GABAergic Maladjustments and Muscimol Corrective Strategies
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GABAergic System Overview and Research Objectives
The GABAergic system plays a crucial role in regulating neural activity throughout the central nervous system. As the primary inhibitory neurotransmitter, gamma-aminobutyric acid (GABA) is essential for maintaining the delicate balance between excitation and inhibition in the brain. This balance is fundamental to normal cognitive function, emotional regulation, and overall neurological health.
Over the past decades, research has revealed the complexity of the GABAergic system, including its various receptor subtypes, synthesis pathways, and regulatory mechanisms. The system's widespread influence extends to numerous neurological and psychiatric conditions, making it a prime target for therapeutic interventions.
Recent advancements in neuroscience have highlighted the importance of GABAergic maladjustments in various disorders, including epilepsy, anxiety, depression, and neurodegenerative diseases. These maladjustments can manifest as alterations in GABA synthesis, release, reuptake, or receptor function, leading to an imbalance in neural circuits.
The focus of this research is to explore the intricate mechanisms underlying GABAergic maladjustments and to investigate the potential of muscimol-based corrective strategies. Muscimol, a potent GABA receptor agonist derived from the Amanita muscaria mushroom, has shown promise in modulating GABAergic activity with high specificity.
Our primary objectives are to elucidate the molecular and cellular processes involved in GABAergic dysregulation, identify key biomarkers for early detection of maladjustments, and develop targeted muscimol-based interventions. We aim to leverage cutting-edge techniques in neuropharmacology, electrophysiology, and neuroimaging to achieve these goals.
Furthermore, this research seeks to explore the potential of combining muscimol with other GABAergic modulators or novel drug delivery systems to enhance therapeutic efficacy while minimizing side effects. The long-term vision is to translate these findings into clinically viable treatments for a range of neurological and psychiatric disorders characterized by GABAergic imbalances.
By advancing our understanding of GABAergic maladjustments and refining muscimol-based corrective strategies, we anticipate making significant contributions to the field of neuroscience and paving the way for innovative therapeutic approaches. This research has the potential to improve the quality of life for millions of individuals affected by disorders linked to GABAergic dysfunction.
Over the past decades, research has revealed the complexity of the GABAergic system, including its various receptor subtypes, synthesis pathways, and regulatory mechanisms. The system's widespread influence extends to numerous neurological and psychiatric conditions, making it a prime target for therapeutic interventions.
Recent advancements in neuroscience have highlighted the importance of GABAergic maladjustments in various disorders, including epilepsy, anxiety, depression, and neurodegenerative diseases. These maladjustments can manifest as alterations in GABA synthesis, release, reuptake, or receptor function, leading to an imbalance in neural circuits.
The focus of this research is to explore the intricate mechanisms underlying GABAergic maladjustments and to investigate the potential of muscimol-based corrective strategies. Muscimol, a potent GABA receptor agonist derived from the Amanita muscaria mushroom, has shown promise in modulating GABAergic activity with high specificity.
Our primary objectives are to elucidate the molecular and cellular processes involved in GABAergic dysregulation, identify key biomarkers for early detection of maladjustments, and develop targeted muscimol-based interventions. We aim to leverage cutting-edge techniques in neuropharmacology, electrophysiology, and neuroimaging to achieve these goals.
Furthermore, this research seeks to explore the potential of combining muscimol with other GABAergic modulators or novel drug delivery systems to enhance therapeutic efficacy while minimizing side effects. The long-term vision is to translate these findings into clinically viable treatments for a range of neurological and psychiatric disorders characterized by GABAergic imbalances.
By advancing our understanding of GABAergic maladjustments and refining muscimol-based corrective strategies, we anticipate making significant contributions to the field of neuroscience and paving the way for innovative therapeutic approaches. This research has the potential to improve the quality of life for millions of individuals affected by disorders linked to GABAergic dysfunction.
Clinical Demand for GABAergic Dysfunction Treatments
The clinical demand for GABAergic dysfunction treatments has been steadily increasing due to the growing prevalence of neurological and psychiatric disorders associated with GABA system imbalances. GABAergic dysfunctions are implicated in a wide range of conditions, including anxiety disorders, epilepsy, insomnia, and certain neurodegenerative diseases. This has led to a significant market need for effective therapeutic interventions targeting the GABAergic system.
Anxiety disorders, which affect approximately 284 million people worldwide, represent a substantial portion of the clinical demand. These disorders are often linked to reduced GABA activity, creating a large patient population that could benefit from GABAergic treatments. Similarly, epilepsy, affecting over 50 million people globally, is frequently associated with GABAergic imbalances, further driving the need for targeted therapies.
The market for sleep disorders, particularly insomnia, also contributes significantly to the demand for GABAergic treatments. With an estimated 10-30% of the global population experiencing chronic insomnia, there is a substantial need for effective sleep-promoting agents that modulate GABAergic activity.
Neurodegenerative diseases, such as Alzheimer's and Parkinson's, have shown links to GABAergic dysfunction. As the global population ages and the incidence of these diseases increases, the demand for treatments addressing GABAergic maladjustments is expected to grow substantially.
The pharmaceutical industry has recognized this clinical need, leading to increased research and development efforts focused on GABAergic modulators. Current market offerings include benzodiazepines and non-benzodiazepine hypnotics, which act on GABA receptors. However, these medications often come with side effects and potential for dependence, creating a demand for safer and more targeted GABAergic treatments.
Emerging research on muscimol, a potent GABA agonist, has sparked interest in its potential as a therapeutic agent. The demand for muscimol-based treatments is driven by its ability to selectively activate GABA receptors, potentially offering more precise control over GABAergic activity with fewer side effects compared to existing medications.
The market trend is shifting towards personalized medicine approaches in GABAergic treatments. This is fueled by advancements in genetic testing and biomarker identification, allowing for more tailored therapeutic strategies based on individual patient profiles of GABAergic dysfunction.
In conclusion, the clinical demand for GABAergic dysfunction treatments spans multiple therapeutic areas and continues to grow. The market seeks innovative solutions that can effectively modulate GABAergic activity while minimizing side effects and improving patient outcomes across various neurological and psychiatric disorders.
Anxiety disorders, which affect approximately 284 million people worldwide, represent a substantial portion of the clinical demand. These disorders are often linked to reduced GABA activity, creating a large patient population that could benefit from GABAergic treatments. Similarly, epilepsy, affecting over 50 million people globally, is frequently associated with GABAergic imbalances, further driving the need for targeted therapies.
The market for sleep disorders, particularly insomnia, also contributes significantly to the demand for GABAergic treatments. With an estimated 10-30% of the global population experiencing chronic insomnia, there is a substantial need for effective sleep-promoting agents that modulate GABAergic activity.
Neurodegenerative diseases, such as Alzheimer's and Parkinson's, have shown links to GABAergic dysfunction. As the global population ages and the incidence of these diseases increases, the demand for treatments addressing GABAergic maladjustments is expected to grow substantially.
The pharmaceutical industry has recognized this clinical need, leading to increased research and development efforts focused on GABAergic modulators. Current market offerings include benzodiazepines and non-benzodiazepine hypnotics, which act on GABA receptors. However, these medications often come with side effects and potential for dependence, creating a demand for safer and more targeted GABAergic treatments.
Emerging research on muscimol, a potent GABA agonist, has sparked interest in its potential as a therapeutic agent. The demand for muscimol-based treatments is driven by its ability to selectively activate GABA receptors, potentially offering more precise control over GABAergic activity with fewer side effects compared to existing medications.
The market trend is shifting towards personalized medicine approaches in GABAergic treatments. This is fueled by advancements in genetic testing and biomarker identification, allowing for more tailored therapeutic strategies based on individual patient profiles of GABAergic dysfunction.
In conclusion, the clinical demand for GABAergic dysfunction treatments spans multiple therapeutic areas and continues to grow. The market seeks innovative solutions that can effectively modulate GABAergic activity while minimizing side effects and improving patient outcomes across various neurological and psychiatric disorders.
Current Challenges in GABAergic System Modulation
The modulation of the GABAergic system presents several significant challenges in current research and clinical applications. One of the primary obstacles is the complexity of the GABAergic system itself, which involves multiple receptor subtypes, various neurotransmitter transporters, and intricate signaling pathways. This complexity makes it difficult to target specific components without affecting others, potentially leading to unintended side effects.
Another major challenge lies in achieving precise spatial and temporal control over GABAergic modulation. The GABAergic system plays diverse roles in different brain regions and at different developmental stages. Consequently, broad-spectrum interventions may not be suitable for addressing specific neurological or psychiatric conditions. Researchers are grappling with the need to develop more targeted approaches that can selectively modulate GABAergic activity in specific neural circuits or brain areas.
The blood-brain barrier (BBB) poses a significant hurdle in delivering GABAergic modulators to the central nervous system. Many potential therapeutic compounds struggle to cross this barrier effectively, limiting their efficacy in treating neurological disorders. Developing drug delivery systems that can overcome the BBB while maintaining the stability and activity of GABAergic modulators remains a critical challenge.
Furthermore, the dynamic nature of GABAergic signaling presents difficulties in maintaining long-term therapeutic effects. The brain's plasticity and adaptive mechanisms can lead to tolerance or compensatory changes in response to chronic GABAergic modulation. This phenomenon necessitates the development of strategies that can provide sustained benefits without triggering maladaptive responses.
Interindividual variability in GABAergic system function and response to modulators is another significant challenge. Genetic differences, environmental factors, and pre-existing conditions can all influence how individuals respond to GABAergic interventions. This variability complicates the development of standardized treatment protocols and highlights the need for personalized approaches in GABAergic modulation strategies.
The potential for off-target effects and interactions with other neurotransmitter systems also presents a considerable challenge. GABAergic neurons are interconnected with various other neural networks, and modulating GABA signaling can have ripple effects throughout the brain. Researchers must carefully consider these potential interactions to minimize adverse effects and optimize therapeutic outcomes.
Lastly, the translation of preclinical findings to clinical applications remains a significant hurdle. Animal models, while valuable, may not fully recapitulate the complexity of human GABAergic systems or neurological disorders. Bridging this gap and developing effective human trials for GABAergic modulators, particularly in the context of muscimol-based strategies, continues to be a pressing challenge in the field.
Another major challenge lies in achieving precise spatial and temporal control over GABAergic modulation. The GABAergic system plays diverse roles in different brain regions and at different developmental stages. Consequently, broad-spectrum interventions may not be suitable for addressing specific neurological or psychiatric conditions. Researchers are grappling with the need to develop more targeted approaches that can selectively modulate GABAergic activity in specific neural circuits or brain areas.
The blood-brain barrier (BBB) poses a significant hurdle in delivering GABAergic modulators to the central nervous system. Many potential therapeutic compounds struggle to cross this barrier effectively, limiting their efficacy in treating neurological disorders. Developing drug delivery systems that can overcome the BBB while maintaining the stability and activity of GABAergic modulators remains a critical challenge.
Furthermore, the dynamic nature of GABAergic signaling presents difficulties in maintaining long-term therapeutic effects. The brain's plasticity and adaptive mechanisms can lead to tolerance or compensatory changes in response to chronic GABAergic modulation. This phenomenon necessitates the development of strategies that can provide sustained benefits without triggering maladaptive responses.
Interindividual variability in GABAergic system function and response to modulators is another significant challenge. Genetic differences, environmental factors, and pre-existing conditions can all influence how individuals respond to GABAergic interventions. This variability complicates the development of standardized treatment protocols and highlights the need for personalized approaches in GABAergic modulation strategies.
The potential for off-target effects and interactions with other neurotransmitter systems also presents a considerable challenge. GABAergic neurons are interconnected with various other neural networks, and modulating GABA signaling can have ripple effects throughout the brain. Researchers must carefully consider these potential interactions to minimize adverse effects and optimize therapeutic outcomes.
Lastly, the translation of preclinical findings to clinical applications remains a significant hurdle. Animal models, while valuable, may not fully recapitulate the complexity of human GABAergic systems or neurological disorders. Bridging this gap and developing effective human trials for GABAergic modulators, particularly in the context of muscimol-based strategies, continues to be a pressing challenge in the field.
Existing Approaches to GABAergic Maladjustment Correction
01 GABAergic system modulation for neurological disorders
Research focuses on modulating the GABAergic system to address various neurological disorders. This approach involves targeting GABA receptors, transporters, or enzymes to correct imbalances in inhibitory neurotransmission. Such modulation can potentially treat conditions like epilepsy, anxiety, and sleep disorders.- GABAergic system modulation for neurological disorders: Research focuses on modulating the GABAergic system to address various neurological disorders. This approach involves targeting GABA receptors, transporters, or enzymes to correct imbalances in inhibitory neurotransmission. Such modulation can potentially treat conditions like epilepsy, anxiety, and sleep disorders.
- Genetic factors influencing GABAergic system maladjustments: Studies investigate genetic variations that may contribute to GABAergic system dysfunction. Identifying specific genes and their mutations helps in understanding the hereditary aspects of GABAergic maladjustments and could lead to targeted gene therapies or personalized treatments for affected individuals.
- Pharmacological interventions for GABAergic system regulation: Development of pharmaceutical compounds that can regulate GABAergic neurotransmission is a key area of research. These interventions aim to enhance or inhibit GABA activity, depending on the specific maladjustment, and may include GABA receptor agonists, antagonists, or drugs that affect GABA metabolism.
- Diagnostic methods for GABAergic system maladjustments: Advanced diagnostic techniques are being developed to identify and characterize GABAergic system maladjustments. These may include neuroimaging methods, biomarker analysis, or electrophysiological measurements to assess GABA levels, receptor function, or synaptic activity in the brain.
- Non-pharmacological approaches to GABAergic system regulation: Research explores non-drug interventions that can influence the GABAergic system. These approaches may include dietary modifications, lifestyle changes, or neuromodulation techniques such as transcranial magnetic stimulation or neurofeedback, aimed at normalizing GABAergic function without pharmaceutical side effects.
02 Genetic factors influencing GABAergic system maladjustments
Studies investigate genetic variations that may contribute to GABAergic system dysfunction. Identifying specific genes and their mutations can provide insights into the underlying causes of GABAergic maladjustments and potentially lead to targeted therapies or diagnostic tools.Expand Specific Solutions03 Pharmacological interventions for GABAergic system regulation
Development of pharmaceutical compounds that can regulate GABAergic neurotransmission. These may include GABA receptor agonists, antagonists, or modulators, as well as drugs that affect GABA synthesis, release, or reuptake. Such interventions aim to correct imbalances in the GABAergic system.Expand Specific Solutions04 Biomarkers for GABAergic system dysfunction
Identification and validation of biomarkers that indicate GABAergic system maladjustments. These biomarkers could include neuroimaging patterns, biochemical indicators, or electrophysiological signatures. They may aid in early diagnosis, treatment monitoring, and personalized medicine approaches for GABAergic disorders.Expand Specific Solutions05 Novel therapeutic approaches for GABAergic system restoration
Exploration of innovative therapeutic strategies to restore GABAergic system function. These may include gene therapy, stem cell treatments, optogenetic interventions, or neuromodulation techniques. Such approaches aim to correct underlying causes of GABAergic maladjustments rather than merely treating symptoms.Expand Specific Solutions
Key Institutions and Researchers in GABAergic Studies
The research on GABAergic maladjustments and muscimol corrective strategies is in a developing stage, with the market showing potential for growth. The field is attracting attention from both academic institutions and pharmaceutical companies, indicating a competitive landscape. Key players include The Regents of the University of California, F. Hoffmann-La Roche Ltd., and SAGE Therapeutics, Inc., each contributing to the advancement of GABAergic research. The technology's maturity is progressing, with companies like Janssen Pharmaceutica NV and Novartis AG investing in related studies. As the understanding of GABAergic systems deepens, the market is expected to expand, driven by the potential applications in treating neurological and psychiatric disorders.
The Regents of the University of California
Technical Solution: The University of California system has been conducting extensive research on GABAergic maladjustments and potential therapeutic strategies. Researchers at UC San Francisco have identified specific GABAergic interneuron subtypes involved in various neurological disorders, providing new targets for therapeutic intervention[11]. At UC Berkeley, scientists are exploring the use of optogenetics to selectively modulate GABAergic circuits, offering potential for highly targeted treatments[12]. UC Davis researchers are investigating the role of GABAergic dysfunction in neurodevelopmental disorders such as autism and schizophrenia, and are developing novel compounds that can modulate specific GABAA receptor subtypes[13]. The UC system's work also includes studying the potential of muscimol and its derivatives as neuroprotective agents and cognitive enhancers.
Strengths: Cutting-edge research facilities, multidisciplinary approach, strong collaboration with industry. Weaknesses: Potential challenges in translating academic research to clinical applications.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has been actively researching GABAergic maladjustments and potential therapeutic strategies. They have developed RO4938581, a selective GABAA α5 inverse agonist, which has shown promise in enhancing cognition in preclinical models of Down syndrome and Alzheimer's disease[4]. This compound aims to address the excessive GABAergic inhibition observed in these conditions. Additionally, Roche is exploring the use of muscimol derivatives as potential treatments for anxiety and sleep disorders, leveraging the compound's ability to enhance GABAergic transmission[5]. Their research also extends to investigating the role of GABAergic interneurons in neurodevelopmental disorders and potential therapeutic interventions[6].
Strengths: Extensive experience in CNS drug development, diverse research portfolio. Weaknesses: Challenges in translating preclinical findings to clinical efficacy.
Muscimol: Mechanism and Therapeutic Potential
Treatment of poriomania
PatentInactiveEP1138332B1
Innovation
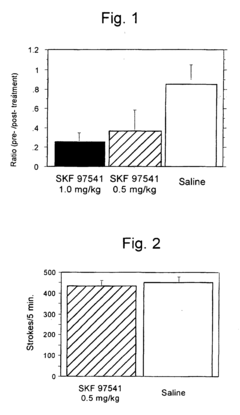
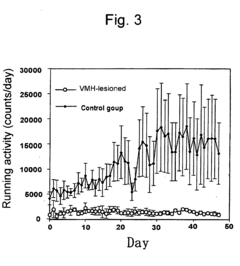
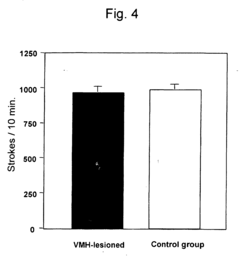
- A pharmaceutical composition using running neuron inhibitory substances like GABA B receptor agonists, GABA A receptor agonists, benzodiazepines, and kainate receptor antagonists to selectively inhibit non-intentional locomotion, specifically targeting the ventromedial nucleus of the hypothalamus to regulate non-intentional behaviors like poriomania without affecting intentional movements.
Treatment of conditions associated with myotonic dystrophy
PatentActiveUS20210023091A1
Innovation
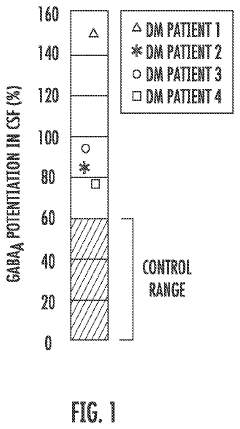
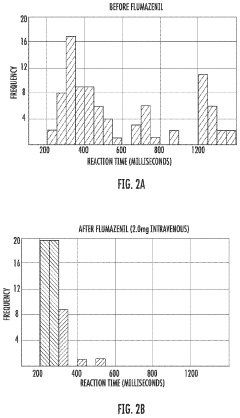
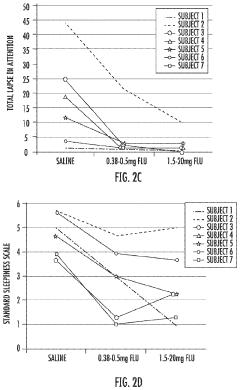
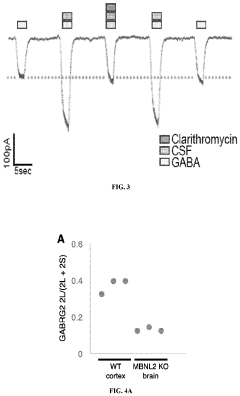
- Administration of a therapeutically effective amount of a GABAA receptor antagonist or inverse agonist, such as flumazenil, clarithromycin, or picrotoxin, to treat conditions like hypersomnia, cognitive impairment, and CNS dysfunction in myotonic dystrophy patients, which can be administered via various routes including intravenous, sublingual, or transdermal methods.
Regulatory Framework for GABAergic Therapeutics
The regulatory framework for GABAergic therapeutics is a complex and evolving landscape that plays a crucial role in the development, approval, and marketing of drugs targeting the GABAergic system. This framework is primarily governed by national and international regulatory bodies, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO).
These regulatory agencies have established stringent guidelines for the research, development, and clinical trials of GABAergic drugs. The process typically begins with preclinical studies, where the safety and efficacy of potential compounds are evaluated in laboratory and animal models. This stage is critical for identifying potential side effects and determining appropriate dosages for human trials.
Once preclinical studies are completed, the regulatory pathway moves into clinical trials. These trials are conducted in phases, with each phase designed to assess different aspects of the drug's safety and efficacy in human subjects. Phase I trials focus on safety and dosage in healthy volunteers, while Phase II and III trials evaluate the drug's effectiveness in treating specific conditions related to GABAergic maladjustments.
Throughout the clinical trial process, regulatory agencies require comprehensive documentation and reporting of all findings, including adverse events and unexpected outcomes. This rigorous approach ensures that only safe and effective GABAergic therapeutics reach the market.
For muscimol-based corrective strategies, additional regulatory considerations may apply due to its potent GABA-A receptor agonist properties. Regulatory bodies often require extensive pharmacokinetic and pharmacodynamic studies to understand the drug's behavior in the body and its potential for abuse or dependence.
Post-market surveillance is another critical component of the regulatory framework. Once a GABAergic therapeutic is approved and marketed, ongoing monitoring and reporting of adverse events are mandatory. This process helps identify any long-term safety concerns or rare side effects that may not have been apparent during clinical trials.
In recent years, there has been a growing emphasis on harmonizing regulatory requirements across different regions to streamline the drug development process. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to achieve greater uniformity in the regulatory processes for new drug approvals.
As research on GABAergic maladjustments and muscimol corrective strategies advances, regulatory frameworks are likely to evolve to address new challenges and opportunities in this field. This may include the development of specific guidelines for novel drug delivery methods, combination therapies, or personalized medicine approaches targeting the GABAergic system.
These regulatory agencies have established stringent guidelines for the research, development, and clinical trials of GABAergic drugs. The process typically begins with preclinical studies, where the safety and efficacy of potential compounds are evaluated in laboratory and animal models. This stage is critical for identifying potential side effects and determining appropriate dosages for human trials.
Once preclinical studies are completed, the regulatory pathway moves into clinical trials. These trials are conducted in phases, with each phase designed to assess different aspects of the drug's safety and efficacy in human subjects. Phase I trials focus on safety and dosage in healthy volunteers, while Phase II and III trials evaluate the drug's effectiveness in treating specific conditions related to GABAergic maladjustments.
Throughout the clinical trial process, regulatory agencies require comprehensive documentation and reporting of all findings, including adverse events and unexpected outcomes. This rigorous approach ensures that only safe and effective GABAergic therapeutics reach the market.
For muscimol-based corrective strategies, additional regulatory considerations may apply due to its potent GABA-A receptor agonist properties. Regulatory bodies often require extensive pharmacokinetic and pharmacodynamic studies to understand the drug's behavior in the body and its potential for abuse or dependence.
Post-market surveillance is another critical component of the regulatory framework. Once a GABAergic therapeutic is approved and marketed, ongoing monitoring and reporting of adverse events are mandatory. This process helps identify any long-term safety concerns or rare side effects that may not have been apparent during clinical trials.
In recent years, there has been a growing emphasis on harmonizing regulatory requirements across different regions to streamline the drug development process. Initiatives such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to achieve greater uniformity in the regulatory processes for new drug approvals.
As research on GABAergic maladjustments and muscimol corrective strategies advances, regulatory frameworks are likely to evolve to address new challenges and opportunities in this field. This may include the development of specific guidelines for novel drug delivery methods, combination therapies, or personalized medicine approaches targeting the GABAergic system.
Ethical Implications of GABAergic System Manipulation
The manipulation of the GABAergic system through research and therapeutic interventions raises significant ethical concerns that must be carefully considered. One primary issue is the potential for unintended consequences on brain function and behavior. GABAergic neurons play a crucial role in maintaining neural balance, and altering this system could lead to unforeseen changes in cognition, emotion, and personality. This raises questions about the extent to which we can ethically modify fundamental aspects of human neurobiology.
Another ethical consideration is the risk of dependency or addiction associated with GABAergic interventions. Muscimol and other GABA-enhancing compounds may have therapeutic benefits, but they also carry the potential for abuse and long-term alterations in brain chemistry. Researchers and clinicians must weigh the short-term benefits against the risk of creating new forms of substance dependence or exacerbating existing addiction vulnerabilities.
The use of GABAergic manipulations in vulnerable populations, such as children or individuals with mental health disorders, presents additional ethical challenges. There is a need to establish clear guidelines for informed consent and to ensure that the rights and well-being of these groups are protected. This includes considering the long-term developmental impacts of GABAergic interventions on the maturing brain and the potential for exacerbating existing mental health conditions.
Privacy and data protection are also critical ethical concerns in GABAergic research. As studies delve deeper into individual brain function and responses to GABAergic modulation, there is a risk of exposing sensitive personal information. Researchers must implement robust safeguards to protect participant data and ensure that findings cannot be misused for discriminatory or exploitative purposes.
The potential for GABAergic interventions to enhance cognitive function or alter mood in healthy individuals raises questions about fairness and social equality. If such treatments become widely available, there is a risk of creating new forms of cognitive inequality or pressure to use these interventions for non-medical purposes, such as academic or professional performance enhancement.
Lastly, the development of GABAergic therapies must consider issues of global justice and access to healthcare. As new treatments emerge, there is an ethical imperative to ensure equitable distribution and avoid exacerbating existing healthcare disparities. This includes addressing the affordability of treatments and the potential for their misuse in regions with less stringent regulatory oversight.
Another ethical consideration is the risk of dependency or addiction associated with GABAergic interventions. Muscimol and other GABA-enhancing compounds may have therapeutic benefits, but they also carry the potential for abuse and long-term alterations in brain chemistry. Researchers and clinicians must weigh the short-term benefits against the risk of creating new forms of substance dependence or exacerbating existing addiction vulnerabilities.
The use of GABAergic manipulations in vulnerable populations, such as children or individuals with mental health disorders, presents additional ethical challenges. There is a need to establish clear guidelines for informed consent and to ensure that the rights and well-being of these groups are protected. This includes considering the long-term developmental impacts of GABAergic interventions on the maturing brain and the potential for exacerbating existing mental health conditions.
Privacy and data protection are also critical ethical concerns in GABAergic research. As studies delve deeper into individual brain function and responses to GABAergic modulation, there is a risk of exposing sensitive personal information. Researchers must implement robust safeguards to protect participant data and ensure that findings cannot be misused for discriminatory or exploitative purposes.
The potential for GABAergic interventions to enhance cognitive function or alter mood in healthy individuals raises questions about fairness and social equality. If such treatments become widely available, there is a risk of creating new forms of cognitive inequality or pressure to use these interventions for non-medical purposes, such as academic or professional performance enhancement.
Lastly, the development of GABAergic therapies must consider issues of global justice and access to healthcare. As new treatments emerge, there is an ethical imperative to ensure equitable distribution and avoid exacerbating existing healthcare disparities. This includes addressing the affordability of treatments and the potential for their misuse in regions with less stringent regulatory oversight.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!