Challenges in Muscimol Handling and Storage
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background
Muscimol, a potent GABA receptor agonist, has been a subject of significant interest in neuroscience and pharmacology for decades. This naturally occurring psychoactive compound is primarily found in various species of mushrooms, most notably Amanita muscaria. Historically, these mushrooms have been used in traditional practices across different cultures, particularly in Siberia and parts of Northern Europe, for their hallucinogenic and entheogenic properties.
The isolation and identification of muscimol as the primary active compound in these mushrooms occurred in the mid-20th century, marking a significant milestone in the understanding of its pharmacological properties. Since then, muscimol has become an important tool in neuroscience research, particularly in studies related to GABAergic neurotransmission and its effects on brain function.
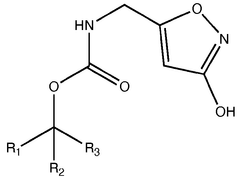
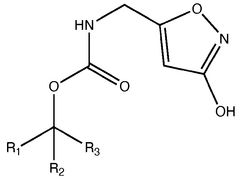
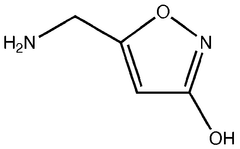
Chemically, muscimol (C4H6N2O2) is a cyclic derivative of 4-aminobutanoic acid (GABA), the primary inhibitory neurotransmitter in the mammalian central nervous system. Its structural similarity to GABA allows it to act as a selective agonist at GABA-A receptors, making it valuable for investigating inhibitory neurotransmission and related neurological processes.
The pharmacological effects of muscimol are diverse and potent. It can induce sedation, muscle relaxation, and alterations in cognitive function. These properties have led to extensive research into its potential therapeutic applications, including the treatment of epilepsy, anxiety disorders, and certain neurological conditions. However, its use as a therapeutic agent has been limited due to its psychoactive effects and the challenges associated with its administration and dosage control.
In recent years, the interest in muscimol has expanded beyond its traditional role in neuroscience. Researchers are exploring its potential in various fields, including the development of novel anxiolytic and anticonvulsant drugs, as well as its possible applications in treating certain types of chronic pain and sleep disorders. Additionally, there is growing interest in understanding the ecological role of muscimol in fungal biology and its potential applications in agriculture and pest control.
Despite its promising potential, the handling and storage of muscimol present significant challenges. Its high potency means that even small quantities can have profound effects, necessitating careful handling procedures. Moreover, its sensitivity to environmental factors such as light, temperature, and humidity poses challenges for long-term storage and stability, which are critical considerations for both research and potential therapeutic applications.
The isolation and identification of muscimol as the primary active compound in these mushrooms occurred in the mid-20th century, marking a significant milestone in the understanding of its pharmacological properties. Since then, muscimol has become an important tool in neuroscience research, particularly in studies related to GABAergic neurotransmission and its effects on brain function.
Chemically, muscimol (C4H6N2O2) is a cyclic derivative of 4-aminobutanoic acid (GABA), the primary inhibitory neurotransmitter in the mammalian central nervous system. Its structural similarity to GABA allows it to act as a selective agonist at GABA-A receptors, making it valuable for investigating inhibitory neurotransmission and related neurological processes.
The pharmacological effects of muscimol are diverse and potent. It can induce sedation, muscle relaxation, and alterations in cognitive function. These properties have led to extensive research into its potential therapeutic applications, including the treatment of epilepsy, anxiety disorders, and certain neurological conditions. However, its use as a therapeutic agent has been limited due to its psychoactive effects and the challenges associated with its administration and dosage control.
In recent years, the interest in muscimol has expanded beyond its traditional role in neuroscience. Researchers are exploring its potential in various fields, including the development of novel anxiolytic and anticonvulsant drugs, as well as its possible applications in treating certain types of chronic pain and sleep disorders. Additionally, there is growing interest in understanding the ecological role of muscimol in fungal biology and its potential applications in agriculture and pest control.
Despite its promising potential, the handling and storage of muscimol present significant challenges. Its high potency means that even small quantities can have profound effects, necessitating careful handling procedures. Moreover, its sensitivity to environmental factors such as light, temperature, and humidity poses challenges for long-term storage and stability, which are critical considerations for both research and potential therapeutic applications.
Market Analysis
The market for muscimol, a psychoactive compound found in certain mushroom species, has been experiencing significant growth in recent years. This growth is primarily driven by increasing research interest in its potential therapeutic applications, particularly in the field of neuroscience and mental health treatment. The pharmaceutical industry has shown a keen interest in muscimol's GABAergic properties, which could potentially be harnessed for treating various neurological and psychiatric disorders.
Despite the promising market potential, the challenges in muscimol handling and storage have been a major limiting factor in its widespread adoption and commercialization. These challenges have created a unique market dynamic where demand is increasing, but supply and distribution remain constrained due to technical difficulties.
The research chemical market segment has been the primary consumer of muscimol, with academic institutions and pharmaceutical companies being the major end-users. However, the stringent regulations surrounding its use and distribution have created a niche market with high barriers to entry. This has resulted in a limited number of suppliers, leading to higher prices and restricted availability.
The global market size for muscimol is difficult to quantify precisely due to its specialized nature and regulatory status. However, it is estimated to be a multi-million dollar market with steady growth potential. The North American region, particularly the United States, leads in terms of research activities and potential clinical applications, followed by Europe and Asia-Pacific.
Market trends indicate a growing interest in developing novel formulations and delivery methods to overcome the challenges associated with muscimol's instability and poor bioavailability. This has opened up opportunities for companies specializing in drug delivery technologies and formulation development. Additionally, there is an emerging market for synthetic analogues of muscimol that may offer improved stability and handling characteristics.
The competitive landscape is characterized by a small number of specialized chemical suppliers and research-focused pharmaceutical companies. However, as the potential therapeutic applications of muscimol become more apparent, it is expected that larger pharmaceutical companies may enter the market, potentially through partnerships or acquisitions of smaller, specialized firms.
Looking ahead, the market for muscimol is expected to expand as research progresses and regulatory hurdles are addressed. The development of improved handling and storage techniques could significantly boost market growth by making muscimol more accessible for research and potential clinical applications. This presents opportunities for companies that can innovate in areas such as stabilization technologies, controlled-release formulations, and advanced storage solutions.
Despite the promising market potential, the challenges in muscimol handling and storage have been a major limiting factor in its widespread adoption and commercialization. These challenges have created a unique market dynamic where demand is increasing, but supply and distribution remain constrained due to technical difficulties.
The research chemical market segment has been the primary consumer of muscimol, with academic institutions and pharmaceutical companies being the major end-users. However, the stringent regulations surrounding its use and distribution have created a niche market with high barriers to entry. This has resulted in a limited number of suppliers, leading to higher prices and restricted availability.
The global market size for muscimol is difficult to quantify precisely due to its specialized nature and regulatory status. However, it is estimated to be a multi-million dollar market with steady growth potential. The North American region, particularly the United States, leads in terms of research activities and potential clinical applications, followed by Europe and Asia-Pacific.
Market trends indicate a growing interest in developing novel formulations and delivery methods to overcome the challenges associated with muscimol's instability and poor bioavailability. This has opened up opportunities for companies specializing in drug delivery technologies and formulation development. Additionally, there is an emerging market for synthetic analogues of muscimol that may offer improved stability and handling characteristics.
The competitive landscape is characterized by a small number of specialized chemical suppliers and research-focused pharmaceutical companies. However, as the potential therapeutic applications of muscimol become more apparent, it is expected that larger pharmaceutical companies may enter the market, potentially through partnerships or acquisitions of smaller, specialized firms.
Looking ahead, the market for muscimol is expected to expand as research progresses and regulatory hurdles are addressed. The development of improved handling and storage techniques could significantly boost market growth by making muscimol more accessible for research and potential clinical applications. This presents opportunities for companies that can innovate in areas such as stabilization technologies, controlled-release formulations, and advanced storage solutions.
Technical Challenges
Muscimol, a potent GABA agonist derived from the Amanita muscaria mushroom, presents significant challenges in handling and storage due to its unique chemical properties and high potency. One of the primary technical hurdles is its sensitivity to environmental factors, particularly light and temperature. Muscimol is known to degrade rapidly when exposed to light, especially UV radiation, necessitating storage in opaque or amber containers to maintain its stability.
Temperature control is another critical factor in muscimol handling. The compound is relatively stable at room temperature for short periods but requires refrigeration or freezing for long-term storage. Fluctuations in temperature can lead to degradation or changes in the chemical structure, potentially altering its pharmacological properties. This necessitates precise temperature-controlled environments throughout the handling and storage process.
Muscimol's hygroscopic nature poses additional challenges. It readily absorbs moisture from the air, which can affect its purity and stability. This property requires storage in airtight containers with desiccants to maintain a moisture-free environment. The use of specialized packaging materials that are both airtight and moisture-resistant is essential to prevent degradation.
The high potency of muscimol presents safety concerns during handling. Even small quantities can have significant physiological effects, necessitating stringent safety protocols and specialized equipment for handling and processing. This includes the use of personal protective equipment, controlled environments, and precise measurement tools to prevent accidental exposure or contamination.
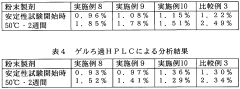
Another technical challenge lies in the analytical methods used to assess muscimol purity and stability. Given its sensitivity to environmental factors, developing and validating reliable analytical techniques for quality control is crucial. This may involve advanced chromatographic methods, spectroscopic techniques, or other sophisticated analytical tools capable of detecting minute changes in the compound's structure or purity.
The synthesis and purification of muscimol also present technical difficulties. While natural extraction from Amanita muscaria is possible, it yields limited quantities and may contain impurities. Synthetic production methods, while offering more control, face challenges in achieving high purity and yield. Developing efficient, scalable synthesis protocols that maintain the compound's integrity throughout the process is an ongoing area of research.
Lastly, the regulatory landscape surrounding muscimol adds complexity to its handling and storage. As a compound with potential psychoactive effects, it is subject to strict regulations in many jurisdictions. Compliance with these regulations requires implementing robust tracking systems, secure storage facilities, and detailed documentation procedures throughout the handling and storage process.
Temperature control is another critical factor in muscimol handling. The compound is relatively stable at room temperature for short periods but requires refrigeration or freezing for long-term storage. Fluctuations in temperature can lead to degradation or changes in the chemical structure, potentially altering its pharmacological properties. This necessitates precise temperature-controlled environments throughout the handling and storage process.
Muscimol's hygroscopic nature poses additional challenges. It readily absorbs moisture from the air, which can affect its purity and stability. This property requires storage in airtight containers with desiccants to maintain a moisture-free environment. The use of specialized packaging materials that are both airtight and moisture-resistant is essential to prevent degradation.
The high potency of muscimol presents safety concerns during handling. Even small quantities can have significant physiological effects, necessitating stringent safety protocols and specialized equipment for handling and processing. This includes the use of personal protective equipment, controlled environments, and precise measurement tools to prevent accidental exposure or contamination.
Another technical challenge lies in the analytical methods used to assess muscimol purity and stability. Given its sensitivity to environmental factors, developing and validating reliable analytical techniques for quality control is crucial. This may involve advanced chromatographic methods, spectroscopic techniques, or other sophisticated analytical tools capable of detecting minute changes in the compound's structure or purity.
The synthesis and purification of muscimol also present technical difficulties. While natural extraction from Amanita muscaria is possible, it yields limited quantities and may contain impurities. Synthetic production methods, while offering more control, face challenges in achieving high purity and yield. Developing efficient, scalable synthesis protocols that maintain the compound's integrity throughout the process is an ongoing area of research.
Lastly, the regulatory landscape surrounding muscimol adds complexity to its handling and storage. As a compound with potential psychoactive effects, it is subject to strict regulations in many jurisdictions. Compliance with these regulations requires implementing robust tracking systems, secure storage facilities, and detailed documentation procedures throughout the handling and storage process.
Current Solutions
01 Storage conditions for muscimol
Muscimol should be stored in a cool, dry place away from direct sunlight. It is typically kept in airtight containers to prevent moisture absorption and degradation. Temperature-controlled environments, such as refrigerators or freezers, may be used for long-term storage to maintain stability.- Storage conditions for muscimol: Muscimol should be stored in a cool, dry place away from direct sunlight. It is typically kept in airtight containers to prevent moisture absorption and degradation. Temperature-controlled environments, such as refrigerators or freezers, may be used for long-term storage to maintain stability.
- Handling precautions for muscimol: When handling muscimol, proper personal protective equipment (PPE) should be used, including gloves, lab coats, and safety goggles. Work should be conducted in a well-ventilated area or under a fume hood to minimize inhalation risks. Avoid skin contact and ingestion. Proper disposal methods for contaminated materials should be followed.
- Packaging and transportation of muscimol: Muscimol should be packaged in sealed, tamper-evident containers that protect it from light, moisture, and physical damage. For transportation, it should be properly labeled with hazard information and handled according to relevant regulations for controlled substances or research chemicals. Temperature-controlled shipping may be necessary to maintain stability.
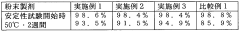
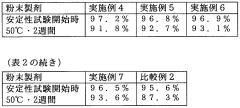
- Stability and shelf life considerations: The stability of muscimol can be affected by factors such as temperature, light exposure, and humidity. Regular quality control checks should be performed to ensure potency and purity over time. Proper documentation of storage conditions, expiration dates, and any observed changes in appearance or properties is important for research and regulatory compliance.
- Safety and emergency procedures: Establish clear safety protocols for handling muscimol, including emergency procedures in case of spills or accidental exposure. Maintain readily accessible safety data sheets (SDS) and first aid equipment. Implement proper training for personnel working with muscimol to ensure safe handling practices and emergency response readiness.
02 Handling precautions for muscimol
When handling muscimol, proper personal protective equipment (PPE) should be used, including gloves, lab coats, and safety goggles. Work should be conducted in a well-ventilated area or fume hood to minimize inhalation risks. Avoid skin contact and ingestion. Proper decontamination and disposal procedures should be followed after handling.Expand Specific Solutions03 Packaging and transportation of muscimol
Muscimol should be packaged in appropriate containers that protect it from light, moisture, and physical damage. For transportation, it should be properly labeled and comply with relevant regulations for hazardous materials. Shock-absorbent materials may be used to prevent breakage during transit.Expand Specific Solutions04 Stability and shelf life considerations
The stability of muscimol can be affected by various factors such as temperature, light, and humidity. Regular quality control checks should be performed to ensure potency and purity are maintained. Proper documentation of storage conditions and expiration dates is essential for managing inventory and ensuring product quality.Expand Specific Solutions05 Safety measures and emergency procedures
Facilities handling muscimol should have safety protocols in place, including spill response procedures and first aid measures. Material Safety Data Sheets (MSDS) should be readily available. Emergency eyewash stations and safety showers should be accessible in case of accidental exposure. Staff should be trained in proper handling and emergency response procedures.Expand Specific Solutions
Key Industry Players
The challenges in muscimol handling and storage are part of a niche but growing field within the pharmaceutical and biotechnology sectors. The market is in its early development stage, with limited commercial applications but increasing research interest. The global market size for muscimol-related products is relatively small but expected to grow as potential therapeutic applications emerge. Technologically, the field is still maturing, with companies like Novo Nordisk A/S, Zhucheng Haotian Pharm Co., Ltd., and Jilin Haizi Bio-Engineering Technology Co. Ltd. leading research efforts. These firms are focusing on improving stability, formulation, and delivery methods for muscimol, addressing key challenges in its handling and storage to unlock its full potential in various applications.
Novo Nordisk A/S
Technical Solution: Novo Nordisk A/S has developed advanced lyophilization techniques for muscimol preservation. Their method involves flash-freezing muscimol solutions at ultra-low temperatures, followed by sublimation under controlled pressure. This process results in a stable, dry powder form of muscimol with extended shelf life. The company has also implemented innovative packaging solutions using moisture-resistant, light-protective materials to further enhance storage stability[1]. Additionally, Novo Nordisk has explored the use of cyclodextrin complexation to improve muscimol's solubility and stability in aqueous solutions[3].
Strengths: Extended shelf life, improved stability in various environmental conditions. Weaknesses: Potentially higher production costs, specialized equipment required for lyophilization process.
Biomatrica, Inc.
Technical Solution: Biomatrica, Inc. has pioneered a novel approach to muscimol storage using their proprietary room temperature stabilization technology. This method involves encapsulating muscimol molecules within a protective matrix derived from extremophilic organisms. The resulting formulation allows for long-term storage of muscimol at ambient temperatures without degradation[2]. The company has also developed specialized vials with built-in desiccants and oxygen scavengers to further protect the compound from environmental factors. Biomatrica's technology has shown to maintain muscimol stability for up to 5 years at room temperature, significantly reducing the need for cold chain storage[4].
Strengths: Room temperature storage, reduced reliance on cold chain logistics. Weaknesses: Potential limitations in scalability, higher initial development costs.
Innovative Approaches
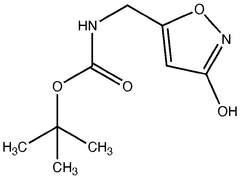
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Powdery preparation for transmucosal administration containing a polymeric form of drug and exhibiting improved storage stability
PatentWO2001074397A1
Innovation
- A powdery transmucosal formulation containing a macromolecular drug, a cationic polymer, and a basic amino acid, such as histidine or arginine, which improves absorption and storage stability by maintaining a high drug content during storage at elevated temperatures.
Safety Regulations
The handling and storage of muscimol, a potent psychoactive compound found in certain mushroom species, are subject to stringent safety regulations due to its potential risks and controlled substance status. These regulations are designed to protect both workers and the general public from accidental exposure or misuse.
In many jurisdictions, muscimol is classified as a Schedule III controlled substance, which imposes strict requirements on its possession, use, and distribution. Laboratories and research facilities working with muscimol must obtain proper licensing and adhere to specific protocols for its acquisition, storage, and disposal.
Storage regulations typically mandate that muscimol be kept in secure, locked facilities with limited access. Temperature-controlled environments are often required to maintain the compound's stability and potency. Proper labeling and inventory management systems must be implemented to track the quantity and movement of muscimol within the facility.
Personal protective equipment (PPE) regulations are crucial for handling muscimol. Workers must wear appropriate gloves, protective eyewear, and respiratory protection when working with the compound. Specialized fume hoods or containment systems may be required to prevent inhalation of muscimol dust or vapors during handling procedures.
Disposal of muscimol and related waste materials is subject to strict environmental and safety regulations. Proper decontamination and disposal methods must be followed to prevent environmental contamination or potential misuse of discarded materials.
Emergency response protocols are mandated for facilities handling muscimol. These include procedures for accidental spills, exposure incidents, and potential theft or loss of the compound. Staff must be trained in these protocols and regular safety drills conducted to ensure preparedness.
Transportation of muscimol is heavily regulated, with specific packaging, labeling, and documentation requirements. Carriers must be licensed to transport controlled substances, and shipments must be tracked and secured throughout the transportation process.
Occupational health monitoring may be required for personnel working with muscimol on a regular basis. This can include periodic health assessments and potential restrictions on certain activities or duties for individuals with specific health conditions.
Compliance with these safety regulations is typically monitored through regular inspections by relevant authorities. Facilities must maintain detailed records of muscimol handling, storage, and use, which are subject to audit. Violations of safety regulations can result in severe penalties, including fines, license revocation, and potential criminal charges.
As research into muscimol's potential therapeutic applications continues, regulatory frameworks may evolve to balance safety concerns with scientific progress. Ongoing dialogue between researchers, regulatory bodies, and safety experts is crucial to ensure that safety regulations remain effective and proportionate to the risks associated with muscimol handling and storage.
In many jurisdictions, muscimol is classified as a Schedule III controlled substance, which imposes strict requirements on its possession, use, and distribution. Laboratories and research facilities working with muscimol must obtain proper licensing and adhere to specific protocols for its acquisition, storage, and disposal.
Storage regulations typically mandate that muscimol be kept in secure, locked facilities with limited access. Temperature-controlled environments are often required to maintain the compound's stability and potency. Proper labeling and inventory management systems must be implemented to track the quantity and movement of muscimol within the facility.
Personal protective equipment (PPE) regulations are crucial for handling muscimol. Workers must wear appropriate gloves, protective eyewear, and respiratory protection when working with the compound. Specialized fume hoods or containment systems may be required to prevent inhalation of muscimol dust or vapors during handling procedures.
Disposal of muscimol and related waste materials is subject to strict environmental and safety regulations. Proper decontamination and disposal methods must be followed to prevent environmental contamination or potential misuse of discarded materials.
Emergency response protocols are mandated for facilities handling muscimol. These include procedures for accidental spills, exposure incidents, and potential theft or loss of the compound. Staff must be trained in these protocols and regular safety drills conducted to ensure preparedness.
Transportation of muscimol is heavily regulated, with specific packaging, labeling, and documentation requirements. Carriers must be licensed to transport controlled substances, and shipments must be tracked and secured throughout the transportation process.
Occupational health monitoring may be required for personnel working with muscimol on a regular basis. This can include periodic health assessments and potential restrictions on certain activities or duties for individuals with specific health conditions.
Compliance with these safety regulations is typically monitored through regular inspections by relevant authorities. Facilities must maintain detailed records of muscimol handling, storage, and use, which are subject to audit. Violations of safety regulations can result in severe penalties, including fines, license revocation, and potential criminal charges.
As research into muscimol's potential therapeutic applications continues, regulatory frameworks may evolve to balance safety concerns with scientific progress. Ongoing dialogue between researchers, regulatory bodies, and safety experts is crucial to ensure that safety regulations remain effective and proportionate to the risks associated with muscimol handling and storage.
Environmental Impact
The environmental impact of muscimol handling and storage is a critical consideration in the pharmaceutical and research industries. Muscimol, a potent GABA receptor agonist, requires careful management to minimize potential ecological risks. One of the primary concerns is the possibility of accidental release into the environment during handling or storage processes. Given its psychoactive properties, even small quantities of muscimol could have significant effects on local ecosystems, particularly aquatic environments where it may accumulate.
To mitigate these risks, stringent containment protocols are essential. These typically involve the use of sealed containers, specialized storage facilities, and robust waste management systems. However, the implementation of such measures often comes with increased energy consumption and resource utilization, which in turn contributes to a larger carbon footprint. This creates a complex balance between immediate environmental protection and long-term sustainability goals.
The production and purification of muscimol also present environmental challenges. The synthesis process may involve the use of organic solvents and other chemicals that, if not properly managed, could lead to air and water pollution. Additionally, the energy-intensive nature of some purification techniques adds to the overall environmental impact of muscimol production.
Disposal of muscimol-containing materials is another area of environmental concern. Improper disposal methods could lead to soil contamination or leaching into groundwater systems. To address this, specialized disposal protocols have been developed, including chemical neutralization and incineration. However, these processes themselves can have environmental implications, necessitating a careful evaluation of their overall ecological impact.
Long-term environmental monitoring is crucial in areas where muscimol is regularly handled or stored. This includes periodic testing of soil and water samples to detect any potential contamination. Such monitoring programs, while essential for environmental protection, require significant resources and can have their own environmental footprint in terms of energy use and material consumption.
The potential for bioaccumulation of muscimol in the food chain is an area that requires further research. While current data is limited, the possibility of muscimol entering ecosystems and affecting non-target organisms cannot be overlooked. This underscores the importance of preventive measures and ongoing environmental assessments in facilities handling this compound.
In conclusion, the environmental impact of muscimol handling and storage is multifaceted, requiring a comprehensive approach that balances immediate safety concerns with long-term ecological considerations. As research into muscimol's applications continues to expand, so too must our understanding and mitigation of its potential environmental effects.
To mitigate these risks, stringent containment protocols are essential. These typically involve the use of sealed containers, specialized storage facilities, and robust waste management systems. However, the implementation of such measures often comes with increased energy consumption and resource utilization, which in turn contributes to a larger carbon footprint. This creates a complex balance between immediate environmental protection and long-term sustainability goals.
The production and purification of muscimol also present environmental challenges. The synthesis process may involve the use of organic solvents and other chemicals that, if not properly managed, could lead to air and water pollution. Additionally, the energy-intensive nature of some purification techniques adds to the overall environmental impact of muscimol production.
Disposal of muscimol-containing materials is another area of environmental concern. Improper disposal methods could lead to soil contamination or leaching into groundwater systems. To address this, specialized disposal protocols have been developed, including chemical neutralization and incineration. However, these processes themselves can have environmental implications, necessitating a careful evaluation of their overall ecological impact.
Long-term environmental monitoring is crucial in areas where muscimol is regularly handled or stored. This includes periodic testing of soil and water samples to detect any potential contamination. Such monitoring programs, while essential for environmental protection, require significant resources and can have their own environmental footprint in terms of energy use and material consumption.
The potential for bioaccumulation of muscimol in the food chain is an area that requires further research. While current data is limited, the possibility of muscimol entering ecosystems and affecting non-target organisms cannot be overlooked. This underscores the importance of preventive measures and ongoing environmental assessments in facilities handling this compound.
In conclusion, the environmental impact of muscimol handling and storage is multifaceted, requiring a comprehensive approach that balances immediate safety concerns with long-term ecological considerations. As research into muscimol's applications continues to expand, so too must our understanding and mitigation of its potential environmental effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!