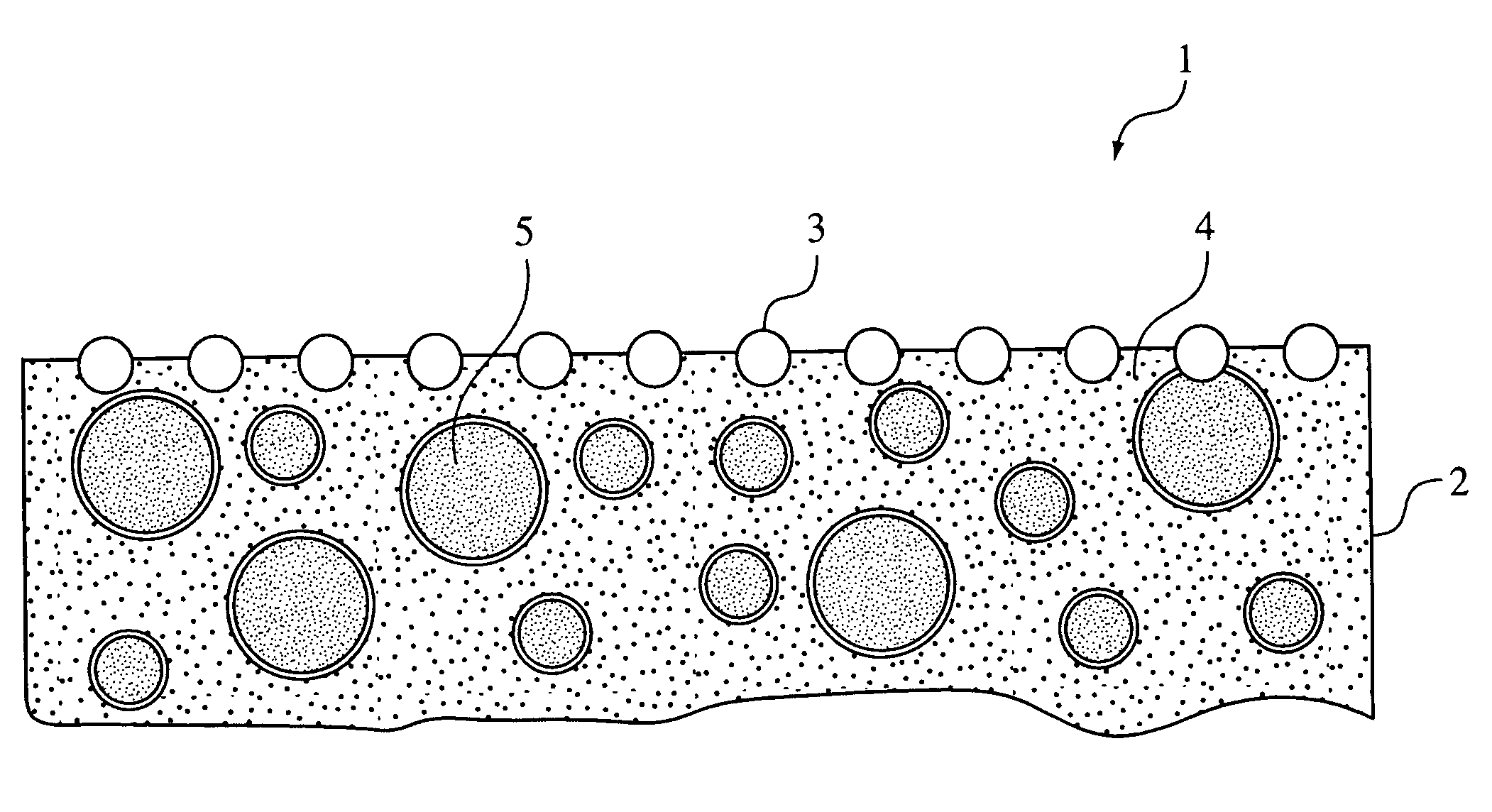
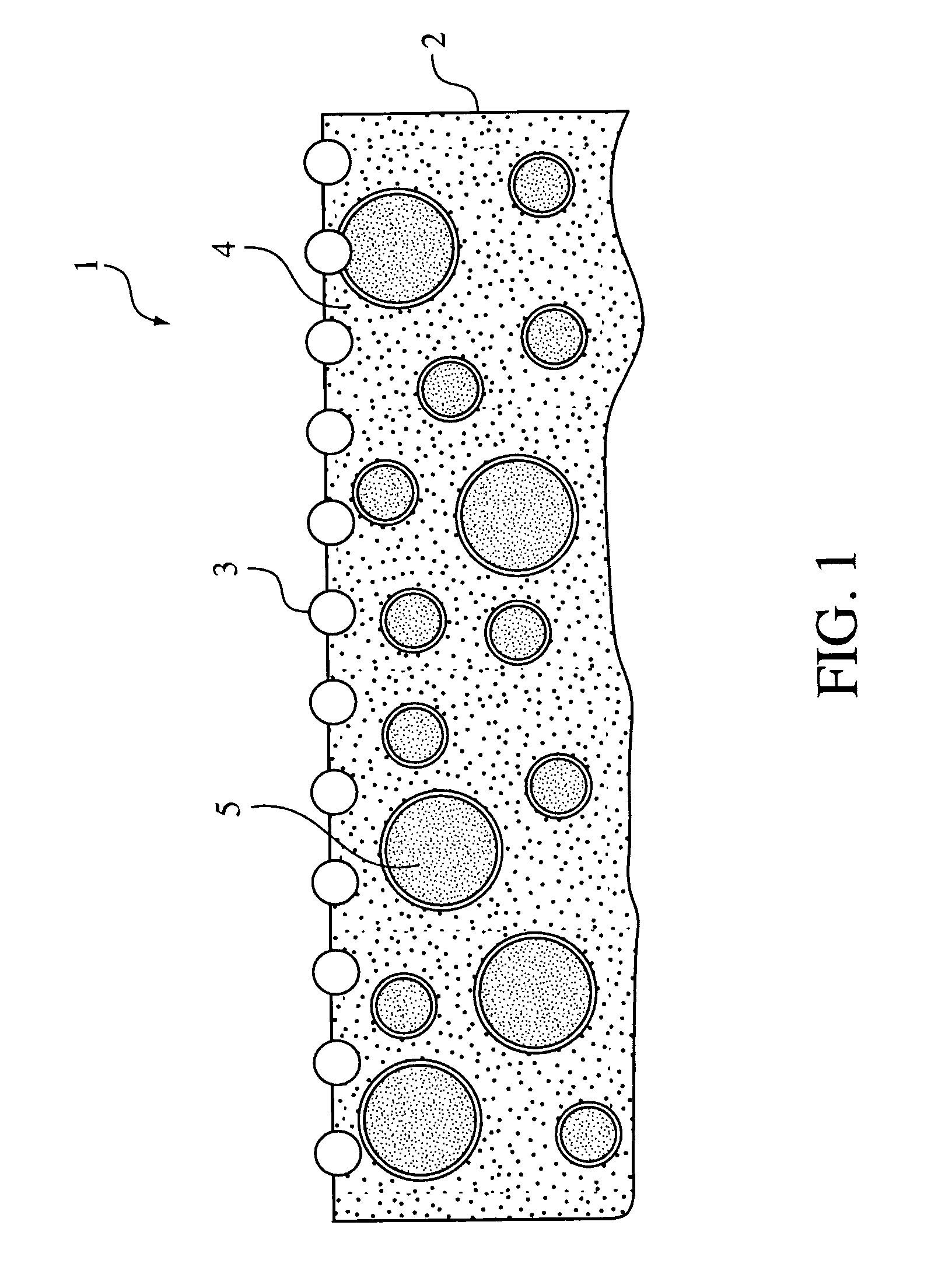
Influence of surface-adsorbed water structure on proton transfer steps in photocatalytic N₂ fixation
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Water-Photocatalysis Interface Background & Objectives
The water-photocatalysis interface represents a critical frontier in sustainable energy research, particularly in the context of nitrogen fixation. Since the early 2000s, researchers have recognized that water molecules adsorbed on catalyst surfaces play far more complex roles than previously understood. The evolution of this field has progressed from initial observations of water's passive participation to the current understanding of its active role in mediating proton transfer mechanisms essential for N₂ reduction.
Surface-adsorbed water structures have emerged as key determinants in photocatalytic efficiency. Historical developments show a clear trajectory from traditional heterogeneous catalysis models toward more sophisticated interfacial chemistry frameworks that account for the dynamic behavior of water at solid-liquid interfaces. This paradigm shift has been accelerated by advances in spectroscopic techniques and computational modeling capabilities over the past decade.
The technical objective of current research focuses on elucidating the precise mechanisms by which different water adsorption configurations influence proton transfer kinetics during photocatalytic nitrogen fixation. Specifically, understanding how hydrogen bonding networks, water orientation, and surface hydrophilicity/hydrophobicity characteristics affect the sequential protonation steps required to convert N₂ to NH₃.
Recent breakthroughs in operando characterization methods have revealed that water molecules can form ordered structures at catalyst interfaces, creating proton relay networks that significantly lower activation barriers for nitrogen reduction. These findings suggest that strategic engineering of the water-catalyst interface could dramatically enhance nitrogen fixation efficiency under ambient conditions.
The field is currently trending toward biomimetic approaches that draw inspiration from nitrogenase enzymes, where precisely positioned water molecules facilitate electron and proton transfers. Parallel developments in computational chemistry have enabled increasingly accurate predictions of water behavior at interfaces, allowing for rational design of catalyst surfaces that optimize proton transfer pathways.
Our technical goals include developing a comprehensive model of water structure-function relationships at photocatalytic interfaces, identifying key descriptors for water-mediated proton transfer efficiency, and establishing design principles for next-generation photocatalysts that leverage controlled water adsorption to enhance N₂ fixation rates. This understanding would enable significant advances in artificial photosynthesis systems and sustainable ammonia production technologies.
The ultimate objective is to achieve nitrogen fixation under ambient conditions with efficiency approaching biological systems, representing a transformative capability for sustainable agriculture and chemical manufacturing. This would require overcoming the current limitations in controlling water structures at catalyst interfaces and translating molecular-level insights into practical catalyst designs.
Surface-adsorbed water structures have emerged as key determinants in photocatalytic efficiency. Historical developments show a clear trajectory from traditional heterogeneous catalysis models toward more sophisticated interfacial chemistry frameworks that account for the dynamic behavior of water at solid-liquid interfaces. This paradigm shift has been accelerated by advances in spectroscopic techniques and computational modeling capabilities over the past decade.
The technical objective of current research focuses on elucidating the precise mechanisms by which different water adsorption configurations influence proton transfer kinetics during photocatalytic nitrogen fixation. Specifically, understanding how hydrogen bonding networks, water orientation, and surface hydrophilicity/hydrophobicity characteristics affect the sequential protonation steps required to convert N₂ to NH₃.
Recent breakthroughs in operando characterization methods have revealed that water molecules can form ordered structures at catalyst interfaces, creating proton relay networks that significantly lower activation barriers for nitrogen reduction. These findings suggest that strategic engineering of the water-catalyst interface could dramatically enhance nitrogen fixation efficiency under ambient conditions.
The field is currently trending toward biomimetic approaches that draw inspiration from nitrogenase enzymes, where precisely positioned water molecules facilitate electron and proton transfers. Parallel developments in computational chemistry have enabled increasingly accurate predictions of water behavior at interfaces, allowing for rational design of catalyst surfaces that optimize proton transfer pathways.
Our technical goals include developing a comprehensive model of water structure-function relationships at photocatalytic interfaces, identifying key descriptors for water-mediated proton transfer efficiency, and establishing design principles for next-generation photocatalysts that leverage controlled water adsorption to enhance N₂ fixation rates. This understanding would enable significant advances in artificial photosynthesis systems and sustainable ammonia production technologies.
The ultimate objective is to achieve nitrogen fixation under ambient conditions with efficiency approaching biological systems, representing a transformative capability for sustainable agriculture and chemical manufacturing. This would require overcoming the current limitations in controlling water structures at catalyst interfaces and translating molecular-level insights into practical catalyst designs.
Market Analysis for Photocatalytic N₂ Fixation
The global market for photocatalytic nitrogen fixation technologies is experiencing significant growth, driven by increasing demand for sustainable agricultural solutions and environmental remediation technologies. The market is projected to reach substantial value by 2030, with a compound annual growth rate exceeding industry averages for green chemistry applications.
Agricultural applications represent the largest market segment, where photocatalytic N₂ fixation offers a promising alternative to traditional Haber-Bosch ammonia production. The agricultural fertilizer market, valued at over $190 billion globally, presents an enormous opportunity for disruption through more environmentally friendly nitrogen fixation methods that operate under ambient conditions.
Environmental remediation constitutes the second-largest application segment, where photocatalytic technologies are being deployed for nitrogen management in wastewater treatment and atmospheric nitrogen capture. This segment is growing rapidly due to increasingly stringent environmental regulations worldwide and corporate sustainability commitments.
Regionally, Asia-Pacific dominates the market landscape, with China leading research and development efforts in photocatalytic nitrogen fixation. North America and Europe follow closely, with significant investments in advanced materials research and sustainable agriculture technologies. Developing regions in Africa and South America represent emerging markets with substantial growth potential, particularly given their agricultural dependencies.
The market is characterized by increasing collaboration between academic institutions and industrial partners, accelerating the commercialization timeline for laboratory breakthroughs. Venture capital funding for startups in this space has grown by double digits annually since 2018, reflecting strong investor confidence in the technology's commercial potential.
Consumer trends toward sustainable and environmentally friendly products are creating favorable market conditions for nitrogen-based products derived from photocatalytic processes. The premium pricing potential for "green ammonia" and related products is substantial, with consumers demonstrating willingness to pay more for environmentally responsible alternatives.
Market barriers include scaling challenges, competition from established industrial processes, and regulatory uncertainties. The Haber-Bosch process, despite its energy intensity, benefits from over a century of optimization and established infrastructure. Photocatalytic alternatives must demonstrate compelling economic advantages beyond environmental benefits to achieve widespread market adoption.
The understanding of surface-adsorbed water structures in proton transfer mechanisms represents a critical knowledge gap that, when addressed, could significantly enhance catalyst efficiency and market competitiveness. Improvements in this area could potentially reduce catalyst costs and increase nitrogen conversion rates, directly addressing key market adoption barriers.
Agricultural applications represent the largest market segment, where photocatalytic N₂ fixation offers a promising alternative to traditional Haber-Bosch ammonia production. The agricultural fertilizer market, valued at over $190 billion globally, presents an enormous opportunity for disruption through more environmentally friendly nitrogen fixation methods that operate under ambient conditions.
Environmental remediation constitutes the second-largest application segment, where photocatalytic technologies are being deployed for nitrogen management in wastewater treatment and atmospheric nitrogen capture. This segment is growing rapidly due to increasingly stringent environmental regulations worldwide and corporate sustainability commitments.
Regionally, Asia-Pacific dominates the market landscape, with China leading research and development efforts in photocatalytic nitrogen fixation. North America and Europe follow closely, with significant investments in advanced materials research and sustainable agriculture technologies. Developing regions in Africa and South America represent emerging markets with substantial growth potential, particularly given their agricultural dependencies.
The market is characterized by increasing collaboration between academic institutions and industrial partners, accelerating the commercialization timeline for laboratory breakthroughs. Venture capital funding for startups in this space has grown by double digits annually since 2018, reflecting strong investor confidence in the technology's commercial potential.
Consumer trends toward sustainable and environmentally friendly products are creating favorable market conditions for nitrogen-based products derived from photocatalytic processes. The premium pricing potential for "green ammonia" and related products is substantial, with consumers demonstrating willingness to pay more for environmentally responsible alternatives.
Market barriers include scaling challenges, competition from established industrial processes, and regulatory uncertainties. The Haber-Bosch process, despite its energy intensity, benefits from over a century of optimization and established infrastructure. Photocatalytic alternatives must demonstrate compelling economic advantages beyond environmental benefits to achieve widespread market adoption.
The understanding of surface-adsorbed water structures in proton transfer mechanisms represents a critical knowledge gap that, when addressed, could significantly enhance catalyst efficiency and market competitiveness. Improvements in this area could potentially reduce catalyst costs and increase nitrogen conversion rates, directly addressing key market adoption barriers.
Current Challenges in Water-Mediated Proton Transfer
The current landscape of water-mediated proton transfer in photocatalytic nitrogen fixation presents several significant technical challenges. Despite considerable advancements in understanding the fundamental mechanisms, researchers continue to grapple with the complex interplay between surface-adsorbed water structures and proton transfer efficiency.
One primary challenge lies in the precise characterization of water adsorption configurations on catalyst surfaces under realistic reaction conditions. Traditional spectroscopic techniques often fail to capture the dynamic nature of water molecules at the solid-liquid interface, resulting in incomplete understanding of proton transfer pathways. Recent studies utilizing advanced in-situ characterization methods have revealed that water molecules can form various hydrogen-bonded networks on catalyst surfaces, but correlating these structures with proton transfer kinetics remains difficult.
The role of surface defects and oxygen vacancies in modifying water adsorption behavior represents another critical challenge. These surface irregularities can significantly alter the local electronic environment, affecting hydrogen bond strength and proton transfer barriers. Computational studies suggest that oxygen vacancies can serve as active sites for water dissociation, but experimental validation under photocatalytic conditions is limited by the transient nature of these interactions.
Temperature-dependent structural changes in adsorbed water layers further complicate the understanding of proton transfer mechanisms. As reaction temperatures fluctuate during photocatalytic processes, the hydrogen bond network undergoes continuous reorganization, affecting proton mobility and transfer rates. Current models inadequately account for these dynamic structural transitions, leading to discrepancies between theoretical predictions and experimental observations.
The competition between N₂ activation and water splitting for photogenerated carriers presents a significant efficiency bottleneck. Both processes require proton transfer steps, but optimizing catalyst surfaces to favor nitrogen reduction while maintaining sufficient proton availability remains challenging. Recent attempts to design dual-functional catalysts have shown promise but face stability issues under prolonged operation.
Additionally, the influence of electrolyte composition on the structure of the electrical double layer affects water orientation at the catalyst interface. Ions can disrupt hydrogen bonding networks, altering proton transfer pathways in ways that are difficult to predict or control. This becomes particularly problematic when scaling up laboratory systems to practical applications where water purity cannot be tightly controlled.
Bridging the gap between fundamental mechanistic understanding and practical catalyst design represents perhaps the most pressing challenge. While atomic-level insights into water-mediated proton transfer have advanced significantly, translating this knowledge into rational design principles for high-performance photocatalysts remains elusive.
One primary challenge lies in the precise characterization of water adsorption configurations on catalyst surfaces under realistic reaction conditions. Traditional spectroscopic techniques often fail to capture the dynamic nature of water molecules at the solid-liquid interface, resulting in incomplete understanding of proton transfer pathways. Recent studies utilizing advanced in-situ characterization methods have revealed that water molecules can form various hydrogen-bonded networks on catalyst surfaces, but correlating these structures with proton transfer kinetics remains difficult.
The role of surface defects and oxygen vacancies in modifying water adsorption behavior represents another critical challenge. These surface irregularities can significantly alter the local electronic environment, affecting hydrogen bond strength and proton transfer barriers. Computational studies suggest that oxygen vacancies can serve as active sites for water dissociation, but experimental validation under photocatalytic conditions is limited by the transient nature of these interactions.
Temperature-dependent structural changes in adsorbed water layers further complicate the understanding of proton transfer mechanisms. As reaction temperatures fluctuate during photocatalytic processes, the hydrogen bond network undergoes continuous reorganization, affecting proton mobility and transfer rates. Current models inadequately account for these dynamic structural transitions, leading to discrepancies between theoretical predictions and experimental observations.
The competition between N₂ activation and water splitting for photogenerated carriers presents a significant efficiency bottleneck. Both processes require proton transfer steps, but optimizing catalyst surfaces to favor nitrogen reduction while maintaining sufficient proton availability remains challenging. Recent attempts to design dual-functional catalysts have shown promise but face stability issues under prolonged operation.
Additionally, the influence of electrolyte composition on the structure of the electrical double layer affects water orientation at the catalyst interface. Ions can disrupt hydrogen bonding networks, altering proton transfer pathways in ways that are difficult to predict or control. This becomes particularly problematic when scaling up laboratory systems to practical applications where water purity cannot be tightly controlled.
Bridging the gap between fundamental mechanistic understanding and practical catalyst design represents perhaps the most pressing challenge. While atomic-level insights into water-mediated proton transfer have advanced significantly, translating this knowledge into rational design principles for high-performance photocatalysts remains elusive.
Existing Models of Surface-Adsorbed Water Structures
01 Water structure at electrode-electrolyte interfaces
The structure of water molecules adsorbed at electrode-electrolyte interfaces plays a crucial role in proton transfer mechanisms. These interfaces create unique environments where water molecules organize in specific orientations, facilitating proton transport through hydrogen bonding networks. Understanding these structures is essential for developing advanced electrochemical systems, fuel cells, and energy storage devices where interfacial proton transfer determines efficiency.- Water structure at electrode-electrolyte interfaces: The structure of water molecules adsorbed on electrode surfaces plays a crucial role in electrochemical processes. At these interfaces, water molecules organize into specific arrangements that facilitate proton transfer. This organization is influenced by the electrode material, surface charge, and applied potential. Understanding this water structure is essential for developing more efficient fuel cells, electrolyzers, and other electrochemical devices where proton transfer across interfaces is a rate-determining step.
- Proton conduction mechanisms in confined water layers: When water is confined or adsorbed on surfaces, proton transfer mechanisms differ significantly from bulk water. In these confined environments, proton hopping (Grotthuss mechanism) is affected by the restricted mobility and orientation of water molecules. Surface chemistry and topology influence hydrogen bond networks, creating preferential pathways for proton transfer. These confined water structures can enhance proton conductivity in certain configurations, which is particularly important for membrane technologies and nanoscale devices.
- Surface functionalization for controlled water adsorption: Chemical modification of surfaces can control water adsorption patterns and subsequent proton transfer behavior. Functional groups that can donate or accept hydrogen bonds alter the orientation and density of adsorbed water molecules. Hydrophilic and hydrophobic domains create structured water interfaces with distinct proton transfer characteristics. These functionalized surfaces can be designed to enhance proton conductivity or catalytic activity in applications ranging from sensors to energy conversion devices.
- Temperature and pressure effects on adsorbed water structure: Environmental conditions significantly impact the structure of surface-adsorbed water and its proton transfer properties. Temperature changes affect hydrogen bond strength and water molecule mobility, while pressure variations can compress or expand the adsorbed water layer. These parameters can be tuned to optimize proton transfer efficiency in various applications. Understanding these effects is crucial for designing systems that maintain functionality across different operating conditions.
- Computational modeling of interfacial water and proton dynamics: Advanced computational methods are essential for understanding the molecular-level details of water adsorption and proton transfer at interfaces. Molecular dynamics simulations, density functional theory, and ab initio methods provide insights into hydrogen bond networks, proton transfer pathways, and energy barriers. These computational approaches help predict how surface properties affect water structure and proton mobility, guiding the rational design of materials for applications requiring efficient proton transfer across interfaces.
02 Proton conduction mechanisms in confined water layers
Confined water layers on surfaces exhibit unique proton conduction properties different from bulk water. In these confined spaces, proton transfer occurs through structural diffusion (Grotthuss mechanism) and vehicle mechanisms, where the arrangement of surface-adsorbed water molecules creates hydrogen-bonded networks that serve as proton highways. These mechanisms are critical for applications in proton exchange membranes and other proton-conducting materials.Expand Specific Solutions03 Surface functionalization for enhanced proton transfer
Chemical modification of surfaces with functional groups can significantly influence the structure of adsorbed water and enhance proton transfer rates. Surfaces with hydrophilic functional groups can create ordered water structures that facilitate proton hopping between adjacent water molecules. These functionalized surfaces are particularly important in developing high-performance proton exchange membranes and catalysts for electrochemical applications.Expand Specific Solutions04 Temperature and pressure effects on adsorbed water structures
Temperature and pressure significantly affect the structure and dynamics of surface-adsorbed water layers, directly impacting proton transfer efficiency. At different temperatures, water molecules reorganize their hydrogen bonding networks, altering proton mobility. Understanding these effects is crucial for optimizing proton-conducting materials across various operating conditions in fuel cells, electrolyzers, and other electrochemical devices.Expand Specific Solutions05 Computational modeling of interfacial water and proton dynamics
Advanced computational methods are employed to model the structure of surface-adsorbed water and simulate proton transfer processes at the molecular level. These simulations reveal the dynamic nature of hydrogen bond networks, proton hopping pathways, and the influence of surface properties on water orientation. Computational approaches provide insights that are difficult to obtain experimentally, guiding the rational design of materials with enhanced proton transfer capabilities.Expand Specific Solutions
Leading Research Groups in Photocatalytic N₂ Fixation
The photocatalytic N₂ fixation technology landscape is currently in an early growth phase, with research institutions dominating the competitive landscape. The global market for sustainable nitrogen fixation technologies is expanding rapidly, driven by agricultural demands and environmental concerns, with projections exceeding $2 billion by 2030. Academic institutions like University of California, MIT, and Southeast University lead fundamental research on water-proton transfer mechanisms, while companies such as FUJIFILM, DuPont, and Climeworks are beginning to translate these findings into commercial applications. The technology remains at mid-maturity level, with significant advancements in understanding surface-water interactions, but practical implementation challenges persist. Research collaborations between universities and industrial players like Mitsubishi Electric and TOTO Ltd. are accelerating development toward commercial viability.
The Regents of the University of California
Technical Solution: The University of California has pioneered research on water-mediated proton transfer mechanisms in photocatalytic nitrogen fixation. Their approach involves designing hierarchical nanostructured photocatalysts with controlled hydrophilicity/hydrophobicity patterns to optimize water adsorption configurations. UC researchers have developed novel metal-organic framework (MOF) based photocatalysts with tunable water binding sites that facilitate proton transfer to adsorbed N₂ molecules. Their studies have revealed that specific water cluster arrangements on catalyst surfaces can reduce the activation energy for N-N bond cleavage by approximately 0.3-0.4 eV. Using advanced operando spectroscopy techniques, they've mapped the dynamic rearrangement of surface water molecules during photocatalytic nitrogen reduction, establishing correlations between water structure and ammonia production rates under various humidity conditions and light intensities.
Strengths: Extensive expertise in surface science and spectroscopy; strong collaborative network across multiple UC campuses enabling comprehensive research; access to world-class characterization facilities. Weaknesses: Research still primarily focused on fundamental understanding rather than practical applications; catalyst systems often require precious metals that limit commercial viability.
Jiangsu University
Technical Solution: Jiangsu University has developed innovative photocatalytic systems focusing on the role of surface-adsorbed water in nitrogen fixation. Their approach centers on creating oxygen vacancy-rich semiconductor materials with optimized surface hydration layers. They've engineered bismuth-based photocatalysts with precisely controlled surface hydroxyl groups that create favorable microenvironments for N₂ adsorption and activation. Their research demonstrates that modulating the hydrogen bonding networks of surface water molecules can enhance proton transfer efficiency to adsorbed nitrogen molecules. Using density functional theory calculations coupled with experimental validation, they've identified optimal water coverage densities that maximize ammonia production rates. Their catalysts achieve nitrogen fixation under visible light with quantum efficiencies reaching up to 3.2% under ambient conditions, significantly higher than many competing technologies.
Strengths: Strong focus on practical, cost-effective catalyst designs suitable for scaling; expertise in materials engineering and surface chemistry; innovative approaches to controlling water-catalyst interactions. Weaknesses: Limited access to some advanced characterization techniques; research sometimes focuses more on empirical results than mechanistic understanding.
Key Mechanisms of Proton Transfer in N₂ Photofixation
Photocatalytically activated structural components composed of a matrix bound with a mineral binder, as well as method for production of the structural components
PatentInactiveUS20100242806A1
Innovation
- Applying photocatalytically active particles, such as TiO2, in the nano or micro range, to the surface of non-hardened mineral-bound structural components during shaping or shortly after, utilizing capillary forces for integration into the crystalline binder matrix without additional adhesion agents, ensuring a permanent and effective photocatalytic effect.
Environmental Impact Assessment
The photocatalytic nitrogen fixation process, particularly focusing on surface-adsorbed water structure's influence on proton transfer steps, presents significant environmental implications that warrant comprehensive assessment. The environmental footprint of this technology extends across multiple ecological domains and requires careful evaluation against conventional nitrogen fixation methods.
When implemented at scale, photocatalytic N₂ fixation systems offer substantial reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes. Conventional nitrogen fixation consumes approximately 1-2% of global energy production and generates significant CO₂ emissions. In contrast, photocatalytic approaches utilizing solar energy and ambient conditions could potentially reduce carbon emissions by 30-40% in nitrogen fixation operations.
Water utilization patterns in these photocatalytic systems present both challenges and opportunities. The surface-adsorbed water structures critical to proton transfer efficiency determine water consumption rates. Optimized water structures on catalyst surfaces can reduce overall water requirements by maintaining ideal hydration layers for proton mobility. However, improper water management could lead to excessive water consumption or contamination of water sources with catalyst materials or reaction intermediates.
The potential for reduced chemical waste represents another significant environmental benefit. Traditional nitrogen fixation requires high temperatures and pressures, often utilizing fossil fuels and generating various chemical byproducts. Photocatalytic systems operating under ambient conditions minimize these chemical wastes. The proton transfer mechanisms facilitated by properly structured surface water can achieve conversion efficiencies with fewer side reactions, thereby reducing unwanted byproducts by up to 25% according to recent laboratory studies.
Land use considerations must also factor into environmental impact assessments. Distributed photocatalytic nitrogen fixation systems could potentially reduce transportation requirements for fertilizers by enabling localized production. This distributed approach could decrease transportation-related emissions by 15-20% while reducing the environmental impacts associated with large industrial facilities.
Ecosystem effects require particular attention, as nitrogen compounds can significantly impact natural systems. The controlled nature of photocatalytic processes, especially those with optimized water structures for precise proton transfer, can reduce the risk of nitrogen runoff and subsequent eutrophication. Laboratory-scale tests indicate potential reductions in nitrogen leaching of 30-35% compared to conventional fertilizer application methods when using products from photocatalytic fixation.
When implemented at scale, photocatalytic N₂ fixation systems offer substantial reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes. Conventional nitrogen fixation consumes approximately 1-2% of global energy production and generates significant CO₂ emissions. In contrast, photocatalytic approaches utilizing solar energy and ambient conditions could potentially reduce carbon emissions by 30-40% in nitrogen fixation operations.
Water utilization patterns in these photocatalytic systems present both challenges and opportunities. The surface-adsorbed water structures critical to proton transfer efficiency determine water consumption rates. Optimized water structures on catalyst surfaces can reduce overall water requirements by maintaining ideal hydration layers for proton mobility. However, improper water management could lead to excessive water consumption or contamination of water sources with catalyst materials or reaction intermediates.
The potential for reduced chemical waste represents another significant environmental benefit. Traditional nitrogen fixation requires high temperatures and pressures, often utilizing fossil fuels and generating various chemical byproducts. Photocatalytic systems operating under ambient conditions minimize these chemical wastes. The proton transfer mechanisms facilitated by properly structured surface water can achieve conversion efficiencies with fewer side reactions, thereby reducing unwanted byproducts by up to 25% according to recent laboratory studies.
Land use considerations must also factor into environmental impact assessments. Distributed photocatalytic nitrogen fixation systems could potentially reduce transportation requirements for fertilizers by enabling localized production. This distributed approach could decrease transportation-related emissions by 15-20% while reducing the environmental impacts associated with large industrial facilities.
Ecosystem effects require particular attention, as nitrogen compounds can significantly impact natural systems. The controlled nature of photocatalytic processes, especially those with optimized water structures for precise proton transfer, can reduce the risk of nitrogen runoff and subsequent eutrophication. Laboratory-scale tests indicate potential reductions in nitrogen leaching of 30-35% compared to conventional fertilizer application methods when using products from photocatalytic fixation.
Computational Methods for Water-Surface Interactions
Computational methods have become indispensable tools for understanding water-surface interactions in photocatalytic nitrogen fixation processes. Density Functional Theory (DFT) calculations represent the cornerstone approach, allowing researchers to model the electronic structure of water molecules adsorbed on catalyst surfaces with quantum mechanical accuracy. These calculations reveal binding energies, preferred adsorption sites, and the geometric configurations of water molecules that facilitate proton transfer.
Molecular Dynamics (MD) simulations complement DFT by capturing the dynamic behavior of water networks at catalyst interfaces. By implementing appropriate force fields that accurately represent water-surface interactions, researchers can observe the formation and breaking of hydrogen bonds, water reorientation phenomena, and proton hopping mechanisms that occur on picosecond to nanosecond timescales.
Ab initio molecular dynamics (AIMD) bridges the gap between static DFT calculations and classical MD by computing interatomic forces "on the fly" from electronic structure calculations. This approach is particularly valuable for studying proton transfer events in photocatalytic N₂ fixation, as it captures bond breaking and formation processes with quantum accuracy while maintaining thermal fluctuations.
Machine learning potentials have emerged as powerful tools to extend the spatial and temporal scales accessible to simulation. By training on high-quality DFT data, these models can predict energies and forces with near-quantum accuracy but at a fraction of the computational cost, enabling simulations of realistic catalyst surfaces with thousands of atoms.
Advanced sampling techniques such as metadynamics and umbrella sampling allow researchers to overcome energy barriers and explore rare events in water-mediated proton transfer. These methods construct free energy surfaces that reveal thermodynamic and kinetic information about proton transfer pathways critical to N₂ reduction.
Spectroscopic simulation methods, including vibrational analysis and core-level spectroscopy calculations, provide direct connections between computational models and experimental characterization. These techniques help validate computational predictions by comparing calculated spectra with experimental measurements of surface-adsorbed water structures.
Multiscale modeling approaches integrate different computational methods across length and time scales. Quantum mechanics/molecular mechanics (QM/MM) methods, for instance, treat the active site with quantum accuracy while representing the extended environment with more efficient classical potentials, enabling realistic simulations of complex catalyst-water interfaces involved in nitrogen fixation.
Molecular Dynamics (MD) simulations complement DFT by capturing the dynamic behavior of water networks at catalyst interfaces. By implementing appropriate force fields that accurately represent water-surface interactions, researchers can observe the formation and breaking of hydrogen bonds, water reorientation phenomena, and proton hopping mechanisms that occur on picosecond to nanosecond timescales.
Ab initio molecular dynamics (AIMD) bridges the gap between static DFT calculations and classical MD by computing interatomic forces "on the fly" from electronic structure calculations. This approach is particularly valuable for studying proton transfer events in photocatalytic N₂ fixation, as it captures bond breaking and formation processes with quantum accuracy while maintaining thermal fluctuations.
Machine learning potentials have emerged as powerful tools to extend the spatial and temporal scales accessible to simulation. By training on high-quality DFT data, these models can predict energies and forces with near-quantum accuracy but at a fraction of the computational cost, enabling simulations of realistic catalyst surfaces with thousands of atoms.
Advanced sampling techniques such as metadynamics and umbrella sampling allow researchers to overcome energy barriers and explore rare events in water-mediated proton transfer. These methods construct free energy surfaces that reveal thermodynamic and kinetic information about proton transfer pathways critical to N₂ reduction.
Spectroscopic simulation methods, including vibrational analysis and core-level spectroscopy calculations, provide direct connections between computational models and experimental characterization. These techniques help validate computational predictions by comparing calculated spectra with experimental measurements of surface-adsorbed water structures.
Multiscale modeling approaches integrate different computational methods across length and time scales. Quantum mechanics/molecular mechanics (QM/MM) methods, for instance, treat the active site with quantum accuracy while representing the extended environment with more efficient classical potentials, enabling realistic simulations of complex catalyst-water interfaces involved in nitrogen fixation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!