Health Impacts of Muscimol in Traditional Dietary Practices
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a psychoactive compound found in certain mushroom species, has been a subject of fascination and research for decades. Its presence in traditional dietary practices across various cultures has sparked significant interest in the scientific community. The evolution of muscimol research can be traced back to the mid-20th century when ethnobotanists first documented its use in indigenous rituals and medicinal practices.
The primary objective of this research is to comprehensively evaluate the health impacts of muscimol consumption within the context of traditional dietary practices. This involves examining both the potential benefits and risks associated with its ingestion, as well as understanding the cultural and historical significance of muscimol-containing fungi in different societies.
As research in this field has progressed, several key trends have emerged. Initially, studies focused primarily on the psychoactive effects of muscimol and its role in religious and spiritual ceremonies. However, recent years have seen a shift towards investigating its potential therapeutic applications, particularly in the treatment of neurological disorders and mental health conditions.
One of the critical aspects of this research is to elucidate the pharmacological mechanisms of muscimol in the human body. This compound is known to act as a potent GABA agonist, which has implications for its effects on the central nervous system. Understanding these mechanisms is crucial for assessing both the short-term and long-term health impacts of muscimol consumption.
The research also aims to explore the variability in muscimol content across different mushroom species and how traditional preparation methods may affect its potency and bioavailability. This is particularly important given the diverse ways in which muscimol-containing fungi are consumed in different cultural contexts.
Another key objective is to investigate the potential interactions between muscimol and other compounds present in traditional diets. This includes examining how these interactions might modulate the health effects of muscimol, either enhancing its beneficial properties or mitigating potential risks.
Furthermore, the research seeks to address the regulatory and ethical considerations surrounding the use of muscimol in traditional practices. This involves evaluating current policies and guidelines, as well as proposing evidence-based recommendations for safe consumption and potential therapeutic applications.
By comprehensively examining these aspects, this research aims to bridge the gap between traditional knowledge and modern scientific understanding, providing valuable insights into the health impacts of muscimol in traditional dietary practices. The findings from this study will not only contribute to the academic discourse but also have practical implications for public health policies and potential pharmaceutical developments.
The primary objective of this research is to comprehensively evaluate the health impacts of muscimol consumption within the context of traditional dietary practices. This involves examining both the potential benefits and risks associated with its ingestion, as well as understanding the cultural and historical significance of muscimol-containing fungi in different societies.
As research in this field has progressed, several key trends have emerged. Initially, studies focused primarily on the psychoactive effects of muscimol and its role in religious and spiritual ceremonies. However, recent years have seen a shift towards investigating its potential therapeutic applications, particularly in the treatment of neurological disorders and mental health conditions.
One of the critical aspects of this research is to elucidate the pharmacological mechanisms of muscimol in the human body. This compound is known to act as a potent GABA agonist, which has implications for its effects on the central nervous system. Understanding these mechanisms is crucial for assessing both the short-term and long-term health impacts of muscimol consumption.
The research also aims to explore the variability in muscimol content across different mushroom species and how traditional preparation methods may affect its potency and bioavailability. This is particularly important given the diverse ways in which muscimol-containing fungi are consumed in different cultural contexts.
Another key objective is to investigate the potential interactions between muscimol and other compounds present in traditional diets. This includes examining how these interactions might modulate the health effects of muscimol, either enhancing its beneficial properties or mitigating potential risks.
Furthermore, the research seeks to address the regulatory and ethical considerations surrounding the use of muscimol in traditional practices. This involves evaluating current policies and guidelines, as well as proposing evidence-based recommendations for safe consumption and potential therapeutic applications.
By comprehensively examining these aspects, this research aims to bridge the gap between traditional knowledge and modern scientific understanding, providing valuable insights into the health impacts of muscimol in traditional dietary practices. The findings from this study will not only contribute to the academic discourse but also have practical implications for public health policies and potential pharmaceutical developments.
Market Analysis of Muscimol-Containing Products
The market for muscimol-containing products is experiencing a notable surge in interest, driven by the growing trend of exploring traditional dietary practices and natural remedies. Muscimol, a psychoactive compound found in certain mushroom species, particularly Amanita muscaria, has garnered attention for its potential health benefits and traditional uses.
The global market for functional mushrooms, which includes muscimol-containing products, is projected to expand significantly in the coming years. This growth is fueled by increasing consumer awareness of the potential health benefits associated with these fungi and their compounds. The market encompasses various product categories, including dietary supplements, functional foods and beverages, and personal care products.
North America and Europe currently dominate the market for muscimol-containing products, with the United States and Canada leading in terms of product innovation and consumer adoption. However, Asia-Pacific is emerging as a rapidly growing market, particularly in countries with a rich history of traditional medicine practices, such as China and Japan.
The primary consumer segments for muscimol-containing products include health-conscious individuals, those interested in alternative medicine, and consumers seeking natural remedies for various health concerns. There is a growing demand for products that claim to support cognitive function, stress relief, and overall well-being.
Key market players in this space include both established nutraceutical companies and emerging startups specializing in functional mushroom products. These companies are investing in research and development to create innovative formulations and delivery methods for muscimol-containing products, such as tinctures, capsules, and powders.
Regulatory challenges remain a significant factor influencing market growth, as the legal status of muscimol varies across different jurisdictions. This has led to a fragmented market landscape, with some regions experiencing more rapid growth than others due to favorable regulatory environments.
Consumer education and awareness campaigns play a crucial role in market development, as many potential customers are unfamiliar with muscimol and its traditional uses. Companies are focusing on marketing strategies that emphasize the natural origins and potential health benefits of muscimol-containing products while addressing safety concerns and promoting responsible use.
The market is also witnessing a trend towards sustainable and ethical sourcing practices, with consumers showing a preference for products that are environmentally friendly and support local communities involved in traditional harvesting practices.
As research into the health impacts of muscimol continues to evolve, the market is expected to see further segmentation and specialization, with products tailored to specific health concerns and consumer preferences. This presents both opportunities and challenges for companies operating in this space, requiring ongoing innovation and adaptation to changing market dynamics and regulatory landscapes.
The global market for functional mushrooms, which includes muscimol-containing products, is projected to expand significantly in the coming years. This growth is fueled by increasing consumer awareness of the potential health benefits associated with these fungi and their compounds. The market encompasses various product categories, including dietary supplements, functional foods and beverages, and personal care products.
North America and Europe currently dominate the market for muscimol-containing products, with the United States and Canada leading in terms of product innovation and consumer adoption. However, Asia-Pacific is emerging as a rapidly growing market, particularly in countries with a rich history of traditional medicine practices, such as China and Japan.
The primary consumer segments for muscimol-containing products include health-conscious individuals, those interested in alternative medicine, and consumers seeking natural remedies for various health concerns. There is a growing demand for products that claim to support cognitive function, stress relief, and overall well-being.
Key market players in this space include both established nutraceutical companies and emerging startups specializing in functional mushroom products. These companies are investing in research and development to create innovative formulations and delivery methods for muscimol-containing products, such as tinctures, capsules, and powders.
Regulatory challenges remain a significant factor influencing market growth, as the legal status of muscimol varies across different jurisdictions. This has led to a fragmented market landscape, with some regions experiencing more rapid growth than others due to favorable regulatory environments.
Consumer education and awareness campaigns play a crucial role in market development, as many potential customers are unfamiliar with muscimol and its traditional uses. Companies are focusing on marketing strategies that emphasize the natural origins and potential health benefits of muscimol-containing products while addressing safety concerns and promoting responsible use.
The market is also witnessing a trend towards sustainable and ethical sourcing practices, with consumers showing a preference for products that are environmentally friendly and support local communities involved in traditional harvesting practices.
As research into the health impacts of muscimol continues to evolve, the market is expected to see further segmentation and specialization, with products tailored to specific health concerns and consumer preferences. This presents both opportunities and challenges for companies operating in this space, requiring ongoing innovation and adaptation to changing market dynamics and regulatory landscapes.
Current Understanding and Challenges in Muscimol Research
Research on muscimol, a psychoactive compound found in certain mushroom species, has made significant strides in recent years. However, the current understanding of its health impacts in traditional dietary practices remains limited, presenting several challenges for researchers and health professionals.
One of the primary obstacles in muscimol research is the scarcity of comprehensive, long-term studies on its effects when consumed as part of traditional diets. While acute effects are relatively well-documented, the chronic impacts of regular, low-dose consumption are not fully understood. This gap in knowledge is particularly concerning given the cultural significance of muscimol-containing mushrooms in some indigenous communities.
Another challenge lies in the variability of muscimol content across different mushroom species and growing conditions. This inconsistency makes it difficult to establish standardized dosages and safety guidelines for traditional consumption practices. Researchers are working to develop more accurate and reliable methods for quantifying muscimol levels in various mushroom samples, but progress has been slow due to the complexity of the compound and its interactions with other mushroom constituents.
The legal status of muscimol-containing mushrooms in many countries further complicates research efforts. Restrictions on cultivation, possession, and use of these mushrooms have limited the scope and scale of scientific studies, particularly those involving human subjects. This regulatory environment has forced many researchers to rely heavily on animal models, which may not always accurately reflect human physiological responses to muscimol.
Despite these challenges, recent advancements in neuroimaging and molecular biology techniques have provided new insights into muscimol's mechanisms of action. Studies have revealed its potent GABA-A receptor agonist properties, suggesting potential therapeutic applications in treating anxiety, epilepsy, and sleep disorders. However, translating these findings into safe, practical applications within traditional dietary contexts remains a significant hurdle.
The interaction between muscimol and other bioactive compounds present in mushrooms is another area of active research. Some studies suggest that these interactions may modulate muscimol's effects, potentially enhancing its beneficial properties or mitigating adverse reactions. Understanding these complex interactions is crucial for assessing the overall health impacts of muscimol consumption in traditional practices.
Ethical considerations also pose challenges in muscimol research, particularly when studying its use in indigenous communities. Balancing scientific inquiry with respect for cultural traditions and practices requires careful navigation and collaboration between researchers and community stakeholders. This delicate balance is essential for conducting meaningful, culturally sensitive research that can inform public health policies and guidelines.
One of the primary obstacles in muscimol research is the scarcity of comprehensive, long-term studies on its effects when consumed as part of traditional diets. While acute effects are relatively well-documented, the chronic impacts of regular, low-dose consumption are not fully understood. This gap in knowledge is particularly concerning given the cultural significance of muscimol-containing mushrooms in some indigenous communities.
Another challenge lies in the variability of muscimol content across different mushroom species and growing conditions. This inconsistency makes it difficult to establish standardized dosages and safety guidelines for traditional consumption practices. Researchers are working to develop more accurate and reliable methods for quantifying muscimol levels in various mushroom samples, but progress has been slow due to the complexity of the compound and its interactions with other mushroom constituents.
The legal status of muscimol-containing mushrooms in many countries further complicates research efforts. Restrictions on cultivation, possession, and use of these mushrooms have limited the scope and scale of scientific studies, particularly those involving human subjects. This regulatory environment has forced many researchers to rely heavily on animal models, which may not always accurately reflect human physiological responses to muscimol.
Despite these challenges, recent advancements in neuroimaging and molecular biology techniques have provided new insights into muscimol's mechanisms of action. Studies have revealed its potent GABA-A receptor agonist properties, suggesting potential therapeutic applications in treating anxiety, epilepsy, and sleep disorders. However, translating these findings into safe, practical applications within traditional dietary contexts remains a significant hurdle.
The interaction between muscimol and other bioactive compounds present in mushrooms is another area of active research. Some studies suggest that these interactions may modulate muscimol's effects, potentially enhancing its beneficial properties or mitigating adverse reactions. Understanding these complex interactions is crucial for assessing the overall health impacts of muscimol consumption in traditional practices.
Ethical considerations also pose challenges in muscimol research, particularly when studying its use in indigenous communities. Balancing scientific inquiry with respect for cultural traditions and practices requires careful navigation and collaboration between researchers and community stakeholders. This delicate balance is essential for conducting meaningful, culturally sensitive research that can inform public health policies and guidelines.
Existing Methods for Muscimol Extraction and Analysis
01 Neurological effects of muscimol
Muscimol, a psychoactive compound found in certain mushrooms, has significant impacts on the central nervous system. It acts as a potent GABA receptor agonist, potentially influencing neurotransmitter activity and brain function. Research suggests it may have both therapeutic potential and risks, including effects on cognition, mood, and motor control.- Neurological effects of muscimol: Muscimol, a psychoactive compound found in certain mushrooms, has significant impacts on the central nervous system. It acts as a potent GABA receptor agonist, potentially influencing neurotransmission, cognitive function, and behavior. Research suggests both therapeutic potential and risks associated with its neurological effects.
- Muscimol in pharmaceutical applications: The compound is being investigated for various pharmaceutical applications due to its unique pharmacological properties. Potential therapeutic uses include treatment of anxiety disorders, epilepsy, and certain neurological conditions. Ongoing research focuses on developing safe and effective formulations for clinical use.
- Safety and toxicity considerations: While muscimol shows promise in medical applications, its use comes with potential risks and side effects. Studies are ongoing to assess its safety profile, including potential for addiction, toxicity at various doses, and interactions with other substances. Proper risk assessment and management strategies are crucial for its responsible use.
- Muscimol's impact on mental health: Research is exploring muscimol's effects on mental health conditions such as depression, PTSD, and addiction. Its ability to modulate GABA activity in the brain may offer new approaches to treating these disorders. However, careful clinical evaluation is necessary to determine its efficacy and appropriate use in psychiatric settings.
- Regulatory and legal aspects of muscimol use: The legal status and regulation of muscimol vary across jurisdictions due to its psychoactive properties. As research progresses, there are ongoing discussions about its classification, control measures, and potential rescheduling. These regulatory aspects significantly impact its availability for research and potential therapeutic applications.
02 Muscimol in pharmaceutical applications
The compound is being investigated for various pharmaceutical applications due to its unique pharmacological properties. Potential therapeutic uses include treatment of anxiety disorders, epilepsy, and certain neurological conditions. However, careful dosing and administration methods are crucial due to its potent effects.Expand Specific Solutions03 Safety and toxicity considerations
While muscimol shows promise in medical applications, its use comes with significant safety concerns. Potential side effects and toxicity risks need to be carefully evaluated. Research is ongoing to determine safe dosage levels, potential interactions with other substances, and long-term health impacts.Expand Specific Solutions04 Muscimol's impact on cognitive function
Studies indicate that muscimol can significantly affect cognitive processes, including memory, attention, and perception. While some effects may be detrimental, there is also interest in its potential to modulate cognitive function in therapeutic contexts, such as in the treatment of certain mental health disorders.Expand Specific Solutions05 Regulatory and legal aspects of muscimol use
The health impacts of muscimol are closely tied to regulatory and legal considerations. Its status as a controlled substance in many jurisdictions affects research, medical use, and public health policies. Ongoing debates and policy changes regarding its classification and permitted uses have significant implications for its potential health applications.Expand Specific Solutions
Key Players in Muscimol Research and Production
The health impacts of muscimol in traditional dietary practices represent an emerging field of research, currently in its early stages of development. The market for related products and research is relatively small but growing, driven by increasing interest in traditional and natural remedies. The technology is still in its infancy, with companies like CaaMTech LLC and Psyched Wellness Ltd. leading the way in exploring muscimol's potential applications. Established pharmaceutical firms such as Vertex Pharmaceuticals and ACADIA Pharmaceuticals are also showing interest, indicating a potential for future market expansion. However, the field remains largely unexplored, with significant opportunities for further research and development.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has conducted comprehensive studies on the traditional use of Amanita muscaria and its active compound, muscimol, in various cultural practices. Their research encompasses ethnobotanical surveys, chemical analysis of muscimol content in different mushroom preparations, and evaluation of potential health impacts. CSIR has investigated the pharmacological properties of muscimol, including its GABAergic effects and potential neuroprotective properties[7]. The organization is also exploring sustainable cultivation methods for Amanita muscaria to ensure a consistent supply for research purposes[8].
Strengths: Multidisciplinary approach combining traditional knowledge and modern scientific methods. Access to diverse research facilities and expertise. Weaknesses: Potential limitations in commercialization and product development compared to private sector entities.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has been exploring muscimol and related GABA receptor modulators for potential therapeutic applications. Their research focuses on the development of selective GABA receptor agonists, including muscimol derivatives, for treating central nervous system disorders. ACADIA has conducted preclinical studies on muscimol's effects on sleep architecture and its potential in treating insomnia[5]. The company is also investigating the use of muscimol-like compounds in managing symptoms of Parkinson's disease psychosis and other neurological conditions[6].
Strengths: Extensive experience in CNS drug development. Broad research pipeline including GABA modulators. Weaknesses: Primary focus on other compounds may limit dedicated muscimol research.
Breakthrough Studies on Muscimol's Health Effects
Health Promoting Dairy and Food Products Containing Mushroom Glucan Produced Through Fermentation of Grifola Frondosa
PatentInactiveUS20080171104A1
Innovation
- Fermentation methods for producing Grifola mycelium and polysaccharides that eliminate the typical fungal taste and smell, ensure consistent quality, reduce protease activity, and enable cost-effective, high-yield production, allowing for the creation of neutral-tasting, safe food and pharmaceutical products.
Lipase Inhibitors
PatentActiveUS20080317821A1
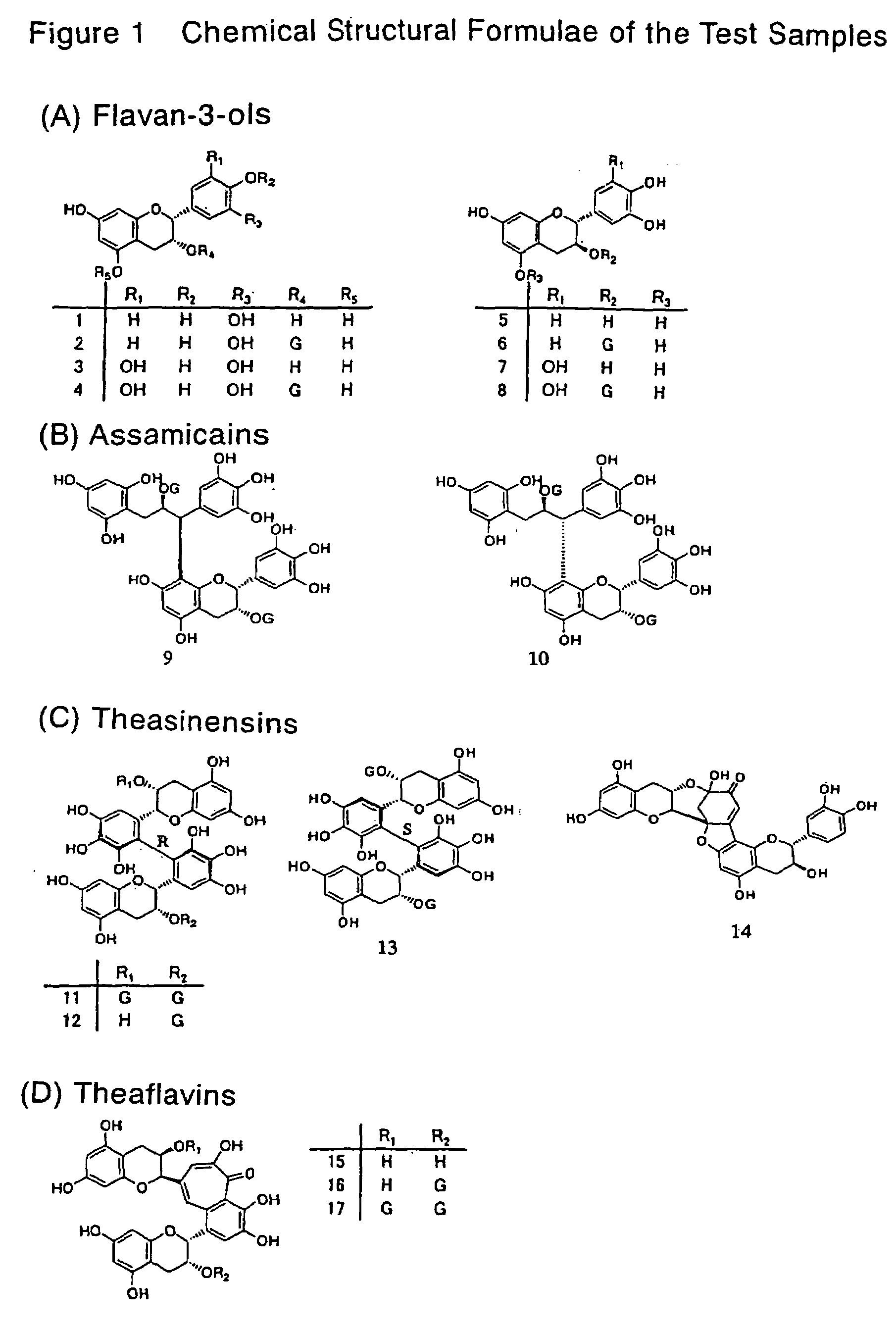
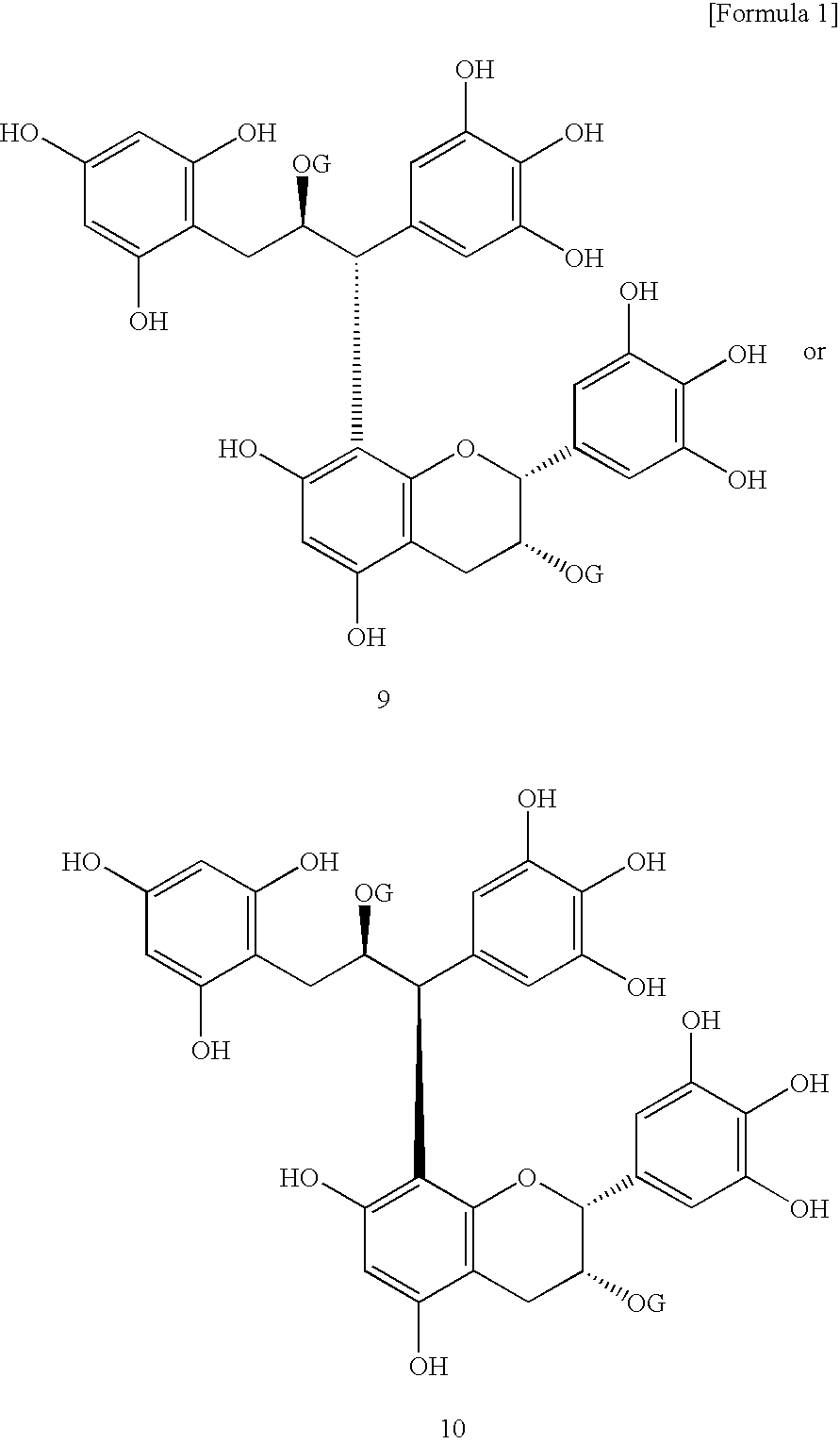
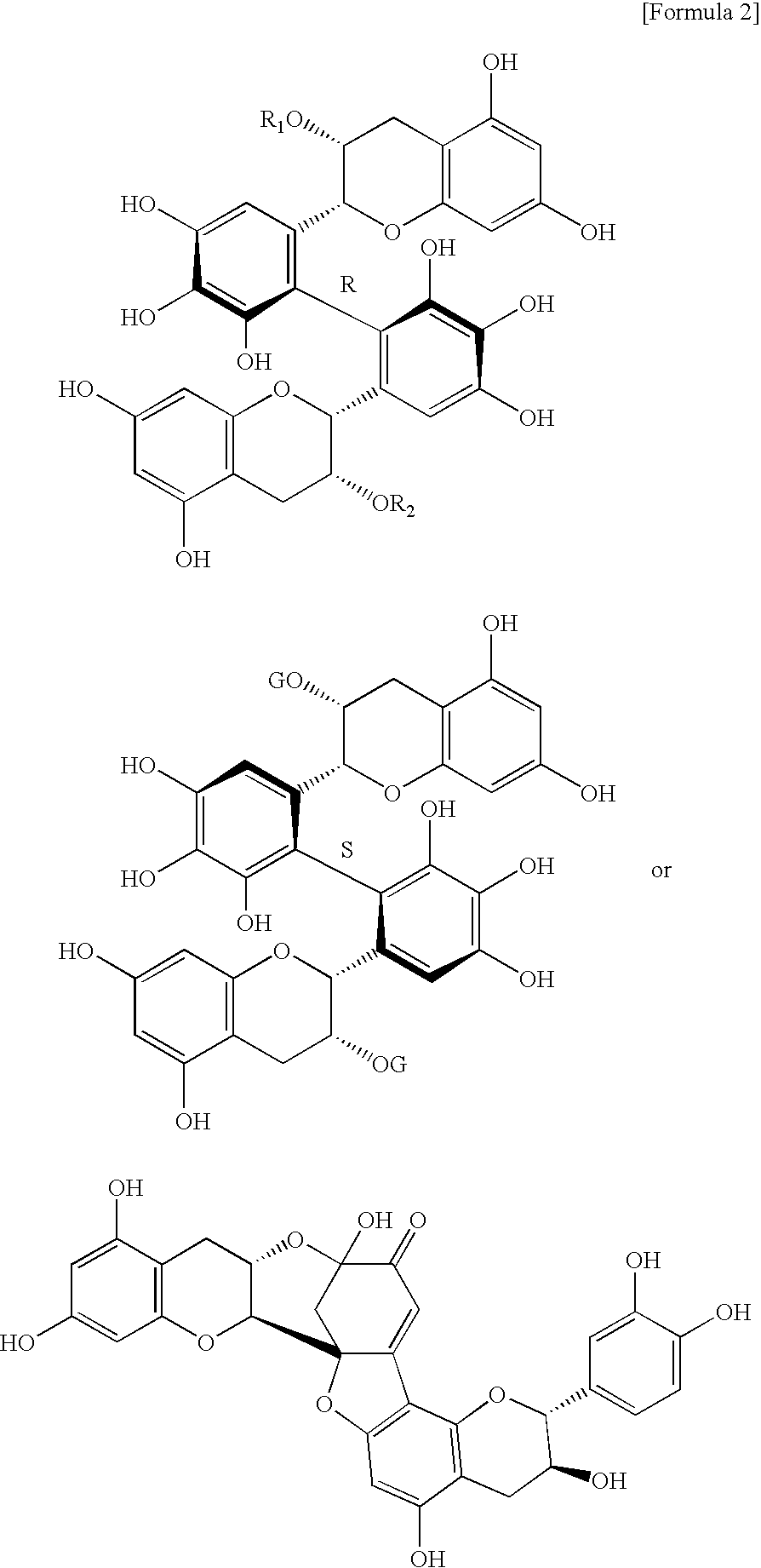
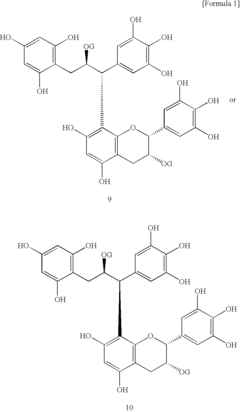
Innovation
- Development of lipase inhibitors containing dimers of flavan-3-ols derived from teas, such as assamicains, theasinensins, and theaflavins, which are commercially available or extracted from green, black, and oolong teas, providing strong lipase inhibitory activity without compromising taste or safety.
Regulatory Framework for Muscimol-Containing Foods
The regulatory framework for muscimol-containing foods is a complex and evolving landscape that varies significantly across different jurisdictions. In many countries, the legal status of muscimol and its source, Amanita muscaria mushrooms, remains ambiguous, falling into a regulatory gray area between food, drug, and controlled substance classifications.
In the United States, the Food and Drug Administration (FDA) has not explicitly approved muscimol or Amanita muscaria for use in food products. However, the mushroom itself is not listed as a controlled substance under federal law. This has led to a situation where some companies market Amanita muscaria products as dietary supplements or "functional foods," operating in a regulatory limbo.
The European Union has taken a more cautious approach. The European Food Safety Authority (EFSA) has not authorized Amanita muscaria or muscimol as a novel food ingredient. Consequently, the intentional addition of muscimol to food products is generally prohibited in EU member states.
In countries with traditional uses of Amanita muscaria, such as certain regions of Russia and Siberia, regulations may be more permissive or simply not address the issue directly. This creates challenges for international trade and harmonization of food safety standards.
Some jurisdictions have implemented specific regulations for muscimol-containing products. For example, the state of Louisiana in the US explicitly banned the possession and sale of Amanita muscaria in 2015, citing public health concerns. This illustrates the potential for regional variations in regulatory approaches, even within a single country.
The regulatory framework is further complicated by the dual nature of muscimol as both a potential food ingredient and a psychoactive compound. This duality raises questions about appropriate dosage, labeling requirements, and potential restrictions on sale and distribution.
As research into the health impacts of muscimol in traditional dietary practices continues to evolve, regulatory bodies are likely to face increasing pressure to develop clear guidelines. Future regulatory frameworks may need to balance traditional uses, potential therapeutic applications, and public health concerns.
Policymakers and food safety authorities are grappling with the challenge of creating regulations that address the unique properties of muscimol while ensuring consumer safety. This may involve developing new testing methods, establishing safe consumption limits, and implementing stringent quality control measures for muscimol-containing food products.
In the United States, the Food and Drug Administration (FDA) has not explicitly approved muscimol or Amanita muscaria for use in food products. However, the mushroom itself is not listed as a controlled substance under federal law. This has led to a situation where some companies market Amanita muscaria products as dietary supplements or "functional foods," operating in a regulatory limbo.
The European Union has taken a more cautious approach. The European Food Safety Authority (EFSA) has not authorized Amanita muscaria or muscimol as a novel food ingredient. Consequently, the intentional addition of muscimol to food products is generally prohibited in EU member states.
In countries with traditional uses of Amanita muscaria, such as certain regions of Russia and Siberia, regulations may be more permissive or simply not address the issue directly. This creates challenges for international trade and harmonization of food safety standards.
Some jurisdictions have implemented specific regulations for muscimol-containing products. For example, the state of Louisiana in the US explicitly banned the possession and sale of Amanita muscaria in 2015, citing public health concerns. This illustrates the potential for regional variations in regulatory approaches, even within a single country.
The regulatory framework is further complicated by the dual nature of muscimol as both a potential food ingredient and a psychoactive compound. This duality raises questions about appropriate dosage, labeling requirements, and potential restrictions on sale and distribution.
As research into the health impacts of muscimol in traditional dietary practices continues to evolve, regulatory bodies are likely to face increasing pressure to develop clear guidelines. Future regulatory frameworks may need to balance traditional uses, potential therapeutic applications, and public health concerns.
Policymakers and food safety authorities are grappling with the challenge of creating regulations that address the unique properties of muscimol while ensuring consumer safety. This may involve developing new testing methods, establishing safe consumption limits, and implementing stringent quality control measures for muscimol-containing food products.
Ethical Considerations in Muscimol Research and Use
The ethical considerations surrounding muscimol research and use are multifaceted and require careful examination. One primary concern is the potential for misuse or abuse of muscimol, given its psychoactive properties. Researchers and policymakers must balance the pursuit of scientific knowledge with the responsibility to protect public health and safety. This necessitates strict protocols for handling and studying muscimol, as well as clear guidelines for its use in traditional dietary practices.
Another critical ethical issue is the impact of muscimol research on indigenous communities that have traditionally used Amanita muscaria mushrooms in their cultural practices. There is a risk of cultural appropriation and exploitation if traditional knowledge is not respected and protected. Researchers must engage in meaningful consultation with these communities and ensure that their rights and interests are safeguarded throughout the research process.
The informed consent of research participants is paramount when studying the health impacts of muscimol. Given the potential for altered states of consciousness, extra care must be taken to ensure that participants fully understand the risks and benefits of their involvement. This includes providing comprehensive information about the effects of muscimol and implementing robust screening procedures to exclude individuals who may be at higher risk of adverse reactions.
Ethical considerations also extend to the dissemination of research findings. There is a responsibility to communicate results accurately and responsibly, avoiding sensationalism that could lead to misunderstanding or misuse of muscimol. Researchers must be transparent about the limitations of their studies and the potential risks associated with muscimol consumption.
The environmental impact of increased interest in Amanita muscaria mushrooms is another ethical concern. Overharvesting could disrupt ecosystems and threaten the sustainability of traditional practices. Conservation efforts and sustainable harvesting methods should be integral to any research or commercial endeavors involving muscimol.
Lastly, there are ethical implications in the potential medicinal applications of muscimol. While research may uncover therapeutic benefits, the development and distribution of muscimol-based treatments must adhere to rigorous ethical standards. This includes ensuring equitable access to any potential treatments and preventing the exploitation of vulnerable populations in clinical trials or marketing efforts.
Another critical ethical issue is the impact of muscimol research on indigenous communities that have traditionally used Amanita muscaria mushrooms in their cultural practices. There is a risk of cultural appropriation and exploitation if traditional knowledge is not respected and protected. Researchers must engage in meaningful consultation with these communities and ensure that their rights and interests are safeguarded throughout the research process.
The informed consent of research participants is paramount when studying the health impacts of muscimol. Given the potential for altered states of consciousness, extra care must be taken to ensure that participants fully understand the risks and benefits of their involvement. This includes providing comprehensive information about the effects of muscimol and implementing robust screening procedures to exclude individuals who may be at higher risk of adverse reactions.
Ethical considerations also extend to the dissemination of research findings. There is a responsibility to communicate results accurately and responsibly, avoiding sensationalism that could lead to misunderstanding or misuse of muscimol. Researchers must be transparent about the limitations of their studies and the potential risks associated with muscimol consumption.
The environmental impact of increased interest in Amanita muscaria mushrooms is another ethical concern. Overharvesting could disrupt ecosystems and threaten the sustainability of traditional practices. Conservation efforts and sustainable harvesting methods should be integral to any research or commercial endeavors involving muscimol.
Lastly, there are ethical implications in the potential medicinal applications of muscimol. While research may uncover therapeutic benefits, the development and distribution of muscimol-based treatments must adhere to rigorous ethical standards. This includes ensuring equitable access to any potential treatments and preventing the exploitation of vulnerable populations in clinical trials or marketing efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!