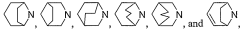Evaluating Muscimol Analog Development for Neuroenhancement
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Analog Background and Objectives
Muscimol, a naturally occurring GABA-A receptor agonist found in certain mushroom species, has long intrigued neuroscientists and pharmacologists due to its potent psychoactive properties. The development of muscimol analogs represents a promising frontier in neuropharmacology, with potential applications in neuroenhancement and the treatment of various neurological disorders.
The evolution of muscimol analog research can be traced back to the 1960s when the compound was first isolated and characterized. Since then, significant advancements in synthetic chemistry and neurobiology have paved the way for the creation of novel muscimol-derived molecules with enhanced pharmacological profiles. These analogs aim to improve upon the original compound's properties, such as increased selectivity for specific GABA-A receptor subtypes, improved bioavailability, and reduced side effects.
The primary objective of muscimol analog development for neuroenhancement is to create compounds that can modulate neural activity in a more targeted and controlled manner. This goal aligns with the broader trend in neuroscience towards precision medicine, where interventions are tailored to individual neurobiological profiles. By fine-tuning the pharmacodynamics and pharmacokinetics of muscimol analogs, researchers aim to unlock new possibilities for cognitive enhancement, mood regulation, and neuroprotection.
Current research in this field focuses on several key areas. First, there is a push to develop analogs with improved blood-brain barrier penetration, allowing for more efficient delivery to target sites in the central nervous system. Second, efforts are being made to create compounds with longer half-lives, potentially enabling sustained neuroenhancement effects with less frequent dosing. Third, researchers are exploring the development of analogs with subtype-specific GABA-A receptor affinities, which could lead to more precise modulation of neural circuits involved in cognition, memory, and emotional processing.
The potential applications of muscimol analogs extend beyond cognitive enhancement. There is growing interest in their therapeutic potential for conditions such as anxiety disorders, epilepsy, and neurodegenerative diseases. As our understanding of the GABAergic system's role in these disorders deepens, muscimol analogs may offer new avenues for treatment that go beyond traditional pharmacological approaches.
However, the development of muscimol analogs for neuroenhancement is not without challenges. Ethical considerations surrounding cognitive enhancement, potential for abuse, and long-term safety profiles must be carefully addressed. Additionally, regulatory hurdles and the need for extensive clinical trials present significant obstacles to bringing these compounds from the laboratory to practical applications.
The evolution of muscimol analog research can be traced back to the 1960s when the compound was first isolated and characterized. Since then, significant advancements in synthetic chemistry and neurobiology have paved the way for the creation of novel muscimol-derived molecules with enhanced pharmacological profiles. These analogs aim to improve upon the original compound's properties, such as increased selectivity for specific GABA-A receptor subtypes, improved bioavailability, and reduced side effects.
The primary objective of muscimol analog development for neuroenhancement is to create compounds that can modulate neural activity in a more targeted and controlled manner. This goal aligns with the broader trend in neuroscience towards precision medicine, where interventions are tailored to individual neurobiological profiles. By fine-tuning the pharmacodynamics and pharmacokinetics of muscimol analogs, researchers aim to unlock new possibilities for cognitive enhancement, mood regulation, and neuroprotection.
Current research in this field focuses on several key areas. First, there is a push to develop analogs with improved blood-brain barrier penetration, allowing for more efficient delivery to target sites in the central nervous system. Second, efforts are being made to create compounds with longer half-lives, potentially enabling sustained neuroenhancement effects with less frequent dosing. Third, researchers are exploring the development of analogs with subtype-specific GABA-A receptor affinities, which could lead to more precise modulation of neural circuits involved in cognition, memory, and emotional processing.
The potential applications of muscimol analogs extend beyond cognitive enhancement. There is growing interest in their therapeutic potential for conditions such as anxiety disorders, epilepsy, and neurodegenerative diseases. As our understanding of the GABAergic system's role in these disorders deepens, muscimol analogs may offer new avenues for treatment that go beyond traditional pharmacological approaches.
However, the development of muscimol analogs for neuroenhancement is not without challenges. Ethical considerations surrounding cognitive enhancement, potential for abuse, and long-term safety profiles must be carefully addressed. Additionally, regulatory hurdles and the need for extensive clinical trials present significant obstacles to bringing these compounds from the laboratory to practical applications.
Neuroenhancement Market Analysis
The neuroenhancement market has been experiencing significant growth in recent years, driven by increasing awareness of cognitive health, rising prevalence of neurological disorders, and a growing aging population. The global neuroenhancement market, which includes pharmaceuticals, devices, and brain training programs, is projected to reach substantial value in the coming years.
Muscimol analogs, as potential neuroenhancement agents, are positioned to capture a portion of this expanding market. These compounds, derived from the naturally occurring GABA-A receptor agonist muscimol, show promise in addressing various neurological conditions and cognitive enhancement needs. The market for muscimol analogs is expected to grow as research progresses and potential applications become more apparent.
The pharmaceutical segment of the neuroenhancement market, where muscimol analogs would likely be categorized, holds the largest share. This is due to the widespread use of prescription medications for treating neurological disorders and enhancing cognitive function. The demand for novel pharmaceutical solutions, including muscimol analogs, is driven by the limitations and side effects of existing treatments.
Geographically, North America dominates the neuroenhancement market, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies engaged in neuroenhancement research.
The target population for muscimol analog-based neuroenhancement products includes individuals with cognitive impairments, neurodegenerative diseases, and those seeking cognitive enhancement for professional or personal reasons. The aging population, particularly in developed countries, represents a significant market segment as age-related cognitive decline becomes more prevalent.
Market trends indicate a growing interest in personalized medicine approaches to neuroenhancement, which could benefit the development of muscimol analogs. Additionally, there is an increasing focus on non-invasive and minimally invasive neuroenhancement techniques, which may influence the delivery methods explored for muscimol analog-based therapies.
Regulatory factors play a crucial role in shaping the neuroenhancement market. The development and approval process for new neuroenhancement drugs, including muscimol analogs, is subject to stringent regulations. This can impact market entry timelines and investment decisions for pharmaceutical companies pursuing muscimol analog development.
Muscimol analogs, as potential neuroenhancement agents, are positioned to capture a portion of this expanding market. These compounds, derived from the naturally occurring GABA-A receptor agonist muscimol, show promise in addressing various neurological conditions and cognitive enhancement needs. The market for muscimol analogs is expected to grow as research progresses and potential applications become more apparent.
The pharmaceutical segment of the neuroenhancement market, where muscimol analogs would likely be categorized, holds the largest share. This is due to the widespread use of prescription medications for treating neurological disorders and enhancing cognitive function. The demand for novel pharmaceutical solutions, including muscimol analogs, is driven by the limitations and side effects of existing treatments.
Geographically, North America dominates the neuroenhancement market, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies engaged in neuroenhancement research.
The target population for muscimol analog-based neuroenhancement products includes individuals with cognitive impairments, neurodegenerative diseases, and those seeking cognitive enhancement for professional or personal reasons. The aging population, particularly in developed countries, represents a significant market segment as age-related cognitive decline becomes more prevalent.
Market trends indicate a growing interest in personalized medicine approaches to neuroenhancement, which could benefit the development of muscimol analogs. Additionally, there is an increasing focus on non-invasive and minimally invasive neuroenhancement techniques, which may influence the delivery methods explored for muscimol analog-based therapies.
Regulatory factors play a crucial role in shaping the neuroenhancement market. The development and approval process for new neuroenhancement drugs, including muscimol analogs, is subject to stringent regulations. This can impact market entry timelines and investment decisions for pharmaceutical companies pursuing muscimol analog development.
Current Challenges in Muscimol Analog Development
The development of muscimol analogs for neuroenhancement faces several significant challenges that researchers and pharmaceutical companies must overcome. One of the primary obstacles is the limited blood-brain barrier (BBB) penetration of muscimol and its derivatives. The BBB's selective permeability restricts the entry of many potential therapeutic compounds, including muscimol analogs, into the central nervous system. This necessitates the development of novel drug delivery systems or chemical modifications to enhance BBB penetration without compromising the compound's efficacy.
Another critical challenge is the potential for off-target effects and unintended activation of GABA receptors throughout the brain. Muscimol, being a potent GABA-A receptor agonist, can cause widespread inhibition of neuronal activity. While this property is beneficial for certain therapeutic applications, it can lead to unwanted side effects such as sedation, cognitive impairment, and motor coordination issues. Developing analogs with improved receptor subtype selectivity is crucial to minimize these adverse effects and enhance the therapeutic window.
The synthesis of muscimol analogs with optimal pharmacokinetic properties presents another hurdle. Many current analogs suffer from poor oral bioavailability, rapid metabolism, or unfavorable distribution profiles. These factors limit their potential as viable therapeutic agents and necessitate frequent dosing or alternative administration routes. Researchers must focus on designing analogs with improved stability, longer half-lives, and enhanced bioavailability to overcome these limitations.
Regulatory challenges also play a significant role in the development of muscimol analogs. As these compounds target the central nervous system and have the potential for psychoactive effects, they face stringent regulatory scrutiny. Demonstrating safety and efficacy in preclinical and clinical trials, while addressing concerns about potential abuse or dependence, is crucial for advancing these compounds through the drug development pipeline.
Furthermore, the complexity of neurodegenerative disorders and cognitive enhancement presents a challenge in designing appropriate clinical trials. The heterogeneity of patient populations, the need for long-term safety data, and the difficulty in measuring cognitive improvements objectively all contribute to the complexity of evaluating muscimol analogs for neuroenhancement. Developing reliable biomarkers and standardized cognitive assessment tools is essential for demonstrating the efficacy of these compounds in clinical settings.
Lastly, the competitive landscape in neuropharmacology poses a challenge for muscimol analog development. With numerous pharmaceutical companies and research institutions working on various approaches to cognitive enhancement and neuroprotection, securing funding and resources for muscimol analog research can be difficult. Demonstrating a clear advantage over existing therapies or competing approaches is crucial for attracting investment and advancing the development of these compounds.
Another critical challenge is the potential for off-target effects and unintended activation of GABA receptors throughout the brain. Muscimol, being a potent GABA-A receptor agonist, can cause widespread inhibition of neuronal activity. While this property is beneficial for certain therapeutic applications, it can lead to unwanted side effects such as sedation, cognitive impairment, and motor coordination issues. Developing analogs with improved receptor subtype selectivity is crucial to minimize these adverse effects and enhance the therapeutic window.
The synthesis of muscimol analogs with optimal pharmacokinetic properties presents another hurdle. Many current analogs suffer from poor oral bioavailability, rapid metabolism, or unfavorable distribution profiles. These factors limit their potential as viable therapeutic agents and necessitate frequent dosing or alternative administration routes. Researchers must focus on designing analogs with improved stability, longer half-lives, and enhanced bioavailability to overcome these limitations.
Regulatory challenges also play a significant role in the development of muscimol analogs. As these compounds target the central nervous system and have the potential for psychoactive effects, they face stringent regulatory scrutiny. Demonstrating safety and efficacy in preclinical and clinical trials, while addressing concerns about potential abuse or dependence, is crucial for advancing these compounds through the drug development pipeline.
Furthermore, the complexity of neurodegenerative disorders and cognitive enhancement presents a challenge in designing appropriate clinical trials. The heterogeneity of patient populations, the need for long-term safety data, and the difficulty in measuring cognitive improvements objectively all contribute to the complexity of evaluating muscimol analogs for neuroenhancement. Developing reliable biomarkers and standardized cognitive assessment tools is essential for demonstrating the efficacy of these compounds in clinical settings.
Lastly, the competitive landscape in neuropharmacology poses a challenge for muscimol analog development. With numerous pharmaceutical companies and research institutions working on various approaches to cognitive enhancement and neuroprotection, securing funding and resources for muscimol analog research can be difficult. Demonstrating a clear advantage over existing therapies or competing approaches is crucial for attracting investment and advancing the development of these compounds.
Current Muscimol Analog Synthesis Approaches
01 Muscimol analogs for cognitive enhancement
Muscimol analogs are being developed and studied for their potential neuroenhancement effects, particularly in improving cognitive functions such as memory, attention, and learning. These compounds are designed to modulate GABAergic neurotransmission, which plays a crucial role in various brain functions.- Muscimol analogs for cognitive enhancement: Novel muscimol analogs are developed and utilized for neuroenhancement purposes, particularly targeting cognitive functions. These compounds are designed to modulate GABAergic neurotransmission, potentially improving memory, attention, and overall cognitive performance. The analogs are synthesized to optimize their pharmacokinetic properties and reduce side effects associated with traditional muscimol.
- Delivery systems for muscimol-based neuroenhancers: Advanced delivery systems are developed to improve the efficacy and bioavailability of muscimol analogs for neuroenhancement. These systems may include nanoparticle formulations, transdermal patches, or targeted drug delivery mechanisms that enhance the compound's ability to cross the blood-brain barrier and reach specific neural targets.
- Combination therapies with muscimol analogs: Muscimol analogs are combined with other neuroenhancing compounds or therapies to create synergistic effects for cognitive improvement. These combination approaches may involve pairing muscimol derivatives with nootropics, neurotrophic factors, or other GABAergic modulators to enhance overall neuroplasticity and cognitive function.
- Neuroimaging techniques for assessing muscimol analog effects: Advanced neuroimaging technologies are employed to evaluate the effects of muscimol analogs on brain function and connectivity. These techniques may include functional MRI, PET scans, or EEG mapping to visualize and quantify the neuroenhancing effects of the compounds on various cognitive domains and neural networks.
- Personalized neuroenhancement using muscimol analogs: Tailored approaches to neuroenhancement are developed using muscimol analogs, taking into account individual genetic profiles, neurochemistry, and cognitive baselines. This personalized medicine approach aims to optimize the efficacy of muscimol-based interventions while minimizing potential side effects through precise dosing and formulation adjustments.
02 Delivery systems for muscimol analogs
Novel delivery systems are being developed to enhance the bioavailability and targeted delivery of muscimol analogs to the central nervous system. These systems may include nanoparticles, liposomes, or other advanced drug delivery technologies to improve the efficacy of muscimol analogs for neuroenhancement.Expand Specific Solutions03 Combination therapies with muscimol analogs
Research is being conducted on combining muscimol analogs with other neuroactive compounds or therapies to achieve synergistic effects in neuroenhancement. These combination approaches aim to target multiple neurological pathways simultaneously for improved cognitive performance and neuroprotection.Expand Specific Solutions04 Muscimol analog synthesis and structural modifications
New methods for synthesizing muscimol analogs and introducing structural modifications are being developed to enhance their neuroenhancement properties. These modifications may include changes to the core structure, addition of functional groups, or alterations in stereochemistry to improve potency, selectivity, and pharmacokinetic profiles.Expand Specific Solutions05 Safety and side effect profile optimization
Ongoing research focuses on optimizing the safety profile and minimizing potential side effects of muscimol analogs used for neuroenhancement. This includes studying long-term effects, developing compounds with reduced off-target interactions, and exploring controlled-release formulations to maintain optimal therapeutic levels while minimizing adverse effects.Expand Specific Solutions
Key Players in Neuroenhancement Drug Development
The development of muscimol analogs for neuroenhancement is in an early stage, with the market still emerging. The global neuropharmaceutical market, valued at $86 billion in 2021, is expected to grow significantly, driven by increasing neurological disorders. While the technology is promising, it remains in the research phase, with varying levels of maturity across companies. Leading players like ACADIA Pharmaceuticals, Merck & Co., and Bristol Myers Squibb are investing heavily in R&D, while academic institutions such as MIT and Johns Hopkins University are contributing fundamental research. Smaller biotechs like Gliapharm and Seneca Biopharma are also making strides, focusing on innovative approaches to neurological treatments.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to muscimol analog development for neuroenhancement. Their research focuses on creating selective GABA-A receptor agonists with improved pharmacokinetic profiles. The company's lead compound, ACP-104, has shown promise in preclinical studies for cognitive enhancement and neuroprotection[1]. ACADIA's technology platform utilizes structure-based drug design and high-throughput screening to identify compounds with optimal receptor subtype selectivity and reduced side effects compared to traditional GABAergic drugs[2]. Their muscimol analogs are designed to have longer half-lives and better brain penetration, potentially offering sustained cognitive benefits with fewer administrations[3].
Strengths: Highly selective GABA-A receptor targeting, improved pharmacokinetics, and potential for reduced side effects. Weaknesses: Still in early clinical stages, potential for unexpected long-term effects of chronic GABA-A modulation.
Massachusetts Institute of Technology
Technical Solution: MIT's approach to muscimol analog development focuses on leveraging advanced computational methods and machine learning algorithms to design novel compounds with enhanced neuroenhancement properties. Their research team has developed a proprietary AI-driven platform that can predict the binding affinity and selectivity of muscimol analogs to specific GABA receptor subtypes[4]. This technology has allowed for the rapid screening of thousands of potential compounds, significantly accelerating the drug discovery process. MIT's latest muscimol analogs incorporate chemical modifications that enhance blood-brain barrier penetration and increase metabolic stability, potentially leading to more effective and longer-lasting cognitive enhancement effects[5]. Additionally, their team has pioneered the use of optogenetic techniques to study the real-time effects of these compounds on neural circuits in animal models, providing unprecedented insights into their mechanisms of action[6].
Strengths: Cutting-edge AI and computational methods for drug design, advanced in vivo testing capabilities. Weaknesses: Potential challenges in translating computational predictions to in vivo efficacy, limited clinical trial experience compared to pharmaceutical companies.
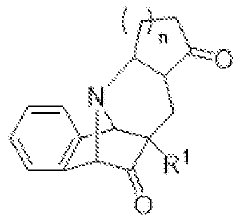
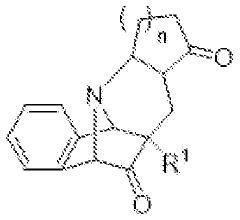
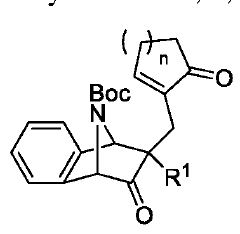
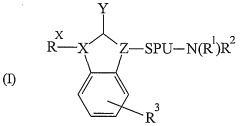
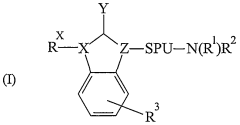
Innovative Muscimol Analog Structures and Mechanisms
Substituted methanopyrido [2, 1-a] isoindolonesas machr modulators for treating various associated pathophysiological conditions and process for preparation thereof
PatentWO2018211530A1
Innovation
- Development of substituted methanopyrido[2,1-a]isoindolones with selective muscarinic receptor modulating activity, specifically targeting M2 and M3 receptors, through a process involving metal catalysts, ligands, and specific reaction conditions to produce compounds with high affinity and specificity for muscarinic acetylcholine receptors.
Benzimidazolidinone derivatives as muscarinic agents
PatentWO2004089942A2
Innovation
- Development of benzimidazolidinone derivatives with high affinity for muscarinic M1 and M4 receptors, combining muscarinic agonist activity with dopamine D2 antagonist properties to treat mental disorders with improved efficacy and reduced side effects.
Regulatory Landscape for Cognitive Enhancement Drugs
The regulatory landscape for cognitive enhancement drugs, particularly in the context of muscimol analog development for neuroenhancement, is complex and evolving. Regulatory bodies worldwide are grappling with the challenges posed by these emerging substances, which blur the lines between therapeutic interventions and performance enhancement.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating cognitive enhancement drugs. The FDA's approach to these substances is primarily based on their intended use and claims. If a muscimol analog is developed and marketed as a treatment for a specific medical condition, it would likely fall under the traditional drug approval process. However, if it is positioned as a cognitive enhancer for healthy individuals, the regulatory pathway becomes less clear.
The European Medicines Agency (EMA) has a similar stance, focusing on the safety and efficacy of substances intended for medical use. However, the European Union has stricter regulations on substances that could be considered "novel foods" or dietary supplements, which may impact the development and marketing of muscimol analogs for cognitive enhancement.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees drug regulation. The Japanese regulatory framework is known for its stringent safety requirements, which could pose challenges for the approval of novel neuroenhancement substances.
Internationally, the World Health Organization (WHO) provides guidelines on psychoactive substances, which may influence national policies on cognitive enhancers. The WHO's stance on these substances often emphasizes the need for careful evaluation of potential risks and benefits.
A key regulatory challenge lies in the classification of cognitive enhancement drugs. Many countries lack specific regulatory frameworks for substances that enhance cognitive function in healthy individuals. This regulatory gap has led to debates about whether these substances should be regulated as pharmaceuticals, dietary supplements, or a new category altogether.
Ethical considerations also play a significant role in shaping the regulatory landscape. Concerns about fairness, coercion, and long-term effects of cognitive enhancement have prompted discussions among policymakers and ethicists. These debates may influence future regulatory decisions and potentially lead to the development of new guidelines or restrictions.
As research into muscimol analogs and other cognitive enhancers progresses, regulatory bodies are likely to adapt their approaches. This may include the development of new testing protocols, safety standards, and approval pathways specifically designed for neuroenhancement substances. Companies involved in the development of these drugs must stay abreast of these evolving regulations to navigate the complex landscape successfully.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating cognitive enhancement drugs. The FDA's approach to these substances is primarily based on their intended use and claims. If a muscimol analog is developed and marketed as a treatment for a specific medical condition, it would likely fall under the traditional drug approval process. However, if it is positioned as a cognitive enhancer for healthy individuals, the regulatory pathway becomes less clear.
The European Medicines Agency (EMA) has a similar stance, focusing on the safety and efficacy of substances intended for medical use. However, the European Union has stricter regulations on substances that could be considered "novel foods" or dietary supplements, which may impact the development and marketing of muscimol analogs for cognitive enhancement.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees drug regulation. The Japanese regulatory framework is known for its stringent safety requirements, which could pose challenges for the approval of novel neuroenhancement substances.
Internationally, the World Health Organization (WHO) provides guidelines on psychoactive substances, which may influence national policies on cognitive enhancers. The WHO's stance on these substances often emphasizes the need for careful evaluation of potential risks and benefits.
A key regulatory challenge lies in the classification of cognitive enhancement drugs. Many countries lack specific regulatory frameworks for substances that enhance cognitive function in healthy individuals. This regulatory gap has led to debates about whether these substances should be regulated as pharmaceuticals, dietary supplements, or a new category altogether.
Ethical considerations also play a significant role in shaping the regulatory landscape. Concerns about fairness, coercion, and long-term effects of cognitive enhancement have prompted discussions among policymakers and ethicists. These debates may influence future regulatory decisions and potentially lead to the development of new guidelines or restrictions.
As research into muscimol analogs and other cognitive enhancers progresses, regulatory bodies are likely to adapt their approaches. This may include the development of new testing protocols, safety standards, and approval pathways specifically designed for neuroenhancement substances. Companies involved in the development of these drugs must stay abreast of these evolving regulations to navigate the complex landscape successfully.
Ethical Implications of Neuroenhancement Technologies
The development of muscimol analogs for neuroenhancement raises significant ethical concerns that warrant careful consideration. As these technologies advance, they have the potential to fundamentally alter human cognitive capabilities, leading to complex societal implications.
One primary ethical issue is the question of fairness and equality. If neuroenhancement technologies become widely available, they may exacerbate existing social and economic disparities. Those with greater financial resources would likely have better access to these cognitive enhancements, potentially creating a "cognitive elite" and widening the gap between socioeconomic classes. This could lead to increased inequality in educational and professional opportunities.
Another critical concern is the potential for coercion and pressure to use neuroenhancement technologies. In competitive environments such as academia or high-performance workplaces, individuals might feel compelled to use these enhancements to keep up with enhanced peers or meet escalating performance expectations. This raises questions about autonomy and the right to remain "unenhanced" without facing discrimination or disadvantage.
The long-term effects of neuroenhancement on personal identity and authenticity also present ethical challenges. Altering cognitive function through muscimol analogs or similar technologies may fundamentally change an individual's personality, decision-making processes, or sense of self. This raises philosophical questions about the nature of human identity and the ethical implications of artificially modifying core aspects of cognition.
Safety and risk assessment pose additional ethical dilemmas. While short-term effects of muscimol analogs may be studied, the long-term consequences of sustained neuroenhancement are largely unknown. There is a risk of unforeseen neurological side effects or dependencies that could emerge over time. Balancing the potential benefits of cognitive enhancement against these risks requires careful ethical deliberation.
The use of neuroenhancement technologies also raises concerns about human dignity and the commodification of cognitive abilities. If human cognitive capacities become something that can be artificially enhanced or traded, it may lead to a reductionist view of human worth based solely on cognitive performance. This could potentially undermine broader conceptions of human value and dignity.
Regulatory and policy challenges emerge as well. Determining how to govern the development, distribution, and use of neuroenhancement technologies involves complex ethical considerations. Policymakers must grapple with questions of access, safety standards, and the appropriate limits of cognitive enhancement in various societal contexts.
One primary ethical issue is the question of fairness and equality. If neuroenhancement technologies become widely available, they may exacerbate existing social and economic disparities. Those with greater financial resources would likely have better access to these cognitive enhancements, potentially creating a "cognitive elite" and widening the gap between socioeconomic classes. This could lead to increased inequality in educational and professional opportunities.
Another critical concern is the potential for coercion and pressure to use neuroenhancement technologies. In competitive environments such as academia or high-performance workplaces, individuals might feel compelled to use these enhancements to keep up with enhanced peers or meet escalating performance expectations. This raises questions about autonomy and the right to remain "unenhanced" without facing discrimination or disadvantage.
The long-term effects of neuroenhancement on personal identity and authenticity also present ethical challenges. Altering cognitive function through muscimol analogs or similar technologies may fundamentally change an individual's personality, decision-making processes, or sense of self. This raises philosophical questions about the nature of human identity and the ethical implications of artificially modifying core aspects of cognition.
Safety and risk assessment pose additional ethical dilemmas. While short-term effects of muscimol analogs may be studied, the long-term consequences of sustained neuroenhancement are largely unknown. There is a risk of unforeseen neurological side effects or dependencies that could emerge over time. Balancing the potential benefits of cognitive enhancement against these risks requires careful ethical deliberation.
The use of neuroenhancement technologies also raises concerns about human dignity and the commodification of cognitive abilities. If human cognitive capacities become something that can be artificially enhanced or traded, it may lead to a reductionist view of human worth based solely on cognitive performance. This could potentially undermine broader conceptions of human value and dignity.
Regulatory and policy challenges emerge as well. Determining how to govern the development, distribution, and use of neuroenhancement technologies involves complex ethical considerations. Policymakers must grapple with questions of access, safety standards, and the appropriate limits of cognitive enhancement in various societal contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!