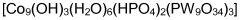Coupling water oxidation catalysts with N₂ reduction photocathodes for overall solar N₂ fixation
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solar N₂ Fixation Background and Objectives
Nitrogen fixation, the process of converting atmospheric nitrogen (N₂) into ammonia (NH₃) or other bioavailable forms, represents one of the most critical processes for sustaining life on Earth. Historically, the Haber-Bosch process has dominated industrial nitrogen fixation since its development in the early 20th century, consuming approximately 1-2% of global energy production and generating significant carbon emissions. This energy-intensive process operates under harsh conditions of high temperature (400-500°C) and pressure (150-300 atm), highlighting the need for more sustainable alternatives.
Solar nitrogen fixation has emerged as a promising approach that harnesses renewable solar energy to drive nitrogen reduction under ambient conditions. This technology aims to mimic natural biological nitrogen fixation while offering advantages in terms of energy efficiency and environmental impact. The evolution of this field has accelerated significantly over the past decade, with breakthrough developments in photocatalysts, electrocatalysts, and integrated systems.
The fundamental challenge in nitrogen fixation lies in breaking the exceptionally strong triple bond of the N₂ molecule (945 kJ/mol), which requires substantial energy input. Solar-driven approaches offer a pathway to overcome this barrier using renewable energy rather than fossil fuels, potentially revolutionizing fertilizer production and addressing global food security challenges in a sustainable manner.
Current research objectives in solar nitrogen fixation focus on developing integrated systems that can efficiently couple water oxidation catalysts with nitrogen reduction photocathodes. This coupling is essential for achieving overall solar nitrogen fixation without external energy inputs. The water oxidation half-reaction provides the necessary electrons and protons for nitrogen reduction while generating oxygen as a benign by-product.
Key technical objectives include enhancing the solar-to-ammonia conversion efficiency, improving catalyst stability under operating conditions, reducing precious metal content in catalysts, and developing scalable manufacturing processes. Current benchmark efficiencies remain below 1%, whereas theoretical calculations suggest potential efficiencies of 5-10% are achievable with optimized systems.
The field is progressing toward more sophisticated architectures that integrate light harvesting, charge separation, and catalytic functions into unified systems. Emerging trends include the development of Z-scheme photocatalysts, plasmonic enhancement strategies, defect engineering in semiconductor materials, and biomimetic approaches inspired by nitrogenase enzymes found in nitrogen-fixing bacteria.
Achieving practical solar nitrogen fixation technology would represent a transformative advancement with far-reaching implications for sustainable agriculture, distributed fertilizer production in remote regions, and significant reduction in greenhouse gas emissions associated with conventional ammonia synthesis.
Solar nitrogen fixation has emerged as a promising approach that harnesses renewable solar energy to drive nitrogen reduction under ambient conditions. This technology aims to mimic natural biological nitrogen fixation while offering advantages in terms of energy efficiency and environmental impact. The evolution of this field has accelerated significantly over the past decade, with breakthrough developments in photocatalysts, electrocatalysts, and integrated systems.
The fundamental challenge in nitrogen fixation lies in breaking the exceptionally strong triple bond of the N₂ molecule (945 kJ/mol), which requires substantial energy input. Solar-driven approaches offer a pathway to overcome this barrier using renewable energy rather than fossil fuels, potentially revolutionizing fertilizer production and addressing global food security challenges in a sustainable manner.
Current research objectives in solar nitrogen fixation focus on developing integrated systems that can efficiently couple water oxidation catalysts with nitrogen reduction photocathodes. This coupling is essential for achieving overall solar nitrogen fixation without external energy inputs. The water oxidation half-reaction provides the necessary electrons and protons for nitrogen reduction while generating oxygen as a benign by-product.
Key technical objectives include enhancing the solar-to-ammonia conversion efficiency, improving catalyst stability under operating conditions, reducing precious metal content in catalysts, and developing scalable manufacturing processes. Current benchmark efficiencies remain below 1%, whereas theoretical calculations suggest potential efficiencies of 5-10% are achievable with optimized systems.
The field is progressing toward more sophisticated architectures that integrate light harvesting, charge separation, and catalytic functions into unified systems. Emerging trends include the development of Z-scheme photocatalysts, plasmonic enhancement strategies, defect engineering in semiconductor materials, and biomimetic approaches inspired by nitrogenase enzymes found in nitrogen-fixing bacteria.
Achieving practical solar nitrogen fixation technology would represent a transformative advancement with far-reaching implications for sustainable agriculture, distributed fertilizer production in remote regions, and significant reduction in greenhouse gas emissions associated with conventional ammonia synthesis.
Market Analysis for Solar Ammonia Production
The global ammonia market is experiencing significant growth, with a market value of $72.5 billion in 2022 and projected to reach $111.7 billion by 2030, growing at a CAGR of 5.6%. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions, creating an urgent need for sustainable alternatives.
Solar ammonia production represents a promising green alternative that aligns with global decarbonization goals. The market for solar-based nitrogen fixation technologies is currently in its nascent stage but shows tremendous potential due to increasing environmental regulations and carbon pricing mechanisms worldwide. Countries with ambitious climate targets, particularly in the European Union, are driving demand for carbon-neutral ammonia production methods.
The agricultural sector remains the largest consumer of ammonia, accounting for approximately 80% of global consumption primarily for fertilizer production. However, emerging applications in energy storage, maritime fuel, and hydrogen carriers are expanding the potential market for green ammonia. The International Energy Agency forecasts that ammonia could constitute up to 45% of maritime fuel demand by 2050, representing a substantial new market opportunity.
Geographically, regions with abundant solar resources and agricultural needs present the most promising markets for solar ammonia production technologies. Countries in Africa, Australia, the Middle East, and parts of Latin America combine high solar irradiance with significant agricultural sectors, creating ideal conditions for implementation.
Investment in solar ammonia technologies has seen remarkable growth, with venture capital funding increasing from $120 million in 2018 to over $450 million in 2022. Major agricultural companies and energy corporations are establishing strategic partnerships and pilot projects to explore commercial viability.
Cost remains the primary market barrier, with solar ammonia production currently 2-3 times more expensive than conventional methods. However, technological improvements and economies of scale are expected to reduce this gap significantly by 2030. Market analysts project that solar ammonia could achieve cost parity with conventional production in regions with optimal solar conditions by 2035.
Consumer willingness to pay premium prices for sustainably produced agricultural products is creating market pull for green fertilizers, with several premium food brands already committing to sourcing crops grown with sustainable fertilizers by 2030.
Solar ammonia production represents a promising green alternative that aligns with global decarbonization goals. The market for solar-based nitrogen fixation technologies is currently in its nascent stage but shows tremendous potential due to increasing environmental regulations and carbon pricing mechanisms worldwide. Countries with ambitious climate targets, particularly in the European Union, are driving demand for carbon-neutral ammonia production methods.
The agricultural sector remains the largest consumer of ammonia, accounting for approximately 80% of global consumption primarily for fertilizer production. However, emerging applications in energy storage, maritime fuel, and hydrogen carriers are expanding the potential market for green ammonia. The International Energy Agency forecasts that ammonia could constitute up to 45% of maritime fuel demand by 2050, representing a substantial new market opportunity.
Geographically, regions with abundant solar resources and agricultural needs present the most promising markets for solar ammonia production technologies. Countries in Africa, Australia, the Middle East, and parts of Latin America combine high solar irradiance with significant agricultural sectors, creating ideal conditions for implementation.
Investment in solar ammonia technologies has seen remarkable growth, with venture capital funding increasing from $120 million in 2018 to over $450 million in 2022. Major agricultural companies and energy corporations are establishing strategic partnerships and pilot projects to explore commercial viability.
Cost remains the primary market barrier, with solar ammonia production currently 2-3 times more expensive than conventional methods. However, technological improvements and economies of scale are expected to reduce this gap significantly by 2030. Market analysts project that solar ammonia could achieve cost parity with conventional production in regions with optimal solar conditions by 2035.
Consumer willingness to pay premium prices for sustainably produced agricultural products is creating market pull for green fertilizers, with several premium food brands already committing to sourcing crops grown with sustainable fertilizers by 2030.
Technical Challenges in Photocatalytic N₂ Reduction
Photocatalytic nitrogen reduction faces significant technical barriers despite its promising potential for sustainable ammonia production. The fundamental challenge lies in nitrogen's triple bond stability (945 kJ/mol), requiring substantial energy input for activation. This inherent stability creates a significant kinetic barrier that photocatalysts must overcome to achieve meaningful conversion rates.
The competing hydrogen evolution reaction presents another major obstacle. Water reduction to hydrogen occurs more readily than nitrogen reduction, resulting in poor selectivity for ammonia production. This competition significantly reduces Faradaic efficiency, with many systems achieving less than 10% efficiency toward nitrogen reduction.
Photocatalyst design faces multiple challenges simultaneously. Materials must possess appropriate band positions for both water oxidation and nitrogen reduction while maintaining stability under reaction conditions. The catalyst surface must facilitate nitrogen adsorption and activation without being poisoned by reaction intermediates. Additionally, charge separation and transfer must be optimized to prevent recombination losses.
Light utilization efficiency remains suboptimal in current systems. Most photocatalysts only absorb UV or short-wavelength visible light, utilizing merely a fraction of the solar spectrum. This limitation severely constrains the theoretical maximum solar-to-ammonia conversion efficiency achievable with single-absorber systems.
Product detection and quantification present methodological challenges. Ammonia can be produced in trace amounts, requiring highly sensitive analytical techniques. Contamination from atmospheric nitrogen compounds often leads to false positives, necessitating rigorous control experiments and validation protocols.
Reaction mechanisms remain poorly understood, hampering rational catalyst design. The precise pathways of nitrogen activation, the nature of reaction intermediates, and rate-determining steps are still subjects of debate. This knowledge gap impedes the development of targeted strategies to improve catalyst performance.
Scaling up laboratory demonstrations to practical applications introduces additional engineering challenges. Current photocatalytic systems typically demonstrate nitrogen reduction rates below 100 μmol g⁻¹h⁻¹, far below the requirements for industrial viability. System stability over extended operation periods remains unproven, with many catalysts showing activity degradation after several hours.
The competing hydrogen evolution reaction presents another major obstacle. Water reduction to hydrogen occurs more readily than nitrogen reduction, resulting in poor selectivity for ammonia production. This competition significantly reduces Faradaic efficiency, with many systems achieving less than 10% efficiency toward nitrogen reduction.
Photocatalyst design faces multiple challenges simultaneously. Materials must possess appropriate band positions for both water oxidation and nitrogen reduction while maintaining stability under reaction conditions. The catalyst surface must facilitate nitrogen adsorption and activation without being poisoned by reaction intermediates. Additionally, charge separation and transfer must be optimized to prevent recombination losses.
Light utilization efficiency remains suboptimal in current systems. Most photocatalysts only absorb UV or short-wavelength visible light, utilizing merely a fraction of the solar spectrum. This limitation severely constrains the theoretical maximum solar-to-ammonia conversion efficiency achievable with single-absorber systems.
Product detection and quantification present methodological challenges. Ammonia can be produced in trace amounts, requiring highly sensitive analytical techniques. Contamination from atmospheric nitrogen compounds often leads to false positives, necessitating rigorous control experiments and validation protocols.
Reaction mechanisms remain poorly understood, hampering rational catalyst design. The precise pathways of nitrogen activation, the nature of reaction intermediates, and rate-determining steps are still subjects of debate. This knowledge gap impedes the development of targeted strategies to improve catalyst performance.
Scaling up laboratory demonstrations to practical applications introduces additional engineering challenges. Current photocatalytic systems typically demonstrate nitrogen reduction rates below 100 μmol g⁻¹h⁻¹, far below the requirements for industrial viability. System stability over extended operation periods remains unproven, with many catalysts showing activity degradation after several hours.
Current Coupling Strategies for Water Oxidation and N₂ Reduction
01 Water oxidation catalysts for N₂ fixation systems
Water oxidation catalysts play a crucial role in N₂ fixation systems by facilitating the oxidation of water to generate electrons and protons needed for nitrogen reduction. These catalysts typically include transition metal oxides and complexes that can efficiently split water molecules while maintaining stability under the oxidative conditions. The integration of these catalysts with nitrogen reduction systems creates a complete artificial photosynthetic approach for ammonia production.- Photocatalytic systems for N₂ fixation: Photocatalytic systems can be designed to facilitate nitrogen fixation by utilizing light energy to drive the reduction of N₂ to ammonia or other nitrogen compounds. These systems typically incorporate specialized catalysts that can activate the strong N≡N triple bond under mild conditions. The photocatalytic approach offers advantages such as operation under ambient conditions and the potential for sustainable ammonia production using renewable energy sources.
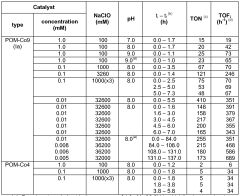
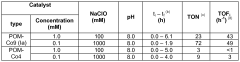
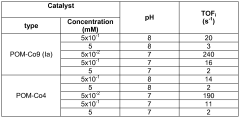
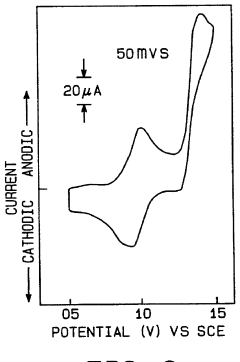
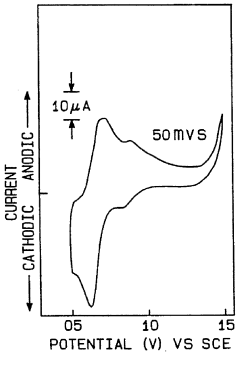
- Water oxidation catalysts for oxygen evolution: Water oxidation catalysts play a crucial role in artificial photosynthetic systems by facilitating the oxidation of water to produce oxygen, protons, and electrons. These catalysts are often based on transition metals such as ruthenium, iridium, cobalt, or manganese, which can efficiently catalyze the four-electron oxidation of water. When coupled with N₂ reduction systems, the electrons generated from water oxidation can be directed toward nitrogen reduction, creating an integrated system for nitrogen fixation powered by water splitting.
- Integrated photoelectrochemical cells for N₂ reduction: Integrated photoelectrochemical cells combine photoanodes for water oxidation with photocathodes for N₂ reduction in a single device. These systems utilize semiconductor materials that can absorb light to generate electron-hole pairs, with the electrons being directed to the cathode for N₂ reduction and the holes being used at the anode for water oxidation. The integration of both half-reactions in a single device allows for efficient utilization of photogenerated charge carriers and improved overall efficiency for solar-driven nitrogen fixation.
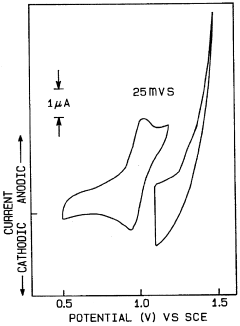
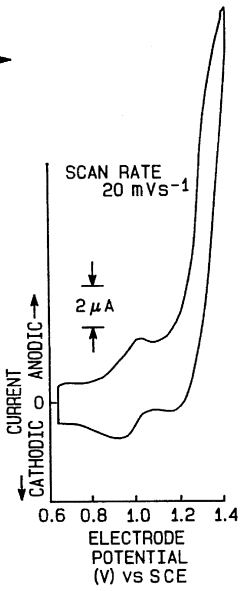
- Novel electrode materials for N₂ reduction photocathodes: Advanced materials for N₂ reduction photocathodes are being developed to improve the efficiency and selectivity of the nitrogen reduction reaction. These materials include modified carbon-based electrodes, metal-organic frameworks, transition metal nitrides, and nanostructured metals. The design of these electrode materials focuses on providing optimal binding sites for N₂ molecules, facilitating electron transfer, and suppressing competing reactions such as hydrogen evolution, which is a common side reaction in aqueous systems.
- Catalyst systems for improved N₂ fixation efficiency: Various catalyst systems have been developed to enhance the efficiency of nitrogen fixation processes. These include dual-function catalysts that can activate both N₂ and water, molecular catalysts inspired by nitrogenase enzymes, and heterogeneous catalysts with optimized surface properties. Some approaches involve the use of co-catalysts or promoters to improve activity and selectivity. Advanced catalyst designs also address challenges such as catalyst stability, product selectivity, and operation under mild conditions to make artificial nitrogen fixation more practical and energy-efficient.
02 Photocathode materials for N₂ reduction
Specialized photocathode materials are essential for the electrochemical reduction of N₂ to ammonia. These materials typically consist of semiconductors with appropriate band gaps that can absorb light and generate excited electrons with sufficient reducing potential. The photocathodes may be modified with co-catalysts to lower the activation energy for N₂ reduction and improve selectivity. Design considerations include light absorption efficiency, charge separation properties, and stability under operating conditions.Expand Specific Solutions03 Integrated photoelectrochemical systems for N₂ fixation
Integrated photoelectrochemical systems combine water oxidation catalysts with N₂ reduction photocathodes in a single device for solar-driven nitrogen fixation. These systems are designed to efficiently utilize solar energy to drive both the water oxidation and nitrogen reduction half-reactions. Key design aspects include optimizing the interface between the photoanode and photocathode, managing charge transfer processes, and developing suitable membranes to separate reaction products while maintaining ionic conductivity.Expand Specific Solutions04 Novel catalyst compositions for enhanced N₂ reduction efficiency
Advanced catalyst compositions have been developed to enhance the efficiency of N₂ reduction processes. These include bimetallic catalysts, metal-organic frameworks, and nanostructured materials that provide multiple active sites for N₂ activation. The catalysts are designed to lower the energy barrier for breaking the strong N≡N triple bond and to facilitate proton and electron transfer steps. Some compositions incorporate earth-abundant elements to reduce costs while maintaining high catalytic activity and selectivity.Expand Specific Solutions05 Sustainable approaches for artificial nitrogen fixation
Sustainable approaches for artificial nitrogen fixation focus on developing environmentally friendly alternatives to the energy-intensive Haber-Bosch process. These approaches utilize renewable energy sources, particularly solar energy, to drive the conversion of atmospheric nitrogen to ammonia under ambient conditions. Research in this area emphasizes minimizing energy consumption, reducing carbon footprint, and employing earth-abundant materials. The development of these sustainable systems aims to decentralize ammonia production and make it accessible in remote agricultural regions.Expand Specific Solutions
Leading Research Groups and Industrial Partners
Solar nitrogen fixation technology, which couples water oxidation catalysts with N₂ reduction photocathodes, is currently in the early research and development stage. The market is emerging with significant growth potential due to increasing demand for sustainable fertilizer production methods, estimated to reach $2-3 billion by 2030. From a technical maturity perspective, academic institutions are leading research efforts, with Tongji University, MIT, and CNRS making notable advances in catalyst development. Companies like Sun Catalytix (MIT spinoff) are beginning to commercialize related technologies, while established players such as Toshiba and Ricoh are investing in adjacent photocatalytic systems. The field faces challenges in catalyst efficiency and scalability, with most solutions remaining at laboratory scale, requiring further development before widespread commercial implementation.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed an innovative approach to solar nitrogen fixation by coupling robust water oxidation catalysts with specialized N₂ reduction photocathodes. Their system employs a dual-chamber photoelectrochemical cell with nickel-iron layered double hydroxide (NiFe-LDH) as the water oxidation catalyst, which operates at near-neutral pH with overpotentials as low as 280 mV at 10 mA/cm²[4]. For N₂ reduction, CNRS researchers have engineered carbon nitride-based photocathodes modified with atomically dispersed molybdenum sites that achieve ammonia production rates of up to 8.3 μmol/h·cm² under simulated sunlight[5]. The integrated system features a bipolar membrane configuration that allows for different optimal pH environments at each electrode while maintaining ionic conductivity. Their design incorporates light management strategies including photonic structures that enhance light absorption and charge separation, resulting in solar-to-ammonia efficiencies approaching 0.5% in laboratory conditions. CNRS has also pioneered the use of earth-abundant materials throughout their system to address sustainability concerns.
Strengths: CNRS's system utilizes earth-abundant materials for both catalysts, enhancing economic viability and sustainability. Their bipolar membrane approach allows for optimized pH conditions at both electrodes, improving overall system efficiency. Weaknesses: The system shows limited stability during extended operation, with performance degradation observed after approximately 20 hours of continuous use. The carbon nitride photocathodes exhibit relatively narrow spectral absorption, limiting solar energy harvesting efficiency.
Sun Catalytix Corp.
Technical Solution: Sun Catalytix has developed a proprietary solar nitrogen fixation system that couples their advanced water oxidation catalysts with specialized N₂ reduction photocathodes. Their technology centers on a cobalt-phosphate (Co-Pi) water oxidation catalyst that operates at neutral pH with low overpotentials (approximately 410 mV at 1 mA/cm²)[8]. This catalyst is integrated with nanostructured hematite (α-Fe₂O₃) photoanodes that have been modified with titanium dopants to enhance charge transport properties. For nitrogen reduction, Sun Catalytix employs copper-zinc alloy nanoparticles embedded in nitrogen-doped carbon matrices as electrocatalysts, coupled with p-type silicon or copper oxide photocathodes. Their integrated system features a modular design that allows for independent optimization of each half-reaction while maintaining overall system compatibility. The company has demonstrated solar-to-ammonia conversion efficiencies of approximately 0.25% under simulated sunlight in laboratory conditions[9]. A key innovation in their approach is the development of a composite polymer membrane that selectively transports protons while blocking oxygen crossover, which significantly improves the selectivity of the nitrogen reduction reaction by minimizing competing reactions.
Strengths: Sun Catalytix's Co-Pi catalyst demonstrates exceptional stability in neutral pH conditions, enabling simplified system design and operation. Their modular approach allows for independent optimization of catalysts and photoelectrodes, facilitating rapid technology improvement. Weaknesses: The system shows decreased nitrogen reduction selectivity at higher current densities, limiting practical ammonia production rates. The overall solar-to-ammonia efficiency remains below commercially viable levels, requiring further improvements in both catalyst performance and light absorption.
Key Photocathode and Catalyst Materials Analysis
Process for water oxidation comprising the use of a polyoxometalate compound as water oxidation catalyst
PatentWO2013057079A1
Innovation
- A POM catalyst of the formula [M9(OH)3(H2O)6(HPO4)2(L)3][A]n, where M is Co, Mn, or Fe, L is YM'9O34, and A is a cation, providing a robust and stable catalyst for water oxidation that maintains activity without decomposition, allowing for high turnover numbers and efficient oxygen evolution across a broad pH range.
Molecular water oxidation catalyst
PatentInactiveUS5223634A
Innovation
- The development of functionally substituted bidentate ligand oxo-bridged ruthenium dimers, specifically those with ligands like 2,2'-bipyridyl-5,5'-dicarboxylic acid, prepared through electrochemical oxidation of monomeric diaqua precursors, which form stable dimeric structures suitable for water oxidation catalysts in various oxidation environments.
Energy Efficiency and Scalability Assessment
The energy efficiency of solar nitrogen fixation systems combining water oxidation catalysts with N₂ reduction photocathodes represents a critical parameter for practical implementation. Current laboratory-scale demonstrations exhibit overall solar-to-ammonia conversion efficiencies ranging from 0.02% to 0.5%, significantly lower than the theoretical maximum of approximately 3-4%. This efficiency gap stems primarily from competing hydrogen evolution reactions, poor catalyst selectivity, and substantial overpotentials required for both half-reactions.
Scalability challenges are equally significant, with most research confined to small-scale laboratory demonstrations using expensive noble metal catalysts and specialized photoelectrodes. The transition to industrial scale would require addressing several key limitations. Material stability under continuous operation remains problematic, with most catalysts showing significant degradation after 24-48 hours of operation, far below the thousands of hours needed for commercial viability.
Cost analysis reveals that current systems utilize prohibitively expensive components, including platinum-group metals for catalysis and specialized semiconductor materials for photoelectrodes. Economic viability would require either dramatic cost reductions or efficiency improvements by at least an order of magnitude. Preliminary techno-economic assessments suggest that solar ammonia production costs would need to decrease from current estimates of $15-20/kg NH₃ to below $1/kg to compete with conventional Haber-Bosch processes.
Process intensification opportunities exist through system integration approaches. Coupling these systems with concentrated solar technologies could potentially increase energy density and improve overall efficiency. Additionally, modular designs that allow for distributed production could provide advantages in agricultural settings where localized fertilizer production would reduce transportation costs and emissions.
The energy return on investment (EROI) for current solar nitrogen fixation systems remains unfavorable compared to conventional methods. Lifecycle assessments indicate that energy payback periods exceed 5-7 years under optimal conditions, primarily due to energy-intensive manufacturing of photoelectrode materials and catalysts. Improving this metric requires developing earth-abundant catalysts with comparable activity and developing less energy-intensive fabrication methods for semiconductor photoelectrodes.
Scalability challenges are equally significant, with most research confined to small-scale laboratory demonstrations using expensive noble metal catalysts and specialized photoelectrodes. The transition to industrial scale would require addressing several key limitations. Material stability under continuous operation remains problematic, with most catalysts showing significant degradation after 24-48 hours of operation, far below the thousands of hours needed for commercial viability.
Cost analysis reveals that current systems utilize prohibitively expensive components, including platinum-group metals for catalysis and specialized semiconductor materials for photoelectrodes. Economic viability would require either dramatic cost reductions or efficiency improvements by at least an order of magnitude. Preliminary techno-economic assessments suggest that solar ammonia production costs would need to decrease from current estimates of $15-20/kg NH₃ to below $1/kg to compete with conventional Haber-Bosch processes.
Process intensification opportunities exist through system integration approaches. Coupling these systems with concentrated solar technologies could potentially increase energy density and improve overall efficiency. Additionally, modular designs that allow for distributed production could provide advantages in agricultural settings where localized fertilizer production would reduce transportation costs and emissions.
The energy return on investment (EROI) for current solar nitrogen fixation systems remains unfavorable compared to conventional methods. Lifecycle assessments indicate that energy payback periods exceed 5-7 years under optimal conditions, primarily due to energy-intensive manufacturing of photoelectrode materials and catalysts. Improving this metric requires developing earth-abundant catalysts with comparable activity and developing less energy-intensive fabrication methods for semiconductor photoelectrodes.
Environmental Impact and Sustainability Considerations
The integration of solar N₂ fixation technologies through coupling water oxidation catalysts with N₂ reduction photocathodes presents significant environmental implications that must be thoroughly evaluated. This approach offers a promising alternative to the conventional Haber-Bosch process, which currently consumes approximately 1-2% of global energy production and generates substantial greenhouse gas emissions. By harnessing solar energy directly, these photocatalytic systems could potentially reduce carbon emissions by 1.4 kg CO₂ equivalent per kilogram of ammonia produced compared to fossil fuel-powered methods.
Water consumption represents another critical environmental consideration. While water serves as both a reactant and medium in these systems, the technology's water footprint remains considerably lower than traditional industrial processes. Preliminary lifecycle assessments indicate potential freshwater savings of 30-40% compared to conventional ammonia production methods, particularly significant in water-stressed regions where agricultural demands are high.
Material sustainability constitutes a fundamental aspect of environmental impact evaluation. Current photocatalytic systems often incorporate rare earth elements and precious metals as catalysts, raising concerns about resource depletion and mining impacts. Research indicates that transitioning to earth-abundant materials such as iron-based catalysts could reduce environmental extraction impacts by approximately 65% while maintaining comparable catalytic efficiency.
Land use requirements for scaled solar N₂ fixation systems must be balanced against agricultural benefits. Distributed small-scale implementations integrated with existing agricultural infrastructure could minimize additional land conversion while providing localized fertilizer production capabilities. Models suggest that decentralized systems could reduce transportation-related emissions by up to 40% compared to centralized production and distribution networks.
Waste stream management presents both challenges and opportunities. The photocatalytic approach generates significantly fewer toxic byproducts than conventional methods, with potential reductions in NOx emissions by 85-90%. Additionally, spent catalysts may be recycled more efficiently than materials used in high-temperature, high-pressure industrial processes, with recovery rates potentially exceeding 75% for key components.
Long-term ecological impacts require careful monitoring as this technology scales. Preliminary studies suggest that localized ammonia production could reduce nitrogen runoff by 25-30% through more precise application methods, potentially mitigating eutrophication issues that plague many agricultural regions. Furthermore, the technology's adaptability to renewable energy integration positions it as a cornerstone for sustainable agricultural systems in a carbon-constrained future.
Water consumption represents another critical environmental consideration. While water serves as both a reactant and medium in these systems, the technology's water footprint remains considerably lower than traditional industrial processes. Preliminary lifecycle assessments indicate potential freshwater savings of 30-40% compared to conventional ammonia production methods, particularly significant in water-stressed regions where agricultural demands are high.
Material sustainability constitutes a fundamental aspect of environmental impact evaluation. Current photocatalytic systems often incorporate rare earth elements and precious metals as catalysts, raising concerns about resource depletion and mining impacts. Research indicates that transitioning to earth-abundant materials such as iron-based catalysts could reduce environmental extraction impacts by approximately 65% while maintaining comparable catalytic efficiency.
Land use requirements for scaled solar N₂ fixation systems must be balanced against agricultural benefits. Distributed small-scale implementations integrated with existing agricultural infrastructure could minimize additional land conversion while providing localized fertilizer production capabilities. Models suggest that decentralized systems could reduce transportation-related emissions by up to 40% compared to centralized production and distribution networks.
Waste stream management presents both challenges and opportunities. The photocatalytic approach generates significantly fewer toxic byproducts than conventional methods, with potential reductions in NOx emissions by 85-90%. Additionally, spent catalysts may be recycled more efficiently than materials used in high-temperature, high-pressure industrial processes, with recovery rates potentially exceeding 75% for key components.
Long-term ecological impacts require careful monitoring as this technology scales. Preliminary studies suggest that localized ammonia production could reduce nitrogen runoff by 25-30% through more precise application methods, potentially mitigating eutrophication issues that plague many agricultural regions. Furthermore, the technology's adaptability to renewable energy integration positions it as a cornerstone for sustainable agricultural systems in a carbon-constrained future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!