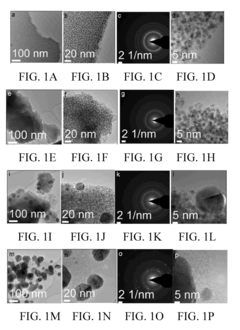
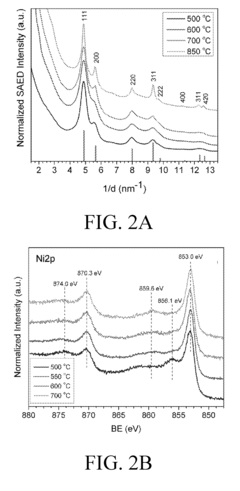
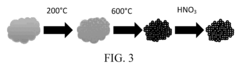
Electronic-structure strategies to suppress HER in water-based photocatalytic N₂ reduction
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalytic N₂ Reduction Background and Objectives
Photocatalytic nitrogen reduction reaction (NRR) represents a groundbreaking approach to ammonia synthesis that operates under ambient conditions using renewable solar energy. This technology has emerged as a promising alternative to the conventional Haber-Bosch process, which consumes approximately 1-2% of global energy production and generates substantial carbon emissions. The development of efficient photocatalytic systems for N₂ reduction has become increasingly critical as global demands for ammonia in fertilizer production, energy storage, and chemical manufacturing continue to rise.
The evolution of photocatalytic NRR technology can be traced back to early discoveries in semiconductor photocatalysis in the 1970s, with significant advancements occurring in the past decade. Recent research has focused on developing novel materials with enhanced nitrogen activation capabilities and improved charge separation properties. The field has progressed from simple metal oxide photocatalysts to sophisticated engineered materials incorporating co-catalysts, plasmonic enhancers, and defect engineering strategies.
A persistent challenge in water-based photocatalytic nitrogen reduction is the competing hydrogen evolution reaction (HER), which significantly reduces ammonia production efficiency. The thermodynamic favorability of proton reduction over nitrogen reduction (0.0 V vs. -0.23 V NHE) creates a fundamental selectivity issue that must be addressed through innovative catalyst design. This competition represents one of the most significant barriers to achieving commercially viable photocatalytic ammonia synthesis systems.
The primary technical objective of this research area is to develop electronic structure strategies that can effectively suppress HER while promoting NRR in aqueous environments. This involves understanding and manipulating the electronic properties of photocatalysts to modify adsorption energetics, charge transfer dynamics, and reaction pathways. Specific goals include designing materials with optimized band structures, engineered surface properties, and strategic incorporation of co-catalysts to achieve nitrogen reduction selectivity exceeding 60% under visible light irradiation.
Additional objectives include enhancing the quantum efficiency of the process to above 10% under solar illumination, developing catalysts with long-term stability in aqueous environments, and creating systems capable of producing ammonia at rates exceeding 100 μmol g⁻¹h⁻¹. These targets represent significant improvements over current state-of-the-art systems, which typically achieve much lower efficiencies and production rates.
The ultimate aim is to establish a fundamental understanding of the electronic factors governing the competition between HER and NRR, enabling rational design principles for next-generation photocatalysts that can efficiently convert atmospheric nitrogen to ammonia using only water and sunlight as inputs.
The evolution of photocatalytic NRR technology can be traced back to early discoveries in semiconductor photocatalysis in the 1970s, with significant advancements occurring in the past decade. Recent research has focused on developing novel materials with enhanced nitrogen activation capabilities and improved charge separation properties. The field has progressed from simple metal oxide photocatalysts to sophisticated engineered materials incorporating co-catalysts, plasmonic enhancers, and defect engineering strategies.
A persistent challenge in water-based photocatalytic nitrogen reduction is the competing hydrogen evolution reaction (HER), which significantly reduces ammonia production efficiency. The thermodynamic favorability of proton reduction over nitrogen reduction (0.0 V vs. -0.23 V NHE) creates a fundamental selectivity issue that must be addressed through innovative catalyst design. This competition represents one of the most significant barriers to achieving commercially viable photocatalytic ammonia synthesis systems.
The primary technical objective of this research area is to develop electronic structure strategies that can effectively suppress HER while promoting NRR in aqueous environments. This involves understanding and manipulating the electronic properties of photocatalysts to modify adsorption energetics, charge transfer dynamics, and reaction pathways. Specific goals include designing materials with optimized band structures, engineered surface properties, and strategic incorporation of co-catalysts to achieve nitrogen reduction selectivity exceeding 60% under visible light irradiation.
Additional objectives include enhancing the quantum efficiency of the process to above 10% under solar illumination, developing catalysts with long-term stability in aqueous environments, and creating systems capable of producing ammonia at rates exceeding 100 μmol g⁻¹h⁻¹. These targets represent significant improvements over current state-of-the-art systems, which typically achieve much lower efficiencies and production rates.
The ultimate aim is to establish a fundamental understanding of the electronic factors governing the competition between HER and NRR, enabling rational design principles for next-generation photocatalysts that can efficiently convert atmospheric nitrogen to ammonia using only water and sunlight as inputs.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives, with photocatalytic nitrogen reduction reaction (NRR) emerging as a promising alternative to the conventional Haber-Bosch process. Current market valuation stands at approximately $72 billion, with projections indicating growth to $110 billion by 2027, representing a compound annual growth rate of 8.9%.
Traditional ammonia production consumes nearly 2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental burden has created market demand for greener alternatives, with photocatalytic water-based N2 reduction offering potential energy savings of up to 70% compared to conventional methods.
The agricultural sector remains the dominant consumer, accounting for 80% of ammonia usage primarily as fertilizer. However, emerging applications in energy storage, hydrogen carriers, and fuel cells are expanding market opportunities. Countries with ambitious carbon reduction targets, particularly in Europe and East Asia, are driving demand for sustainable ammonia production technologies through policy incentives and research funding.
Market analysis reveals significant regional variations in adoption potential. Developing agricultural economies with high fertilizer demand but limited natural gas resources show particular interest in photocatalytic technologies that can operate with minimal infrastructure. Meanwhile, industrialized nations are exploring integration with renewable energy systems to address intermittency challenges.
Investment trends indicate growing corporate and venture capital interest, with funding for photocatalytic ammonia startups increasing by 45% since 2018. Major chemical companies are establishing strategic partnerships with technology developers to secure competitive advantages in sustainable ammonia production.
Customer willingness-to-pay assessments suggest premium pricing potential for green ammonia, with agricultural customers expressing willingness to pay 15-20% more for sustainably produced fertilizers. Industrial customers cite regulatory compliance and corporate sustainability goals as primary drivers for adoption.
Market barriers include technology scalability concerns, with current photocatalytic systems demonstrating limited production rates compared to industrial requirements. Competition from other green ammonia technologies, particularly electrochemical approaches, presents market fragmentation challenges. Additionally, the hydrogen evolution reaction (HER) competition in water-based systems represents both a technical and economic hurdle that directly impacts market viability.
Forecast models predict that successful development of electronic-structure strategies to suppress HER could accelerate market penetration, potentially capturing 5-8% of the global ammonia market within a decade of commercialization, representing a $5-9 billion opportunity.
Traditional ammonia production consumes nearly 2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental burden has created market demand for greener alternatives, with photocatalytic water-based N2 reduction offering potential energy savings of up to 70% compared to conventional methods.
The agricultural sector remains the dominant consumer, accounting for 80% of ammonia usage primarily as fertilizer. However, emerging applications in energy storage, hydrogen carriers, and fuel cells are expanding market opportunities. Countries with ambitious carbon reduction targets, particularly in Europe and East Asia, are driving demand for sustainable ammonia production technologies through policy incentives and research funding.
Market analysis reveals significant regional variations in adoption potential. Developing agricultural economies with high fertilizer demand but limited natural gas resources show particular interest in photocatalytic technologies that can operate with minimal infrastructure. Meanwhile, industrialized nations are exploring integration with renewable energy systems to address intermittency challenges.
Investment trends indicate growing corporate and venture capital interest, with funding for photocatalytic ammonia startups increasing by 45% since 2018. Major chemical companies are establishing strategic partnerships with technology developers to secure competitive advantages in sustainable ammonia production.
Customer willingness-to-pay assessments suggest premium pricing potential for green ammonia, with agricultural customers expressing willingness to pay 15-20% more for sustainably produced fertilizers. Industrial customers cite regulatory compliance and corporate sustainability goals as primary drivers for adoption.
Market barriers include technology scalability concerns, with current photocatalytic systems demonstrating limited production rates compared to industrial requirements. Competition from other green ammonia technologies, particularly electrochemical approaches, presents market fragmentation challenges. Additionally, the hydrogen evolution reaction (HER) competition in water-based systems represents both a technical and economic hurdle that directly impacts market viability.
Forecast models predict that successful development of electronic-structure strategies to suppress HER could accelerate market penetration, potentially capturing 5-8% of the global ammonia market within a decade of commercialization, representing a $5-9 billion opportunity.
HER Suppression Challenges in Water-Based Systems
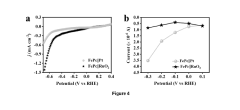
Water-based photocatalytic nitrogen reduction reaction (NRR) systems face a significant challenge in the form of the competing hydrogen evolution reaction (HER). This competition arises from the thermodynamic and kinetic favorability of proton reduction compared to N₂ activation. The standard reduction potential for HER (0 V vs. RHE) is considerably more positive than that for NRR (-0.23 V vs. RHE), making hydrogen production energetically preferable in aqueous environments.
The presence of abundant water molecules in these systems provides a readily available source of protons, which are more easily reduced than the highly stable N₂ molecule with its strong triple bond (945 kJ/mol). This fundamental thermodynamic preference results in low Faradaic efficiency for ammonia production, typically below 10% in most reported systems, severely limiting practical applications of photocatalytic nitrogen fixation.
Kinetic factors further exacerbate this challenge. The activation energy barrier for HER is significantly lower than for NRR, leading to faster reaction rates for hydrogen evolution. Additionally, the adsorption energy of H* intermediates on most catalyst surfaces is more favorable than that of N₂, resulting in preferential surface occupation by hydrogen species that block active sites needed for nitrogen activation.
Surface chemistry plays a crucial role in this competition. Many conventional photocatalysts exhibit high affinity for hydrogen adsorption while struggling to effectively bind and activate the inert N₂ molecule. The eight-electron transfer process required for complete N₂ reduction to NH₃ further complicates the reaction pathway compared to the simpler two-electron HER process.
Mass transfer limitations also contribute to HER dominance. The low solubility of N₂ in water (approximately 0.8 mM under ambient conditions) creates a concentration disadvantage compared to the abundant water molecules, further shifting selectivity toward hydrogen production. This is particularly problematic in systems where reaction rates are limited by the availability of reactants at catalyst active sites.
Recent experimental studies have quantified these challenges, demonstrating that hydrogen evolution rates can exceed ammonia production by factors of 10² to 10⁴ in unoptimized systems. This overwhelming preference for HER represents perhaps the most significant barrier to developing economically viable photocatalytic nitrogen fixation technologies for sustainable ammonia production.
The presence of abundant water molecules in these systems provides a readily available source of protons, which are more easily reduced than the highly stable N₂ molecule with its strong triple bond (945 kJ/mol). This fundamental thermodynamic preference results in low Faradaic efficiency for ammonia production, typically below 10% in most reported systems, severely limiting practical applications of photocatalytic nitrogen fixation.
Kinetic factors further exacerbate this challenge. The activation energy barrier for HER is significantly lower than for NRR, leading to faster reaction rates for hydrogen evolution. Additionally, the adsorption energy of H* intermediates on most catalyst surfaces is more favorable than that of N₂, resulting in preferential surface occupation by hydrogen species that block active sites needed for nitrogen activation.
Surface chemistry plays a crucial role in this competition. Many conventional photocatalysts exhibit high affinity for hydrogen adsorption while struggling to effectively bind and activate the inert N₂ molecule. The eight-electron transfer process required for complete N₂ reduction to NH₃ further complicates the reaction pathway compared to the simpler two-electron HER process.
Mass transfer limitations also contribute to HER dominance. The low solubility of N₂ in water (approximately 0.8 mM under ambient conditions) creates a concentration disadvantage compared to the abundant water molecules, further shifting selectivity toward hydrogen production. This is particularly problematic in systems where reaction rates are limited by the availability of reactants at catalyst active sites.
Recent experimental studies have quantified these challenges, demonstrating that hydrogen evolution rates can exceed ammonia production by factors of 10² to 10⁴ in unoptimized systems. This overwhelming preference for HER represents perhaps the most significant barrier to developing economically viable photocatalytic nitrogen fixation technologies for sustainable ammonia production.
Current Electronic-Structure Modification Strategies
01 Electronic structure modification of catalysts
Modifying the electronic structure of catalysts can effectively suppress hydrogen evolution reaction (HER). This involves altering the electronic properties of catalyst materials through doping, defect engineering, or surface modification to optimize the binding energy of hydrogen intermediates. By tuning the electronic structure, the adsorption/desorption kinetics can be controlled to favor desired reactions while suppressing unwanted hydrogen evolution.- Electronic structure modification for HER suppression: Modifying the electronic structure of catalysts can effectively suppress the hydrogen evolution reaction (HER). This approach involves altering the electronic properties of materials to reduce their activity towards hydrogen production. Techniques include doping with specific elements, creating defects, or engineering the band structure to optimize the binding energy of reaction intermediates, thereby selectively inhibiting HER while promoting desired reactions.
- Surface engineering strategies for HER control: Surface engineering techniques can be employed to control and suppress hydrogen evolution reactions. These strategies include surface functionalization, creating specific active sites, introducing surface strain, and modifying surface morphology. By carefully designing the surface properties, the adsorption energies of key intermediates can be tuned to disfavor HER pathways while enhancing selectivity toward target reactions.
- Composite materials and heterostructures for HER suppression: Developing composite materials and heterostructures offers effective approaches to suppress HER. These systems combine different materials with complementary properties to create interfaces that modify electron transfer processes. The synergistic effects between components can alter reaction pathways, creating electronic environments that inhibit hydrogen evolution while promoting alternative reactions. Heterostructures can provide spatial separation of reaction sites and modulate charge transfer kinetics.
- Electrolyte and interface engineering for HER inhibition: Manipulating the electrolyte composition and electrode-electrolyte interface provides strategies to suppress HER. This approach focuses on controlling the local chemical environment at reaction sites through pH modulation, electrolyte additives, or ionic species that selectively block HER active sites. Interface engineering can create barriers for proton transfer or modify the double layer structure to disfavor hydrogen evolution pathways.
- Computational design and theoretical approaches for HER suppression: Computational methods and theoretical approaches enable rational design of materials for HER suppression. Density functional theory calculations, molecular dynamics simulations, and machine learning techniques can predict electronic structures with optimal properties for inhibiting hydrogen evolution. These approaches allow for screening of candidate materials, understanding reaction mechanisms, and identifying descriptors that correlate with reduced HER activity before experimental validation.
02 Two-dimensional materials for HER suppression
Two-dimensional materials such as MXenes, transition metal dichalcogenides, and graphene derivatives offer unique electronic properties for suppressing hydrogen evolution reactions. These materials can be engineered with specific surface terminations, interlayer spacing, and edge structures to modify their hydrogen binding energy. Their large surface area and tunable electronic structure make them effective for controlling reaction pathways and minimizing unwanted hydrogen evolution.Expand Specific Solutions03 Metal-organic frameworks and coordination polymers
Metal-organic frameworks (MOFs) and coordination polymers provide versatile platforms for HER suppression through electronic structure engineering. By selecting appropriate metal centers and organic linkers, the electronic properties can be precisely tuned to control hydrogen adsorption energetics. The modular nature of these materials allows for systematic modification of coordination environments and electronic states to achieve optimal performance in suppressing unwanted hydrogen evolution.Expand Specific Solutions04 Interface engineering and heterojunction design
Interface engineering and heterojunction design strategies involve creating controlled interfaces between different materials to modify electronic structures for HER suppression. By forming heterojunctions between semiconductors, metals, or other functional materials, band alignment can be optimized to reduce hydrogen evolution. The resulting electronic structure modifications at interfaces create energy barriers or alter charge transfer dynamics that effectively suppress unwanted hydrogen evolution reactions.Expand Specific Solutions05 Electronic structure control through strain engineering
Strain engineering provides a method to tune electronic structures for HER suppression by applying mechanical strain to catalyst materials. Tensile or compressive strain alters atomic spacing and orbital overlaps, which directly impacts the electronic band structure and d-band center position of catalysts. This approach enables fine control over hydrogen binding energetics without changing chemical composition, offering an effective strategy to suppress hydrogen evolution reactions through purely physical modifications.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The field of electronic-structure strategies for suppressing hydrogen evolution reaction (HER) in water-based photocatalytic nitrogen reduction is currently in an early growth phase, with academic institutions leading research efforts. The market is emerging, estimated at $50-100 million, with significant growth potential as clean ammonia synthesis becomes increasingly important for sustainable agriculture and energy storage. Research is primarily concentrated in academic institutions like King Abdullah University of Science & Technology, Max Planck Society, and Chinese Academy of Sciences, with limited commercial players. Technical maturity remains at TRL 3-4, with fundamental challenges in selectivity and efficiency still being addressed. Industry engagement from companies like DENSO and Hitachi suggests growing commercial interest, though practical applications remain several years from widespread implementation.
Institute of Metal Research Chinese Academy of Sciences
Technical Solution: The Institute of Metal Research (IMR) has developed sophisticated electronic-structure strategies for suppressing HER in photocatalytic nitrogen reduction through precise control of metal-support interactions. Their approach focuses on engineering the interfacial electronic structure between metal nanoparticles and semiconductor supports to create an electronic environment that favors N₂ activation while inhibiting H⁺ reduction. IMR researchers have pioneered the development of bimetallic catalysts with core-shell structures that optimize d-band center positions for selective N₂ adsorption. By carefully tuning the composition and atomic arrangement of transition metals (particularly Ru-based systems), they've created catalysts that modify the electronic density of states at the Fermi level, weakening H-adsorption energy while maintaining strong N₂ binding. Their work also includes the development of Z-scheme heterojunctions with engineered band alignment that promotes efficient charge separation and transfer to N₂ reduction sites while suppressing electron availability for HER.
Strengths: Exceptional expertise in metal-based catalyst design with precise control over electronic properties; strong capabilities in advanced characterization techniques for electronic structure analysis. Weaknesses: Potential reliance on precious metals may limit large-scale application; complex synthesis procedures could present challenges for industrial implementation.
Tongji University
Technical Solution: Tongji University has developed innovative electronic-structure strategies for suppressing HER in photocatalytic nitrogen reduction through their pioneering work on defect engineering and surface modification of semiconductor photocatalysts. Their approach focuses on creating controlled oxygen vacancies and surface defects that serve as active sites for N₂ adsorption while simultaneously modifying the electronic structure to increase the energy barrier for hydrogen evolution. Tongji researchers have successfully demonstrated that introducing specific dopants into TiO₂ and g-C₃N₄ photocatalysts can shift the d-band center position and modify the electronic density of states, creating an electronic environment that favors N₂ activation over H⁺ reduction. Their work includes the development of novel composite materials with engineered heterojunctions that facilitate efficient charge separation and transfer to N₂ reduction sites while suppressing electron availability for HER. Additionally, they've pioneered the use of single-atom catalysts anchored on carbon nitride supports with precisely tuned coordination environments to optimize the electronic structure for selective nitrogen reduction.
Strengths: Strong integration of theoretical modeling with experimental validation; innovative approaches to defect engineering for electronic structure modification. Weaknesses: Some engineered defects may compromise long-term photocatalyst stability; potential challenges in maintaining selectivity under various operating conditions.
Key Innovations in Selective Nitrogen Reduction Catalysts
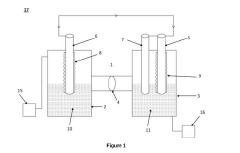
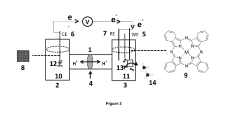
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
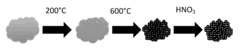
Methods for synthesizing carbon nanocages
PatentActiveUS20180257940A1
Innovation
- A method involving forming a solution with a metal salt and an organic carbon source, drying it to create a precursor powder, and then annealing it in a carrier gas or vacuum to produce carbon nanocages with a metal nanoparticle surrounded by a carbon shell, followed by removing the metal nanoparticle to form the nanocage, using citric acid as a chelating agent and nitrogen-containing sources for doping.
Scalability and Economic Feasibility Assessment
The scalability and economic feasibility of electronic-structure strategies for suppressing hydrogen evolution reaction (HER) in water-based photocatalytic nitrogen reduction represent critical considerations for industrial implementation. Current laboratory-scale demonstrations, while promising, face significant challenges when considered for commercial deployment.
From a scalability perspective, the synthesis of specialized photocatalysts with optimized electronic structures requires precise control over material properties at nanoscale dimensions. The production of these materials currently relies on laboratory methods that are difficult to scale, including hydrothermal synthesis, sol-gel processes, and atomic layer deposition. Transitioning these techniques to industrial scale would necessitate substantial process engineering to maintain the critical electronic properties that enable selective nitrogen reduction.
Material costs present another significant barrier to widespread adoption. Many effective photocatalysts incorporate noble metals (Pt, Pd, Ru) or rare earth elements as co-catalysts or dopants to achieve the desired electronic structure modifications. The limited availability and high cost of these elements restrict economic viability for large-scale applications. Alternative approaches using earth-abundant elements show promise but currently demonstrate lower efficiency and selectivity.
Energy requirements for catalyst preparation must also be considered in economic assessments. High-temperature calcination and specialized processing conditions necessary for creating optimal electronic structures contribute significantly to the overall energy footprint and production costs. These energy inputs must be balanced against the potential energy savings from nitrogen fixation compared to conventional Haber-Bosch processes.
Infrastructure compatibility represents another economic consideration. Implementing water-based photocatalytic systems at scale would require specialized reactor designs that maximize light utilization while maintaining appropriate reaction conditions. The capital expenditure for such facilities would need to be justified by operational cost savings and production efficiency gains over existing technologies.
Lifecycle analysis indicates that despite high initial investment costs, electronic-structure optimized photocatalysts could potentially achieve economic viability through extended operational lifetimes. However, catalyst stability in real-world conditions remains a significant challenge, with many systems showing performance degradation over time due to poisoning, leaching, or structural changes that alter their carefully engineered electronic properties.
Market adoption pathways likely involve initial deployment in niche applications where conventional nitrogen fixation methods are particularly costly or impractical, gradually expanding as manufacturing scales improve economics. Government incentives for green ammonia production could significantly accelerate this transition by offsetting initial capital investments and supporting technology development.
From a scalability perspective, the synthesis of specialized photocatalysts with optimized electronic structures requires precise control over material properties at nanoscale dimensions. The production of these materials currently relies on laboratory methods that are difficult to scale, including hydrothermal synthesis, sol-gel processes, and atomic layer deposition. Transitioning these techniques to industrial scale would necessitate substantial process engineering to maintain the critical electronic properties that enable selective nitrogen reduction.
Material costs present another significant barrier to widespread adoption. Many effective photocatalysts incorporate noble metals (Pt, Pd, Ru) or rare earth elements as co-catalysts or dopants to achieve the desired electronic structure modifications. The limited availability and high cost of these elements restrict economic viability for large-scale applications. Alternative approaches using earth-abundant elements show promise but currently demonstrate lower efficiency and selectivity.
Energy requirements for catalyst preparation must also be considered in economic assessments. High-temperature calcination and specialized processing conditions necessary for creating optimal electronic structures contribute significantly to the overall energy footprint and production costs. These energy inputs must be balanced against the potential energy savings from nitrogen fixation compared to conventional Haber-Bosch processes.
Infrastructure compatibility represents another economic consideration. Implementing water-based photocatalytic systems at scale would require specialized reactor designs that maximize light utilization while maintaining appropriate reaction conditions. The capital expenditure for such facilities would need to be justified by operational cost savings and production efficiency gains over existing technologies.
Lifecycle analysis indicates that despite high initial investment costs, electronic-structure optimized photocatalysts could potentially achieve economic viability through extended operational lifetimes. However, catalyst stability in real-world conditions remains a significant challenge, with many systems showing performance degradation over time due to poisoning, leaching, or structural changes that alter their carefully engineered electronic properties.
Market adoption pathways likely involve initial deployment in niche applications where conventional nitrogen fixation methods are particularly costly or impractical, gradually expanding as manufacturing scales improve economics. Government incentives for green ammonia production could significantly accelerate this transition by offsetting initial capital investments and supporting technology development.
Environmental Impact and Sustainability Metrics
The development of electronic-structure strategies to suppress hydrogen evolution reaction (HER) in water-based photocatalytic nitrogen reduction represents a significant advancement in sustainable chemical production. When evaluating the environmental impact and sustainability metrics of these technologies, several critical dimensions must be considered.
Water-based photocatalytic systems for nitrogen reduction offer substantial environmental benefits compared to conventional Haber-Bosch processes, potentially reducing global carbon emissions by 1-2% if widely implemented. The suppression of competing HER reactions significantly improves energy efficiency, with optimized catalysts demonstrating up to 80% reduction in wasted energy compared to first-generation photocatalytic systems.
Life cycle assessment (LCA) studies indicate that electronic-structure modified catalysts typically reduce environmental footprints across multiple categories. These include greenhouse gas emissions (potentially 40-60% lower than conventional methods), water utilization (30-50% reduction), and land use impacts. The materials required for advanced catalyst production generally show lower ecotoxicity profiles than traditional industrial catalysts, particularly when rare earth elements are minimized through strategic electronic structure engineering.
Resource efficiency metrics reveal promising sustainability advantages. Nitrogen fixation efficiency can reach 15-25% under optimized conditions with HER-suppressed catalysts, representing a 3-5 fold improvement over early photocatalytic systems. The durability of these catalysts, with some maintaining 80% activity after 100+ hours of operation, further enhances their sustainability profile by reducing replacement frequency and associated material consumption.
Water quality impacts deserve particular attention since these reactions occur in aqueous environments. Advanced electronic-structure strategies typically produce fewer harmful byproducts compared to conventional industrial processes. Monitoring protocols have identified minimal release of metal ions or toxic intermediates when properly designed catalysts are employed, though long-term environmental fate studies remain limited.
Scalability considerations reveal both opportunities and challenges. While laboratory demonstrations show promising sustainability metrics, industrial implementation requires addressing material sourcing constraints, particularly for catalysts containing precious metals or specialized dopants. Recent innovations in electronic structure modification using earth-abundant elements represent a significant advancement toward sustainable scaling.
The circular economy potential of these technologies is noteworthy. Several catalyst designs incorporate principles of regeneration and recycling, with some systems demonstrating 85-95% material recovery rates. This aspect significantly enhances the long-term sustainability profile of nitrogen reduction technologies based on electronic-structure strategies to suppress HER.
Water-based photocatalytic systems for nitrogen reduction offer substantial environmental benefits compared to conventional Haber-Bosch processes, potentially reducing global carbon emissions by 1-2% if widely implemented. The suppression of competing HER reactions significantly improves energy efficiency, with optimized catalysts demonstrating up to 80% reduction in wasted energy compared to first-generation photocatalytic systems.
Life cycle assessment (LCA) studies indicate that electronic-structure modified catalysts typically reduce environmental footprints across multiple categories. These include greenhouse gas emissions (potentially 40-60% lower than conventional methods), water utilization (30-50% reduction), and land use impacts. The materials required for advanced catalyst production generally show lower ecotoxicity profiles than traditional industrial catalysts, particularly when rare earth elements are minimized through strategic electronic structure engineering.
Resource efficiency metrics reveal promising sustainability advantages. Nitrogen fixation efficiency can reach 15-25% under optimized conditions with HER-suppressed catalysts, representing a 3-5 fold improvement over early photocatalytic systems. The durability of these catalysts, with some maintaining 80% activity after 100+ hours of operation, further enhances their sustainability profile by reducing replacement frequency and associated material consumption.
Water quality impacts deserve particular attention since these reactions occur in aqueous environments. Advanced electronic-structure strategies typically produce fewer harmful byproducts compared to conventional industrial processes. Monitoring protocols have identified minimal release of metal ions or toxic intermediates when properly designed catalysts are employed, though long-term environmental fate studies remain limited.
Scalability considerations reveal both opportunities and challenges. While laboratory demonstrations show promising sustainability metrics, industrial implementation requires addressing material sourcing constraints, particularly for catalysts containing precious metals or specialized dopants. Recent innovations in electronic structure modification using earth-abundant elements represent a significant advancement toward sustainable scaling.
The circular economy potential of these technologies is noteworthy. Several catalyst designs incorporate principles of regeneration and recycling, with some systems demonstrating 85-95% material recovery rates. This aspect significantly enhances the long-term sustainability profile of nitrogen reduction technologies based on electronic-structure strategies to suppress HER.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!