Carbolic Acid in Organic Synthesis: Efficiency and Limitations
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Evolution
Carbolic acid, also known as phenol, has undergone a significant evolution in its role within organic synthesis over the past century. Initially discovered in coal tar during the 19th century, phenol's potential as a versatile chemical intermediate was quickly recognized. Early applications focused on its use as an antiseptic and in the production of plastics, particularly Bakelite, the first fully synthetic plastic.
The mid-20th century saw a surge in phenol's importance in organic synthesis. Its reactivity, attributed to the hydroxyl group and the aromatic ring, made it a valuable starting material for various transformations. The Kolbe-Schmitt reaction, discovered in 1860, became a cornerstone in the synthesis of salicylic acid, a precursor to aspirin. This reaction demonstrated phenol's ability to undergo carboxylation, opening new avenues in pharmaceutical synthesis.
As synthetic methodologies advanced, phenol's role expanded beyond simple transformations. The development of transition metal-catalyzed reactions in the latter half of the 20th century further enhanced its utility. Palladium-catalyzed cross-coupling reactions, such as the Heck and Suzuki couplings, allowed for the functionalization of phenol derivatives, enabling the synthesis of complex molecules with applications in pharmaceuticals and materials science.
The late 20th and early 21st centuries witnessed a shift towards more environmentally friendly and efficient processes. This led to the exploration of phenol as a renewable feedstock, derived from biomass rather than petroleum sources. Researchers began investigating the conversion of lignin, a major component of plant biomass, into phenol and its derivatives, aligning with the principles of green chemistry.
Recent advancements have focused on overcoming the limitations of phenol in organic synthesis. These include developing milder reaction conditions to address its corrosive nature and exploring catalytic systems to improve selectivity in transformations. The emergence of flow chemistry has also impacted phenol chemistry, allowing for safer handling and more efficient processes, particularly in large-scale applications.
The evolution of carbolic acid in organic synthesis reflects broader trends in chemistry, moving from simple, often harsh reactions to more sophisticated, selective, and sustainable processes. As research continues, phenol remains a crucial building block in organic synthesis, with ongoing efforts to enhance its efficiency and mitigate its limitations, ensuring its relevance in the face of evolving synthetic challenges and environmental considerations.
The mid-20th century saw a surge in phenol's importance in organic synthesis. Its reactivity, attributed to the hydroxyl group and the aromatic ring, made it a valuable starting material for various transformations. The Kolbe-Schmitt reaction, discovered in 1860, became a cornerstone in the synthesis of salicylic acid, a precursor to aspirin. This reaction demonstrated phenol's ability to undergo carboxylation, opening new avenues in pharmaceutical synthesis.
As synthetic methodologies advanced, phenol's role expanded beyond simple transformations. The development of transition metal-catalyzed reactions in the latter half of the 20th century further enhanced its utility. Palladium-catalyzed cross-coupling reactions, such as the Heck and Suzuki couplings, allowed for the functionalization of phenol derivatives, enabling the synthesis of complex molecules with applications in pharmaceuticals and materials science.
The late 20th and early 21st centuries witnessed a shift towards more environmentally friendly and efficient processes. This led to the exploration of phenol as a renewable feedstock, derived from biomass rather than petroleum sources. Researchers began investigating the conversion of lignin, a major component of plant biomass, into phenol and its derivatives, aligning with the principles of green chemistry.
Recent advancements have focused on overcoming the limitations of phenol in organic synthesis. These include developing milder reaction conditions to address its corrosive nature and exploring catalytic systems to improve selectivity in transformations. The emergence of flow chemistry has also impacted phenol chemistry, allowing for safer handling and more efficient processes, particularly in large-scale applications.
The evolution of carbolic acid in organic synthesis reflects broader trends in chemistry, moving from simple, often harsh reactions to more sophisticated, selective, and sustainable processes. As research continues, phenol remains a crucial building block in organic synthesis, with ongoing efforts to enhance its efficiency and mitigate its limitations, ensuring its relevance in the face of evolving synthetic challenges and environmental considerations.
Organic Synthesis Demand
The demand for organic synthesis in the chemical industry has been steadily growing, driven by the increasing need for complex organic compounds in various sectors. Carbolic acid, also known as phenol, plays a significant role in this landscape due to its versatility as a precursor in numerous synthetic pathways. The market for organic synthesis, particularly involving carbolic acid, is primarily fueled by the pharmaceutical, agrochemical, and materials science industries.
In the pharmaceutical sector, the demand for carbolic acid-based organic synthesis is particularly strong. Many drug molecules require phenol derivatives as key building blocks, making carbolic acid an essential starting material. The growing emphasis on developing new and more effective drugs, especially in areas such as oncology and neurology, continues to drive this demand. Additionally, the trend towards personalized medicine and the need for more targeted therapies further amplifies the requirement for diverse organic compounds, many of which can be synthesized using carbolic acid as a precursor.
The agrochemical industry also contributes significantly to the demand for carbolic acid in organic synthesis. Pesticides, herbicides, and fungicides often incorporate phenol-based structures, necessitating efficient synthetic routes starting from carbolic acid. As global agricultural practices evolve to meet the challenges of feeding a growing population while minimizing environmental impact, the demand for more sophisticated and targeted agrochemicals is expected to rise, further boosting the need for carbolic acid-based syntheses.
In materials science, carbolic acid serves as a crucial starting point for the synthesis of various polymers and resins. The production of phenolic resins, which find applications in diverse fields such as electronics, automotive, and construction, relies heavily on carbolic acid. The ongoing research into advanced materials, including high-performance plastics and composites, continues to explore new synthetic pathways involving carbolic acid, driving demand in this sector.
The efficiency of carbolic acid in organic synthesis is a key factor in its widespread use. Its reactivity, particularly in electrophilic aromatic substitution reactions, makes it a valuable building block for creating more complex molecules. However, the limitations of carbolic acid, such as its corrosive nature and potential environmental concerns, have led to ongoing research into alternative synthetic routes and greener chemistry approaches.
As industries strive for more sustainable practices, there is a growing interest in developing bio-based sources of carbolic acid and exploring more environmentally friendly synthetic methodologies. This trend is likely to shape the future demand for carbolic acid in organic synthesis, potentially leading to new market opportunities and technological innovations in the field.
In the pharmaceutical sector, the demand for carbolic acid-based organic synthesis is particularly strong. Many drug molecules require phenol derivatives as key building blocks, making carbolic acid an essential starting material. The growing emphasis on developing new and more effective drugs, especially in areas such as oncology and neurology, continues to drive this demand. Additionally, the trend towards personalized medicine and the need for more targeted therapies further amplifies the requirement for diverse organic compounds, many of which can be synthesized using carbolic acid as a precursor.
The agrochemical industry also contributes significantly to the demand for carbolic acid in organic synthesis. Pesticides, herbicides, and fungicides often incorporate phenol-based structures, necessitating efficient synthetic routes starting from carbolic acid. As global agricultural practices evolve to meet the challenges of feeding a growing population while minimizing environmental impact, the demand for more sophisticated and targeted agrochemicals is expected to rise, further boosting the need for carbolic acid-based syntheses.
In materials science, carbolic acid serves as a crucial starting point for the synthesis of various polymers and resins. The production of phenolic resins, which find applications in diverse fields such as electronics, automotive, and construction, relies heavily on carbolic acid. The ongoing research into advanced materials, including high-performance plastics and composites, continues to explore new synthetic pathways involving carbolic acid, driving demand in this sector.
The efficiency of carbolic acid in organic synthesis is a key factor in its widespread use. Its reactivity, particularly in electrophilic aromatic substitution reactions, makes it a valuable building block for creating more complex molecules. However, the limitations of carbolic acid, such as its corrosive nature and potential environmental concerns, have led to ongoing research into alternative synthetic routes and greener chemistry approaches.
As industries strive for more sustainable practices, there is a growing interest in developing bio-based sources of carbolic acid and exploring more environmentally friendly synthetic methodologies. This trend is likely to shape the future demand for carbolic acid in organic synthesis, potentially leading to new market opportunities and technological innovations in the field.
Current Challenges
Carbolic acid, also known as phenol, faces several challenges in its application to organic synthesis, despite its historical significance and widespread use. One of the primary limitations is its toxicity and corrosive nature, which poses significant health and safety risks to researchers and laboratory personnel. This necessitates stringent handling protocols and protective measures, potentially limiting its use in certain research environments or industrial settings.
Another challenge lies in the reactivity of carbolic acid. While its high reactivity can be advantageous in some synthetic processes, it can also lead to unwanted side reactions or over-functionalization of target molecules. This lack of selectivity can result in reduced yields and the formation of complex mixtures, making product isolation and purification more difficult and time-consuming.
The environmental impact of carbolic acid production and use is also a growing concern. Traditional methods of phenol synthesis often involve petroleum-based feedstocks and energy-intensive processes, contributing to carbon emissions and environmental pollution. This has led to increased pressure on the chemical industry to develop more sustainable and eco-friendly alternatives or production methods.
From an economic perspective, the fluctuating costs of raw materials and energy required for carbolic acid production can impact its viability in large-scale industrial applications. This volatility in production costs can make it challenging for manufacturers to maintain consistent pricing and profitability, potentially limiting its use in cost-sensitive industries.
The regulatory landscape surrounding carbolic acid usage presents another hurdle. Stringent regulations on its production, transportation, and disposal due to its hazardous nature can increase operational costs and complexity for businesses. Compliance with these regulations requires significant investment in safety equipment, training, and waste management systems.
In terms of synthetic efficiency, while carbolic acid is a versatile reagent, it often requires harsh reaction conditions or the use of additional catalysts to achieve desired transformations. This can limit its applicability in sensitive or complex synthetic routes, particularly those involving delicate functional groups or biomolecules.
The development of alternative synthetic methodologies and greener chemistry approaches has also challenged the traditional role of carbolic acid in organic synthesis. As researchers explore more selective, milder, and environmentally benign reagents, the relative advantages of carbolic acid in certain reactions may diminish, necessitating a reevaluation of its place in modern synthetic strategies.
Another challenge lies in the reactivity of carbolic acid. While its high reactivity can be advantageous in some synthetic processes, it can also lead to unwanted side reactions or over-functionalization of target molecules. This lack of selectivity can result in reduced yields and the formation of complex mixtures, making product isolation and purification more difficult and time-consuming.
The environmental impact of carbolic acid production and use is also a growing concern. Traditional methods of phenol synthesis often involve petroleum-based feedstocks and energy-intensive processes, contributing to carbon emissions and environmental pollution. This has led to increased pressure on the chemical industry to develop more sustainable and eco-friendly alternatives or production methods.
From an economic perspective, the fluctuating costs of raw materials and energy required for carbolic acid production can impact its viability in large-scale industrial applications. This volatility in production costs can make it challenging for manufacturers to maintain consistent pricing and profitability, potentially limiting its use in cost-sensitive industries.
The regulatory landscape surrounding carbolic acid usage presents another hurdle. Stringent regulations on its production, transportation, and disposal due to its hazardous nature can increase operational costs and complexity for businesses. Compliance with these regulations requires significant investment in safety equipment, training, and waste management systems.
In terms of synthetic efficiency, while carbolic acid is a versatile reagent, it often requires harsh reaction conditions or the use of additional catalysts to achieve desired transformations. This can limit its applicability in sensitive or complex synthetic routes, particularly those involving delicate functional groups or biomolecules.
The development of alternative synthetic methodologies and greener chemistry approaches has also challenged the traditional role of carbolic acid in organic synthesis. As researchers explore more selective, milder, and environmentally benign reagents, the relative advantages of carbolic acid in certain reactions may diminish, necessitating a reevaluation of its place in modern synthetic strategies.
Existing Applications
01 Carbolic acid in disinfection and sterilization
Carbolic acid, also known as phenol, is widely used for its disinfectant and sterilizing properties. It is effective in killing various microorganisms, making it suitable for use in medical and industrial settings. The efficiency of carbolic acid in disinfection depends on factors such as concentration, contact time, and the type of microorganisms targeted.- Carbolic acid in disinfection applications: Carbolic acid, also known as phenol, is widely used in disinfection applications due to its high efficiency in killing bacteria and other microorganisms. It is particularly effective in medical and industrial settings where thorough sanitization is crucial. The efficiency of carbolic acid in disinfection is attributed to its ability to denature proteins and disrupt cell membranes.
- Carbolic acid in water treatment systems: Carbolic acid is utilized in water treatment systems to improve efficiency in removing contaminants and purifying water. Its strong antimicrobial properties make it effective in treating both industrial and municipal water supplies. The integration of carbolic acid in water treatment processes enhances the overall purification efficiency and helps maintain water quality standards.
- Carbolic acid in polymer production: The efficiency of carbolic acid in polymer production is significant, particularly in the synthesis of phenolic resins. It serves as a key raw material in the manufacturing of various polymers, including adhesives, coatings, and molding compounds. The use of carbolic acid in polymer production contributes to improved product performance and durability.
- Carbolic acid in pharmaceutical applications: Carbolic acid demonstrates efficiency in pharmaceutical applications, particularly in the production of certain medications and as an active ingredient in topical treatments. Its antiseptic properties make it valuable in the formulation of various medical products. The pharmaceutical industry utilizes carbolic acid to enhance the efficacy of specific drug formulations.
- Carbolic acid in industrial cleaning processes: The efficiency of carbolic acid in industrial cleaning processes is notable, especially in heavy-duty degreasing and sanitizing applications. It is effective in removing stubborn contaminants and providing thorough disinfection in industrial settings. The use of carbolic acid in cleaning formulations enhances the overall efficiency of industrial maintenance and sanitation procedures.
02 Carbolic acid in water treatment
Carbolic acid is utilized in water treatment processes to remove contaminants and improve water quality. Its efficiency in water treatment applications is attributed to its ability to break down organic compounds and eliminate harmful microorganisms. The use of carbolic acid in water treatment systems can enhance the overall purification process.Expand Specific Solutions03 Carbolic acid in industrial cleaning
The efficiency of carbolic acid in industrial cleaning applications is notable due to its strong solvent properties and ability to dissolve various substances. It is particularly effective in removing grease, oil, and other stubborn contaminants from surfaces. The use of carbolic acid in industrial cleaning formulations can improve cleaning efficiency and reduce the time required for cleaning processes.Expand Specific Solutions04 Carbolic acid in pharmaceutical applications
Carbolic acid demonstrates efficiency in various pharmaceutical applications, including as an active ingredient in topical medications and as a preservative in drug formulations. Its antiseptic properties make it useful in treating skin conditions and preventing bacterial growth in pharmaceutical products. The efficiency of carbolic acid in these applications is dependent on proper formulation and dosage.Expand Specific Solutions05 Carbolic acid in polymer production
Carbolic acid plays a role in the production of various polymers, demonstrating efficiency in the synthesis process. It serves as a precursor for the manufacture of plastics, resins, and other synthetic materials. The efficiency of carbolic acid in polymer production is influenced by factors such as reaction conditions, catalysts, and the specific polymer being synthesized.Expand Specific Solutions
Key Industry Players
The competitive landscape for carbolic acid in organic synthesis is characterized by a mature market with established players and ongoing research efforts. The global phenol market, which includes carbolic acid, is projected to reach a substantial size, driven by demand in various industries. Technologically, the field is well-developed but continues to evolve, with companies like BASF, Evonik, and SABIC leading in innovation. Academic institutions such as Hainan University and Ludwig-Maximilians-Universität München contribute to advancing the technology. While efficiency improvements are ongoing, limitations in selectivity and environmental concerns present challenges, spurring research into greener alternatives and catalytic processes.
BASF Corp.
Technical Solution: BASF has developed innovative approaches for utilizing carbolic acid (phenol) in organic synthesis. They have implemented a proprietary process for the production of bisphenol A, a key intermediate for polycarbonates and epoxy resins, using phenol and acetone as raw materials[1]. This process employs advanced catalysts and reactor designs to achieve high yields and selectivity. Additionally, BASF has explored the use of phenol in the synthesis of alkylphenols through alkylation reactions, optimizing reaction conditions to minimize byproduct formation[2]. The company has also invested in research on phenol-formaldehyde resins, developing new formulations with improved thermal and mechanical properties for various industrial applications[3].
Strengths: Extensive R&D capabilities, large-scale production facilities, and a diverse product portfolio. Weaknesses: Dependence on petrochemical feedstocks and potential environmental concerns associated with phenol-based processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant advancements in the utilization of carbolic acid for organic synthesis. They have developed a novel process for the production of cyclohexanone, a key intermediate for nylon production, using phenol as a starting material[4]. This process employs a proprietary catalyst system that achieves high conversion rates and selectivity. Sinopec has also explored the use of phenol in the synthesis of various fine chemicals, including salicylic acid derivatives and alkylphenols[5]. Their research has focused on improving reaction efficiency and reducing environmental impact through the development of greener catalysts and process intensification techniques.
Strengths: Strong integration with petrochemical feedstocks, large-scale production capabilities, and a focus on process optimization. Weaknesses: Potential challenges in meeting stringent environmental regulations and adapting to shifting market demands for more sustainable products.
Core Reaction Mechanisms
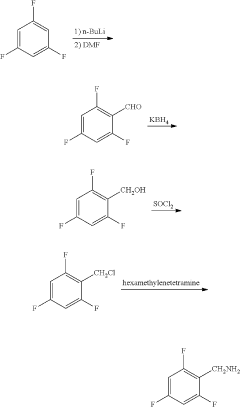
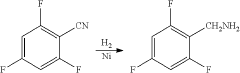
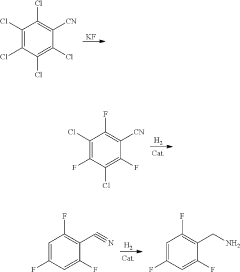
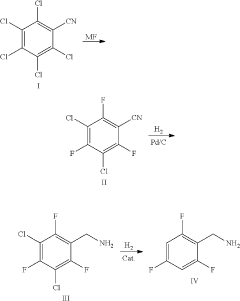
Synthesis method of 2,4,6-trifluorobenzylamine
PatentActiveUS20200207702A1
Innovation
- A three-step synthesis method using pentachlorobenzonitrile as the raw material, involving fluoridation, hydrogenation, and reductive dechlorination, where 2,4,6-trifluoro-3,5-dichlorobenzonitrile serves as both the fluoridation reaction solvent and product, reducing the need for additional solvents and simplifying the process, with palladium carbon and organic acids used as catalysts to enhance reaction efficiency.
Green Chemistry Impact
The impact of green chemistry principles on the use of carbolic acid (phenol) in organic synthesis has been significant, driving innovations in both efficiency and sustainability. As environmental concerns have grown, researchers and industries have sought to develop more eco-friendly approaches to chemical processes involving carbolic acid.
One of the primary focuses has been on reducing the environmental footprint of carbolic acid production. Traditional methods often involve energy-intensive processes and generate substantial waste. Green chemistry initiatives have led to the development of catalytic processes that operate at lower temperatures and pressures, significantly reducing energy consumption. Additionally, bio-based routes for phenol production from renewable resources, such as lignin, have gained traction as sustainable alternatives to petroleum-based methods.
In organic synthesis applications, green chemistry has influenced the way carbolic acid is utilized. Efforts have been made to minimize the use of toxic solvents and reagents often associated with phenol-based reactions. Water-based systems and ionic liquids have emerged as greener alternatives, offering improved safety profiles and reduced environmental impact. Moreover, the development of recyclable catalysts and support materials has enhanced the atom economy and reduced waste generation in carbolic acid-mediated transformations.
The principles of green chemistry have also driven advancements in reaction efficiency. Microwave-assisted and flow chemistry techniques have been applied to phenol-based reactions, leading to shorter reaction times, improved yields, and reduced energy consumption. These methods often allow for precise control over reaction conditions, minimizing side product formation and improving overall process sustainability.
Furthermore, green chemistry has spurred research into alternative reagents and methodologies that can replace or complement carbolic acid in certain applications. Biocatalysis, for instance, has shown promise in performing selective transformations traditionally achieved with phenol derivatives, often under milder conditions and with enhanced stereoselectivity.
The limitations of carbolic acid use have also been addressed through green chemistry approaches. Efforts to mitigate the toxicity and corrosive nature of phenol have led to the development of safer handling protocols and improved personal protective equipment. Additionally, research into less hazardous phenol analogues and bio-based alternatives has expanded the toolkit available to organic chemists, providing options that align more closely with green chemistry principles.
In conclusion, the impact of green chemistry on carbolic acid use in organic synthesis has been multifaceted, driving improvements in production methods, reaction efficiency, and overall sustainability. While challenges remain, the ongoing integration of green chemistry principles continues to shape the future of phenol-based chemistry, pushing towards more environmentally benign and economically viable processes.
One of the primary focuses has been on reducing the environmental footprint of carbolic acid production. Traditional methods often involve energy-intensive processes and generate substantial waste. Green chemistry initiatives have led to the development of catalytic processes that operate at lower temperatures and pressures, significantly reducing energy consumption. Additionally, bio-based routes for phenol production from renewable resources, such as lignin, have gained traction as sustainable alternatives to petroleum-based methods.
In organic synthesis applications, green chemistry has influenced the way carbolic acid is utilized. Efforts have been made to minimize the use of toxic solvents and reagents often associated with phenol-based reactions. Water-based systems and ionic liquids have emerged as greener alternatives, offering improved safety profiles and reduced environmental impact. Moreover, the development of recyclable catalysts and support materials has enhanced the atom economy and reduced waste generation in carbolic acid-mediated transformations.
The principles of green chemistry have also driven advancements in reaction efficiency. Microwave-assisted and flow chemistry techniques have been applied to phenol-based reactions, leading to shorter reaction times, improved yields, and reduced energy consumption. These methods often allow for precise control over reaction conditions, minimizing side product formation and improving overall process sustainability.
Furthermore, green chemistry has spurred research into alternative reagents and methodologies that can replace or complement carbolic acid in certain applications. Biocatalysis, for instance, has shown promise in performing selective transformations traditionally achieved with phenol derivatives, often under milder conditions and with enhanced stereoselectivity.
The limitations of carbolic acid use have also been addressed through green chemistry approaches. Efforts to mitigate the toxicity and corrosive nature of phenol have led to the development of safer handling protocols and improved personal protective equipment. Additionally, research into less hazardous phenol analogues and bio-based alternatives has expanded the toolkit available to organic chemists, providing options that align more closely with green chemistry principles.
In conclusion, the impact of green chemistry on carbolic acid use in organic synthesis has been multifaceted, driving improvements in production methods, reaction efficiency, and overall sustainability. While challenges remain, the ongoing integration of green chemistry principles continues to shape the future of phenol-based chemistry, pushing towards more environmentally benign and economically viable processes.
Safety Considerations
Safety considerations are paramount when working with carbolic acid (phenol) in organic synthesis due to its corrosive and toxic nature. Proper handling and storage procedures must be strictly adhered to in order to minimize risks to personnel and the environment. Personal protective equipment (PPE) is essential, including chemical-resistant gloves, safety goggles, and lab coats. A well-ventilated workspace or fume hood is necessary to prevent inhalation of vapors.
Carbolic acid can cause severe burns upon skin contact, and its vapors can irritate the respiratory system. In case of exposure, immediate decontamination procedures should be followed, including thorough washing with water for at least 15 minutes. Eye wash stations and safety showers must be readily accessible in the laboratory.
Storage of carbolic acid requires special attention. It should be kept in tightly sealed containers made of compatible materials, such as glass or certain plastics, and stored in a cool, dry place away from direct sunlight and heat sources. Segregation from incompatible substances, particularly oxidizing agents and strong acids or bases, is crucial to prevent dangerous reactions.
Proper disposal of carbolic acid waste is essential to prevent environmental contamination. It should never be poured down the drain or disposed of in regular trash. Instead, it must be collected in designated waste containers and handled by certified waste management professionals.
When using carbolic acid in organic synthesis, researchers should be aware of its potential to form explosive compounds when mixed with certain substances. Careful planning of reactions and thorough risk assessments are necessary before proceeding with any experiment involving this compound.
Emergency response protocols specific to carbolic acid incidents should be established and regularly reviewed. This includes training personnel on proper spill containment and cleanup procedures, as well as first aid measures for potential exposures.
Monitoring and record-keeping are important aspects of safety management when working with carbolic acid. Regular safety audits, equipment inspections, and maintenance of safety data sheets (SDS) help ensure ongoing compliance with safety standards and regulations.
By implementing comprehensive safety measures and fostering a culture of safety awareness, researchers can effectively mitigate the risks associated with carbolic acid use in organic synthesis, allowing for the exploration of its efficiency and limitations while prioritizing the well-being of laboratory personnel and the environment.
Carbolic acid can cause severe burns upon skin contact, and its vapors can irritate the respiratory system. In case of exposure, immediate decontamination procedures should be followed, including thorough washing with water for at least 15 minutes. Eye wash stations and safety showers must be readily accessible in the laboratory.
Storage of carbolic acid requires special attention. It should be kept in tightly sealed containers made of compatible materials, such as glass or certain plastics, and stored in a cool, dry place away from direct sunlight and heat sources. Segregation from incompatible substances, particularly oxidizing agents and strong acids or bases, is crucial to prevent dangerous reactions.
Proper disposal of carbolic acid waste is essential to prevent environmental contamination. It should never be poured down the drain or disposed of in regular trash. Instead, it must be collected in designated waste containers and handled by certified waste management professionals.
When using carbolic acid in organic synthesis, researchers should be aware of its potential to form explosive compounds when mixed with certain substances. Careful planning of reactions and thorough risk assessments are necessary before proceeding with any experiment involving this compound.
Emergency response protocols specific to carbolic acid incidents should be established and regularly reviewed. This includes training personnel on proper spill containment and cleanup procedures, as well as first aid measures for potential exposures.
Monitoring and record-keeping are important aspects of safety management when working with carbolic acid. Regular safety audits, equipment inspections, and maintenance of safety data sheets (SDS) help ensure ongoing compliance with safety standards and regulations.
By implementing comprehensive safety measures and fostering a culture of safety awareness, researchers can effectively mitigate the risks associated with carbolic acid use in organic synthesis, allowing for the exploration of its efficiency and limitations while prioritizing the well-being of laboratory personnel and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!