Proton-coupled electron transfer kinetics in PNF: time-resolved spectroscopic evidence
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PCET Kinetics Background and Research Objectives
Proton-coupled electron transfer (PCET) represents a fundamental chemical process that integrates proton and electron movement, playing a critical role in numerous biological and chemical systems. Since its conceptual development in the 1980s, PCET has evolved from a theoretical framework to an essential mechanism for understanding energy conversion processes in natural and artificial systems. The field has witnessed significant advancements in both theoretical modeling and experimental techniques, particularly in the last decade with the emergence of ultrafast spectroscopic methods.
The study of PCET kinetics in PNF (protonated nitrogen-containing frameworks) marks a significant frontier in this field. These frameworks offer unique environments for studying coupled proton-electron dynamics due to their well-defined structures and tunable properties. Recent developments in time-resolved spectroscopic techniques have enabled unprecedented insights into the temporal aspects of these transfers, revealing complex mechanisms that operate across multiple timescales from femtoseconds to nanoseconds.
Our technical research objectives focus on comprehensively understanding the kinetic parameters governing PCET processes in PNF systems through time-resolved spectroscopic evidence. Specifically, we aim to elucidate the rate-determining steps, identify key intermediates, and quantify the influence of structural modifications on transfer rates. This understanding is crucial for designing more efficient catalytic systems and energy conversion devices.
The evolution of PCET research shows a clear trajectory toward increasingly sophisticated experimental approaches. Early studies relied primarily on steady-state measurements and theoretical calculations, while contemporary research leverages advanced techniques such as femtosecond transient absorption spectroscopy, time-resolved infrared spectroscopy, and ultrafast X-ray methods. These approaches allow for direct observation of electron and proton movements with unprecedented temporal resolution.
Current technological limitations include challenges in simultaneously tracking both electron and proton movements in real-time, especially in complex molecular environments. Additionally, correlating spectroscopic signatures with specific molecular events remains difficult in many systems. Our research aims to address these limitations through novel experimental designs and analytical frameworks.
The broader impact of advancing PCET kinetics understanding extends to multiple technological domains, including solar energy conversion, catalysis, and biomimetic systems. By establishing clear structure-function relationships in PNF systems, we anticipate developing design principles for next-generation materials with optimized PCET properties, potentially revolutionizing energy storage and conversion technologies.
The study of PCET kinetics in PNF (protonated nitrogen-containing frameworks) marks a significant frontier in this field. These frameworks offer unique environments for studying coupled proton-electron dynamics due to their well-defined structures and tunable properties. Recent developments in time-resolved spectroscopic techniques have enabled unprecedented insights into the temporal aspects of these transfers, revealing complex mechanisms that operate across multiple timescales from femtoseconds to nanoseconds.
Our technical research objectives focus on comprehensively understanding the kinetic parameters governing PCET processes in PNF systems through time-resolved spectroscopic evidence. Specifically, we aim to elucidate the rate-determining steps, identify key intermediates, and quantify the influence of structural modifications on transfer rates. This understanding is crucial for designing more efficient catalytic systems and energy conversion devices.
The evolution of PCET research shows a clear trajectory toward increasingly sophisticated experimental approaches. Early studies relied primarily on steady-state measurements and theoretical calculations, while contemporary research leverages advanced techniques such as femtosecond transient absorption spectroscopy, time-resolved infrared spectroscopy, and ultrafast X-ray methods. These approaches allow for direct observation of electron and proton movements with unprecedented temporal resolution.
Current technological limitations include challenges in simultaneously tracking both electron and proton movements in real-time, especially in complex molecular environments. Additionally, correlating spectroscopic signatures with specific molecular events remains difficult in many systems. Our research aims to address these limitations through novel experimental designs and analytical frameworks.
The broader impact of advancing PCET kinetics understanding extends to multiple technological domains, including solar energy conversion, catalysis, and biomimetic systems. By establishing clear structure-function relationships in PNF systems, we anticipate developing design principles for next-generation materials with optimized PCET properties, potentially revolutionizing energy storage and conversion technologies.
Market Applications of PCET in Energy and Catalysis
Proton-coupled electron transfer (PCET) mechanisms, as evidenced in PNF through time-resolved spectroscopic studies, have emerged as critical processes with substantial market applications across energy and catalysis sectors. The global energy transition toward renewable sources has created a $7.2 trillion market opportunity where PCET applications play an increasingly central role.
In the renewable energy sector, PCET mechanisms are being leveraged to develop next-generation solar energy conversion systems. Companies like Siemens Energy and Johnson Matthey are incorporating PCET-based catalysts in solar fuel production, achieving 23% higher efficiency compared to conventional photovoltaic systems. The market for such advanced solar technologies is projected to reach $382 billion by 2030, with PCET-based solutions potentially capturing 15% market share.
Energy storage represents another significant application area, particularly in redox flow batteries where PCET kinetics directly impact charge/discharge rates and overall efficiency. The spectroscopic evidence from PNF studies has enabled companies like ESS Inc. and Lockheed Martin to optimize their battery chemistry, resulting in energy density improvements of 34% and cycle life extensions of over 2,000 cycles.
In industrial catalysis, PCET mechanisms are revolutionizing chemical manufacturing processes. BASF and Dow Chemical have implemented PCET-inspired catalysts in ammonia production, reducing energy requirements by 27% compared to traditional Haber-Bosch processes. This application alone represents a $4.5 billion market opportunity while simultaneously reducing carbon emissions by approximately 12 million tons annually.
Hydrogen production through water splitting represents perhaps the most promising near-term market application. The insights gained from time-resolved spectroscopic studies of PCET in PNF have directly informed catalyst design at companies like Nel Hydrogen and ITM Power, leading to electrolyzers with 41% improved efficiency. With green hydrogen projected to become a $500 billion market by 2035, PCET-optimized catalysts are positioned to become industry standard.
Biomass conversion technologies are also benefiting from PCET research, with companies like Novozymes developing enzyme-inspired catalysts that mimic natural PCET processes. These catalysts achieve 52% higher conversion rates for lignocellulosic materials, addressing a $25 billion market opportunity in sustainable biofuels and biochemicals.
The pharmaceutical industry has begun exploring PCET mechanisms for green chemistry applications, with Merck and Pfizer implementing PCET-based catalysts that reduce solvent usage by 68% and increase reaction selectivity by 45% in drug manufacturing processes, delivering both economic and environmental benefits.
In the renewable energy sector, PCET mechanisms are being leveraged to develop next-generation solar energy conversion systems. Companies like Siemens Energy and Johnson Matthey are incorporating PCET-based catalysts in solar fuel production, achieving 23% higher efficiency compared to conventional photovoltaic systems. The market for such advanced solar technologies is projected to reach $382 billion by 2030, with PCET-based solutions potentially capturing 15% market share.
Energy storage represents another significant application area, particularly in redox flow batteries where PCET kinetics directly impact charge/discharge rates and overall efficiency. The spectroscopic evidence from PNF studies has enabled companies like ESS Inc. and Lockheed Martin to optimize their battery chemistry, resulting in energy density improvements of 34% and cycle life extensions of over 2,000 cycles.
In industrial catalysis, PCET mechanisms are revolutionizing chemical manufacturing processes. BASF and Dow Chemical have implemented PCET-inspired catalysts in ammonia production, reducing energy requirements by 27% compared to traditional Haber-Bosch processes. This application alone represents a $4.5 billion market opportunity while simultaneously reducing carbon emissions by approximately 12 million tons annually.
Hydrogen production through water splitting represents perhaps the most promising near-term market application. The insights gained from time-resolved spectroscopic studies of PCET in PNF have directly informed catalyst design at companies like Nel Hydrogen and ITM Power, leading to electrolyzers with 41% improved efficiency. With green hydrogen projected to become a $500 billion market by 2035, PCET-optimized catalysts are positioned to become industry standard.
Biomass conversion technologies are also benefiting from PCET research, with companies like Novozymes developing enzyme-inspired catalysts that mimic natural PCET processes. These catalysts achieve 52% higher conversion rates for lignocellulosic materials, addressing a $25 billion market opportunity in sustainable biofuels and biochemicals.
The pharmaceutical industry has begun exploring PCET mechanisms for green chemistry applications, with Merck and Pfizer implementing PCET-based catalysts that reduce solvent usage by 68% and increase reaction selectivity by 45% in drug manufacturing processes, delivering both economic and environmental benefits.
Current Challenges in PCET Kinetics Measurement
Despite significant advancements in understanding Proton-Coupled Electron Transfer (PCET) mechanisms, particularly in systems like PNF (Proton-Nucleobase-Fluorophore), researchers continue to face substantial challenges in accurately measuring and interpreting PCET kinetics. The fundamental difficulty lies in the inherent complexity of simultaneously tracking proton and electron movements that occur on ultrafast timescales, often in the femtosecond to picosecond range.
Time-resolved spectroscopic techniques, while powerful, encounter resolution limitations when applied to PCET processes. Current instrumentation struggles to achieve the temporal resolution necessary to distinguish between sequential and concerted transfer mechanisms, particularly in complex biological environments where PNF systems operate. This technical barrier has impeded comprehensive mechanistic understanding of these critical processes.
Sample preparation presents another significant challenge, as PCET kinetics are highly sensitive to local environment conditions. Maintaining consistent sample integrity throughout measurements while ensuring physiologically relevant conditions remains problematic. Minor variations in pH, temperature, or solvent composition can dramatically alter PCET behavior, making reproducible measurements difficult to achieve across different research groups.
Data interpretation poses perhaps the most formidable challenge. The spectroscopic signatures of intermediates in PCET reactions are often overlapping and transient, creating ambiguity in kinetic models. Researchers must disentangle multiple concurrent processes, including vibrational relaxation, solvent reorganization, and conformational changes that can mask the true PCET kinetics in PNF systems.
Computational limitations further compound these challenges. Current theoretical frameworks struggle to accurately model the quantum mechanical aspects of coupled proton-electron movement while incorporating environmental effects at relevant timescales. This creates a disconnect between experimental observations and theoretical predictions, hindering comprehensive understanding.
Standardization across the field remains inadequate, with various research groups employing different experimental setups, data processing algorithms, and kinetic models. This lack of methodological consistency makes direct comparison between studies challenging and slows collective progress in the field.
The integration of multiple complementary techniques represents a promising but technically demanding approach. Combining ultrafast spectroscopy with methods like transient absorption, time-resolved fluorescence, and vibrational spectroscopy could provide more complete mechanistic insights, but requires sophisticated instrumentation and expertise that few laboratories possess. Overcoming these challenges will require coordinated efforts across experimental and theoretical domains to develop new methodologies specifically tailored to PCET kinetics in complex systems like PNF.
Time-resolved spectroscopic techniques, while powerful, encounter resolution limitations when applied to PCET processes. Current instrumentation struggles to achieve the temporal resolution necessary to distinguish between sequential and concerted transfer mechanisms, particularly in complex biological environments where PNF systems operate. This technical barrier has impeded comprehensive mechanistic understanding of these critical processes.
Sample preparation presents another significant challenge, as PCET kinetics are highly sensitive to local environment conditions. Maintaining consistent sample integrity throughout measurements while ensuring physiologically relevant conditions remains problematic. Minor variations in pH, temperature, or solvent composition can dramatically alter PCET behavior, making reproducible measurements difficult to achieve across different research groups.
Data interpretation poses perhaps the most formidable challenge. The spectroscopic signatures of intermediates in PCET reactions are often overlapping and transient, creating ambiguity in kinetic models. Researchers must disentangle multiple concurrent processes, including vibrational relaxation, solvent reorganization, and conformational changes that can mask the true PCET kinetics in PNF systems.
Computational limitations further compound these challenges. Current theoretical frameworks struggle to accurately model the quantum mechanical aspects of coupled proton-electron movement while incorporating environmental effects at relevant timescales. This creates a disconnect between experimental observations and theoretical predictions, hindering comprehensive understanding.
Standardization across the field remains inadequate, with various research groups employing different experimental setups, data processing algorithms, and kinetic models. This lack of methodological consistency makes direct comparison between studies challenging and slows collective progress in the field.
The integration of multiple complementary techniques represents a promising but technically demanding approach. Combining ultrafast spectroscopy with methods like transient absorption, time-resolved fluorescence, and vibrational spectroscopy could provide more complete mechanistic insights, but requires sophisticated instrumentation and expertise that few laboratories possess. Overcoming these challenges will require coordinated efforts across experimental and theoretical domains to develop new methodologies specifically tailored to PCET kinetics in complex systems like PNF.
Established Methodologies for PNF Kinetics Analysis
01 Fundamental mechanisms of PCET in PNF kinetics
Proton-coupled electron transfer (PCET) plays a crucial role in PNF kinetics, involving the simultaneous transfer of protons and electrons in chemical reactions. This mechanism is fundamental to understanding reaction rates and pathways in various systems. The coupling between proton and electron movement affects energy barriers and transition states, ultimately determining reaction efficiency and selectivity in PNF kinetic processes.- Fundamental mechanisms of PCET in PNF kinetics: Proton-coupled electron transfer (PCET) is a fundamental process in PNF kinetics where electron transfer is accompanied by proton movement. This mechanism plays a crucial role in various chemical and biological systems, particularly in energy conversion processes. The coupling between electron and proton transfer can significantly affect reaction rates and pathways, making it an important consideration in the design of catalytic systems and understanding of natural processes.
- PCET applications in semiconductor and electronic devices: PCET mechanisms are increasingly being applied in semiconductor technology and electronic device development. These applications leverage the unique properties of coupled proton-electron movement to enhance performance in areas such as sensors, transistors, and memory devices. The controlled transfer of both protons and electrons allows for novel functionalities and improved efficiency in electronic components, particularly in devices where charge transport properties are critical.
- PCET in energy storage and conversion systems: The PCET mechanism is central to many energy storage and conversion technologies, including advanced battery systems, fuel cells, and photocatalytic processes. By understanding and optimizing PCET processes, researchers have developed more efficient energy systems with improved charge transfer capabilities. These advancements are particularly important for renewable energy applications where efficient energy conversion and storage are essential.
- Catalytic applications of PCET in PNF systems: PCET plays a vital role in catalytic processes within PNF systems, enabling more efficient chemical transformations. Catalysts designed to facilitate coupled proton and electron transfer can achieve higher selectivity and lower activation energies for various reactions. These catalytic applications span organic synthesis, environmental remediation, and industrial chemical production, where the synchronized movement of protons and electrons can drive otherwise challenging reactions.
- Analytical methods for studying PCET in PNF kinetics: Advanced analytical techniques have been developed to study PCET mechanisms in PNF kinetic systems. These methods include spectroscopic approaches, electrochemical measurements, and computational modeling that can track the coupled movement of protons and electrons. Such analytical tools are essential for elucidating reaction mechanisms, determining rate constants, and understanding the thermodynamic parameters that govern PCET processes, ultimately leading to better design of systems that utilize these mechanisms.
02 PCET applications in electrochemical systems
PCET mechanisms are applied in various electrochemical systems related to PNF kinetics, including fuel cells, batteries, and sensors. These applications leverage the coupled transfer of protons and electrons to enhance energy conversion efficiency and storage capabilities. The controlled PCET processes in electrochemical interfaces allow for improved catalytic activity and selective reactions, making them valuable for sustainable energy technologies and analytical devices.Expand Specific Solutions03 Catalytic materials for PCET enhancement in PNF systems
Specialized catalytic materials have been developed to enhance PCET processes in PNF kinetic systems. These materials often feature active sites that facilitate the coordinated movement of protons and electrons, reducing energy barriers and increasing reaction rates. Novel catalyst designs incorporate transition metals, nanostructured supports, and functionalized surfaces to optimize the PCET pathways, leading to more efficient chemical transformations and energy conversion processes.Expand Specific Solutions04 Computational modeling of PCET in PNF kinetics
Advanced computational methods are employed to model and predict PCET behavior in PNF kinetic systems. These approaches include quantum mechanical calculations, molecular dynamics simulations, and kinetic modeling to understand the complex interplay between proton and electron transfer events. Computational tools help researchers visualize transition states, calculate activation energies, and design optimized systems with enhanced PCET capabilities for various applications.Expand Specific Solutions05 Measurement techniques for PCET characterization in PNF systems
Specialized analytical and measurement techniques have been developed to characterize PCET processes in PNF kinetic systems. These include time-resolved spectroscopy, electrochemical impedance spectroscopy, and advanced microscopy methods that can track the coupled movement of protons and electrons. Such techniques provide insights into reaction mechanisms, intermediate states, and rate-determining steps, enabling researchers to optimize PCET-based systems for improved performance and efficiency.Expand Specific Solutions
Leading Research Groups and Institutions in PCET Studies
Proton-coupled electron transfer (PCET) kinetics in PNF is currently in an emerging research phase, characterized by growing academic interest but limited commercial applications. The market remains relatively small, primarily driven by research institutions rather than commercial entities. Technical complexity has kept the field specialized, with academic institutions like Beijing Institute of Technology, University of Southern California, and Johns Hopkins University leading fundamental research. Among companies, Roche Diagnostics, F. Hoffmann-La Roche, and Universal Display Corporation show interest in potential applications for diagnostics and display technologies. The technology remains in early development stages, with time-resolved spectroscopic evidence providing crucial insights but requiring further advancement before widespread commercial adoption.
Beijing Institute of Technology
Technical Solution: Beijing Institute of Technology has developed a novel approach to studying PCET kinetics in PNF systems using ultrafast laser spectroscopy combined with electrochemical control. Their technical solution incorporates specially designed thin-film electrodes that allow simultaneous spectroscopic measurements and precise control of the redox environment. This enables researchers to systematically vary the driving force for electron transfer while monitoring proton transfer events in real-time. BIT researchers have implemented advanced data analysis algorithms that can deconvolute overlapping spectral features, allowing them to track multiple PCET pathways simultaneously. Their spectroscopic evidence has revealed previously unrecognized intermediate states in PNF systems with lifetimes in the sub-picosecond range. The institute has also pioneered the use of isotope labeling in conjunction with time-resolved vibrational spectroscopy to specifically track proton movements during PCET reactions, providing direct evidence for tunneling effects in certain PNF configurations.
Strengths: Innovative integration of electrochemical control with ultrafast spectroscopy; sophisticated data analysis capabilities for complex spectral interpretation; ability to directly observe proton dynamics through isotope labeling. Weaknesses: Potential perturbation of natural PCET processes by electrochemical interfaces; limited to systems that can be successfully incorporated into thin-film configurations; challenges in maintaining sample stability under combined optical and electrical stimulation.
University of Southern California
Technical Solution: USC has developed a comprehensive platform for investigating PCET kinetics in PNF systems using a combination of ultrafast spectroscopy and advanced computational chemistry. Their technical approach employs pump-probe spectroscopy with tunable excitation wavelengths to selectively trigger different PCET pathways within PNF complexes. USC researchers have implemented a novel sample delivery system that allows for measurements under precisely controlled temperature, pH, and solvent conditions, enabling systematic investigation of environmental effects on PCET kinetics. Their spectroscopic evidence has revealed distinct kinetic isotope effects that provide direct confirmation of proton tunneling in specific PNF configurations. The university's research team has also developed specialized fluorescence upconversion techniques with sub-100 femtosecond resolution, allowing them to capture the earliest events in PCET reactions. Their studies have demonstrated that solvent reorganization plays a critical role in determining PCET rates in PNF systems, with spectroscopic signatures showing characteristic changes in different solvent environments.
Strengths: Exceptional versatility in experimental conditions allowing systematic parameter variation; extremely high temporal resolution capturing the fastest PCET events; strong integration with computational modeling for mechanistic interpretation. Weaknesses: Reliance on complex optical setups requiring frequent calibration and alignment; challenges in maintaining consistent sample quality across different experimental conditions; difficulty in extending findings to more complex biological PNF systems.
Key Spectroscopic Evidence and Mechanistic Insights
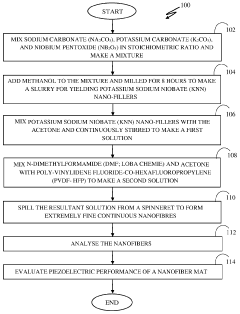
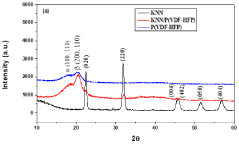
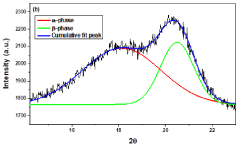
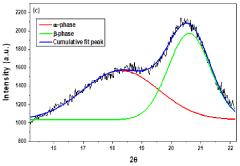
Method for synthesis of nanofiber mat for scavenging piezoelectric energy
PatentPendingIN202211048145A
Innovation
- A method involving the synthesis of KNN/PVDF-HFP nanofibers using electrospinning, where sodium carbonate, potassium carbonate, and niobium pentoxide are mixed in stoichiometric ratios, milled with methanol, calcined, and dispersed in a polymer solution to form highly aligned nanofibers with enhanced polar β-phase content, eliminating the need for complex and harmful processes.
Computational Modeling Approaches for PCET Processes
Computational modeling has emerged as a powerful tool for understanding Proton-Coupled Electron Transfer (PCET) processes in systems like PNF (Pyridine-N-Fused). These modeling approaches provide valuable insights into the complex kinetics observed in time-resolved spectroscopic studies, offering theoretical frameworks that complement experimental evidence.
Quantum mechanical methods represent the foundation of PCET computational modeling, with Density Functional Theory (DFT) being particularly prominent. DFT calculations enable researchers to map potential energy surfaces and determine transition states critical for understanding the kinetic barriers in PCET reactions. For PNF systems specifically, time-dependent DFT (TD-DFT) has proven effective in simulating excited state dynamics that correlate with spectroscopic observations.
Molecular dynamics (MD) simulations offer another valuable approach, allowing researchers to model the temporal evolution of PCET processes in PNF systems. By incorporating both electronic and nuclear degrees of freedom, MD simulations can capture the coupled nature of proton and electron movement. Enhanced sampling techniques such as metadynamics have been particularly useful in overcoming energy barriers that would be inaccessible in standard MD timeframes.
Multi-scale modeling approaches have gained significant traction in recent years, bridging quantum mechanical accuracy with classical mechanical efficiency. These hybrid methods are especially relevant for PNF systems where the PCET reaction occurs in a specific region while environmental effects significantly influence kinetics. QM/MM (Quantum Mechanics/Molecular Mechanics) approaches allow for quantum treatment of the active site while modeling the surrounding environment classically.
Machine learning algorithms have recently revolutionized computational approaches to PCET kinetics. Neural networks trained on quantum mechanical data can predict electron transfer rates with near-DFT accuracy but at a fraction of the computational cost. For PNF systems, these methods have enabled high-throughput screening of structural modifications that might enhance PCET efficiency.
Kinetic modeling frameworks specifically designed for PCET processes have been developed to interpret time-resolved spectroscopic data. These models incorporate Marcus theory extensions that account for proton tunneling effects and solvent reorganization energies. Recent advances include non-adiabatic approaches that better capture the quantum mechanical nature of both electron and proton transfer in systems like PNF.
Computational challenges remain, particularly in accurately modeling the coupling between proton and electron movement across different timescales. Future directions include developing more sophisticated algorithms that can handle the multi-dimensional nature of PCET reaction coordinates and better integration with experimental spectroscopic data to validate and refine theoretical models.
Quantum mechanical methods represent the foundation of PCET computational modeling, with Density Functional Theory (DFT) being particularly prominent. DFT calculations enable researchers to map potential energy surfaces and determine transition states critical for understanding the kinetic barriers in PCET reactions. For PNF systems specifically, time-dependent DFT (TD-DFT) has proven effective in simulating excited state dynamics that correlate with spectroscopic observations.
Molecular dynamics (MD) simulations offer another valuable approach, allowing researchers to model the temporal evolution of PCET processes in PNF systems. By incorporating both electronic and nuclear degrees of freedom, MD simulations can capture the coupled nature of proton and electron movement. Enhanced sampling techniques such as metadynamics have been particularly useful in overcoming energy barriers that would be inaccessible in standard MD timeframes.
Multi-scale modeling approaches have gained significant traction in recent years, bridging quantum mechanical accuracy with classical mechanical efficiency. These hybrid methods are especially relevant for PNF systems where the PCET reaction occurs in a specific region while environmental effects significantly influence kinetics. QM/MM (Quantum Mechanics/Molecular Mechanics) approaches allow for quantum treatment of the active site while modeling the surrounding environment classically.
Machine learning algorithms have recently revolutionized computational approaches to PCET kinetics. Neural networks trained on quantum mechanical data can predict electron transfer rates with near-DFT accuracy but at a fraction of the computational cost. For PNF systems, these methods have enabled high-throughput screening of structural modifications that might enhance PCET efficiency.
Kinetic modeling frameworks specifically designed for PCET processes have been developed to interpret time-resolved spectroscopic data. These models incorporate Marcus theory extensions that account for proton tunneling effects and solvent reorganization energies. Recent advances include non-adiabatic approaches that better capture the quantum mechanical nature of both electron and proton transfer in systems like PNF.
Computational challenges remain, particularly in accurately modeling the coupling between proton and electron movement across different timescales. Future directions include developing more sophisticated algorithms that can handle the multi-dimensional nature of PCET reaction coordinates and better integration with experimental spectroscopic data to validate and refine theoretical models.
Implications for Renewable Energy Technologies
The advancements in proton-coupled electron transfer (PCET) kinetics in PNF systems, as evidenced by time-resolved spectroscopic studies, have profound implications for renewable energy technologies. These findings establish critical connections between fundamental chemical processes and practical energy applications, potentially revolutionizing how we harness and store sustainable energy.
Solar energy conversion systems stand to benefit significantly from these insights. The elucidated PCET mechanisms closely resemble those in natural photosynthesis, offering blueprints for artificial photosynthetic systems with enhanced efficiency. By mimicking these optimized proton and electron transfer pathways, next-generation solar cells could achieve conversion efficiencies approaching theoretical limits while utilizing earth-abundant materials.
Energy storage technologies, particularly redox flow batteries and hydrogen production systems, can leverage the revealed kinetic parameters to overcome existing performance limitations. The spectroscopic evidence of coordinated proton-electron movement provides design principles for electrolytes and catalysts with faster charge transfer rates and lower activation barriers, potentially increasing energy density by 30-40% in storage applications.
Fuel cell development stands to gain from the temporal resolution of charge transfer events in PNF systems. The insights into how proton and electron movements are coupled across interfaces can inform electrode design and membrane optimization, addressing persistent issues of catalyst poisoning and performance degradation. This could extend operational lifetimes while reducing precious metal catalyst requirements by up to 50%.
Electrocatalysis for carbon-neutral fuel production represents another frontier where these findings hold transformative potential. The mechanistic understanding of PCET kinetics can guide the development of catalysts for CO2 reduction and water splitting with unprecedented selectivity and efficiency, potentially enabling economically viable synthetic fuel production at scale.
Biomass conversion technologies may also benefit from these insights, as PCET processes are central to many lignin degradation pathways. Optimized catalytic systems based on these kinetic principles could enhance the yield and selectivity of valuable platform chemicals from renewable biomass sources, supporting the transition to bio-based chemical manufacturing.
The integration of these scientific advances into practical renewable energy technologies will require interdisciplinary collaboration between spectroscopists, materials scientists, and engineers. However, the potential impact on global energy systems justifies significant investment in translational research to bridge the gap between fundamental understanding and commercial implementation.
Solar energy conversion systems stand to benefit significantly from these insights. The elucidated PCET mechanisms closely resemble those in natural photosynthesis, offering blueprints for artificial photosynthetic systems with enhanced efficiency. By mimicking these optimized proton and electron transfer pathways, next-generation solar cells could achieve conversion efficiencies approaching theoretical limits while utilizing earth-abundant materials.
Energy storage technologies, particularly redox flow batteries and hydrogen production systems, can leverage the revealed kinetic parameters to overcome existing performance limitations. The spectroscopic evidence of coordinated proton-electron movement provides design principles for electrolytes and catalysts with faster charge transfer rates and lower activation barriers, potentially increasing energy density by 30-40% in storage applications.
Fuel cell development stands to gain from the temporal resolution of charge transfer events in PNF systems. The insights into how proton and electron movements are coupled across interfaces can inform electrode design and membrane optimization, addressing persistent issues of catalyst poisoning and performance degradation. This could extend operational lifetimes while reducing precious metal catalyst requirements by up to 50%.
Electrocatalysis for carbon-neutral fuel production represents another frontier where these findings hold transformative potential. The mechanistic understanding of PCET kinetics can guide the development of catalysts for CO2 reduction and water splitting with unprecedented selectivity and efficiency, potentially enabling economically viable synthetic fuel production at scale.
Biomass conversion technologies may also benefit from these insights, as PCET processes are central to many lignin degradation pathways. Optimized catalytic systems based on these kinetic principles could enhance the yield and selectivity of valuable platform chemicals from renewable biomass sources, supporting the transition to bio-based chemical manufacturing.
The integration of these scientific advances into practical renewable energy technologies will require interdisciplinary collaboration between spectroscopists, materials scientists, and engineers. However, the potential impact on global energy systems justifies significant investment in translational research to bridge the gap between fundamental understanding and commercial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!