the sustainability of carbolic acid derivative synthesis. Catalysts can enable reactions to occur under milder conditions, reducing energy requirements and improving selectivity. This not only decreases the environmental footprint but also often leads to improved product yields and purity.
Continuous flow chemistry is emerging as a valuable tool for reducing the environmental impact of pharmaceutical synthesis. This approach allows for better control of reaction parameters, leading to increased efficiency and reduced waste generation. Additionally, the smaller reactor volumes used in flow chemistry can enhance safety and minimize the potential for environmental contamination in case of accidents.
One of the primary environmental concerns in carbolic acid derivative synthesis is the generation of toxic by-products and waste streams. Conventional methods frequently employ strong acids, bases, and organic solvents, which can lead to the formation of harmful effluents. These waste products require extensive treatment before disposal, increasing the overall environmental footprint of the process. Additionally, the use of volatile organic compounds (VOCs) as solvents contributes to air pollution and poses health risks to workers and surrounding communities.
Energy consumption is another significant factor in the environmental impact of synthesis methods. Many traditional processes require high temperatures and pressures, resulting in substantial energy usage and associated greenhouse gas emissions. The carbon footprint of these energy-intensive methods is a growing concern in the context of climate change and the need for more sustainable industrial practices.
Water usage and contamination are also important considerations in the environmental assessment of carbolic acid derivative synthesis. Some methods require large volumes of water for reactions, cooling, and purification steps. The resulting wastewater often contains trace amounts of pharmaceuticals and other chemicals, which can have detrimental effects on aquatic ecosystems if not properly treated.
To address these environmental challenges, green chemistry principles are being increasingly applied to the synthesis of carbolic acid derivatives. These approaches focus on reducing or eliminating the use of hazardous substances, improving atom economy, and minimizing waste generation. For example, the development of solvent-free reactions and the use of bio-based solvents can significantly reduce the environmental impact of synthesis processes.
The implementation of catalytic systems is another promising strategy for enhancing the sustainability of carbolic acid derivative synthesis. Catalysts can enable reactions to occur under milder conditions, reducing energy requirements and improving selectivity. This not only decreases the environmental footprint but also often leads to improved product yields and purity.
Continuous flow chemistry is emerging as a valuable tool for reducing the environmental impact of pharmaceutical synthesis. This approach allows for better control of reaction parameters, leading to increased efficiency and reduced waste generation. Additionally, the smaller reactor volumes used in flow chemistry can enhance safety and minimize the potential for environmental contamination in case of accidents.
Regulatory Framework for Green Pharmaceutical Production
The regulatory framework for green pharmaceutical production is evolving rapidly to address the growing concerns about environmental sustainability in the pharmaceutical industry. This framework encompasses a wide range of regulations, guidelines, and incentives aimed at promoting the adoption of greener practices in pharmaceutical synthesis, including the use of carbolic acid derivatives.
At the international level, organizations such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) have been instrumental in developing guidelines that encourage the implementation of green chemistry principles in pharmaceutical manufacturing. The ICH Q7 guideline on Good Manufacturing Practice for Active Pharmaceutical Ingredients, for instance, emphasizes the importance of environmental considerations in the production process.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in promoting the use of safer chemicals, including greener alternatives in pharmaceutical synthesis. REACH requires manufacturers to assess and manage the risks associated with the substances they produce or import, encouraging the development and use of more environmentally friendly compounds.
The United States Environmental Protection Agency (EPA) has also been proactive in promoting green chemistry in the pharmaceutical sector. The EPA's Green Chemistry Program provides resources and recognition for innovative green chemistry technologies, including those related to pharmaceutical synthesis. Additionally, the FDA's Quality by Design (QbD) initiative indirectly supports green pharmaceutical production by encouraging more efficient and sustainable manufacturing processes.
In developing countries, regulatory frameworks for green pharmaceutical production are still in their early stages. However, there is a growing recognition of the need to align pharmaceutical manufacturing practices with environmental sustainability goals. Countries like India and China, which are major producers of pharmaceutical ingredients, are gradually implementing stricter environmental regulations for the industry.
One of the key challenges in the regulatory landscape is the need to balance environmental considerations with the imperative of ensuring drug safety and efficacy. Regulators must ensure that the push for greener production methods does not compromise the quality and reliability of pharmaceutical products. This has led to the development of guidelines that specifically address the use of green solvents and reagents in pharmaceutical synthesis, including the application of carbolic acid derivatives.
Looking ahead, the regulatory framework for green pharmaceutical production is likely to become more comprehensive and stringent. There is a growing trend towards the integration of lifecycle assessment approaches in regulatory decision-making, which could further incentivize the adoption of greener synthesis methods. Additionally, the concept of extended producer responsibility is gaining traction, potentially leading to regulations that hold pharmaceutical companies accountable for the environmental impact of their products throughout their lifecycle.
At the international level, organizations such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) have been instrumental in developing guidelines that encourage the implementation of green chemistry principles in pharmaceutical manufacturing. The ICH Q7 guideline on Good Manufacturing Practice for Active Pharmaceutical Ingredients, for instance, emphasizes the importance of environmental considerations in the production process.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in promoting the use of safer chemicals, including greener alternatives in pharmaceutical synthesis. REACH requires manufacturers to assess and manage the risks associated with the substances they produce or import, encouraging the development and use of more environmentally friendly compounds.
The United States Environmental Protection Agency (EPA) has also been proactive in promoting green chemistry in the pharmaceutical sector. The EPA's Green Chemistry Program provides resources and recognition for innovative green chemistry technologies, including those related to pharmaceutical synthesis. Additionally, the FDA's Quality by Design (QbD) initiative indirectly supports green pharmaceutical production by encouraging more efficient and sustainable manufacturing processes.
In developing countries, regulatory frameworks for green pharmaceutical production are still in their early stages. However, there is a growing recognition of the need to align pharmaceutical manufacturing practices with environmental sustainability goals. Countries like India and China, which are major producers of pharmaceutical ingredients, are gradually implementing stricter environmental regulations for the industry.
One of the key challenges in the regulatory landscape is the need to balance environmental considerations with the imperative of ensuring drug safety and efficacy. Regulators must ensure that the push for greener production methods does not compromise the quality and reliability of pharmaceutical products. This has led to the development of guidelines that specifically address the use of green solvents and reagents in pharmaceutical synthesis, including the application of carbolic acid derivatives.
Looking ahead, the regulatory framework for green pharmaceutical production is likely to become more comprehensive and stringent. There is a growing trend towards the integration of lifecycle assessment approaches in regulatory decision-making, which could further incentivize the adoption of greener synthesis methods. Additionally, the concept of extended producer responsibility is gaining traction, potentially leading to regulations that hold pharmaceutical companies accountable for the environmental impact of their products throughout their lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!