Role of adsorbed oxygen species on selectivity and deactivation in aerobic photocatalytic N₂ fixation
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalytic N₂ Fixation Background and Objectives
Photocatalytic nitrogen fixation represents a revolutionary approach to ammonia synthesis that operates under ambient conditions, offering a sustainable alternative to the energy-intensive Haber-Bosch process. The evolution of this technology traces back to the early 1980s when researchers first demonstrated the possibility of converting atmospheric nitrogen to ammonia using semiconductor photocatalysts. Since then, significant advancements have occurred, particularly in the last decade, with the development of various photocatalytic materials including TiO2-based composites, graphitic carbon nitride, and plasmonic metal nanostructures.
The technological trajectory has been characterized by increasing conversion efficiencies, improved selectivity, and enhanced stability of photocatalysts. Recent breakthroughs in 2018-2022 have demonstrated quantum efficiencies approaching 1% under visible light, representing orders of magnitude improvement over early systems. However, these values remain significantly below the theoretical maximum and commercial viability thresholds.
A critical aspect of photocatalytic N₂ fixation involves understanding the complex role of oxygen species in the reaction mechanism. While traditional approaches have focused on oxygen-free environments to prevent competitive reactions, emerging research suggests that certain adsorbed oxygen species may actually facilitate nitrogen activation through cooperative binding mechanisms and electron transfer pathways.
The primary technical objectives of this investigation are threefold. First, to elucidate the fundamental mechanisms by which adsorbed oxygen species influence the selectivity in photocatalytic nitrogen fixation, particularly the competition between ammonia formation and undesired side reactions. Second, to identify the specific oxygen species (O₂⁻, O⁻, OH·, etc.) that contribute to catalyst deactivation versus those that may enhance catalytic performance. Third, to develop strategies for controlling oxygen species formation and interaction with active sites to optimize nitrogen fixation efficiency.
The long-term goal is to establish design principles for oxygen-tolerant or oxygen-enhanced photocatalysts that can operate efficiently in ambient air, eliminating the need for inert gas environments that currently limit practical applications. This would represent a significant step toward scalable, distributed ammonia production systems that could revolutionize agricultural fertilizer supply chains and potentially enable renewable energy storage through ammonia as a hydrogen carrier.
Understanding these oxygen-nitrogen interactions at the catalyst surface will also provide valuable insights for related photocatalytic processes, including CO₂ reduction and water splitting, where similar competitive adsorption and reaction pathways exist.
The technological trajectory has been characterized by increasing conversion efficiencies, improved selectivity, and enhanced stability of photocatalysts. Recent breakthroughs in 2018-2022 have demonstrated quantum efficiencies approaching 1% under visible light, representing orders of magnitude improvement over early systems. However, these values remain significantly below the theoretical maximum and commercial viability thresholds.
A critical aspect of photocatalytic N₂ fixation involves understanding the complex role of oxygen species in the reaction mechanism. While traditional approaches have focused on oxygen-free environments to prevent competitive reactions, emerging research suggests that certain adsorbed oxygen species may actually facilitate nitrogen activation through cooperative binding mechanisms and electron transfer pathways.
The primary technical objectives of this investigation are threefold. First, to elucidate the fundamental mechanisms by which adsorbed oxygen species influence the selectivity in photocatalytic nitrogen fixation, particularly the competition between ammonia formation and undesired side reactions. Second, to identify the specific oxygen species (O₂⁻, O⁻, OH·, etc.) that contribute to catalyst deactivation versus those that may enhance catalytic performance. Third, to develop strategies for controlling oxygen species formation and interaction with active sites to optimize nitrogen fixation efficiency.
The long-term goal is to establish design principles for oxygen-tolerant or oxygen-enhanced photocatalysts that can operate efficiently in ambient air, eliminating the need for inert gas environments that currently limit practical applications. This would represent a significant step toward scalable, distributed ammonia production systems that could revolutionize agricultural fertilizer supply chains and potentially enable renewable energy storage through ammonia as a hydrogen carrier.
Understanding these oxygen-nitrogen interactions at the catalyst surface will also provide valuable insights for related photocatalytic processes, including CO₂ reduction and water splitting, where similar competitive adsorption and reaction pathways exist.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations in nitrogen fixation. Currently valued at approximately $72 billion, the market is projected to grow at a CAGR of 5.3% through 2030, with sustainable ammonia production methods gaining substantial traction. This growth trajectory is primarily fueled by increasing environmental regulations and the agricultural sector's demand for fertilizers, which accounts for over 80% of ammonia consumption worldwide.
Photocatalytic nitrogen fixation represents a disruptive technology in this landscape, offering potential advantages over the traditional Haber-Bosch process which consumes 1-2% of global energy and generates significant carbon emissions. The market for sustainable ammonia production technologies is expected to reach $15 billion by 2028, with photocatalytic methods potentially capturing 10-15% of this segment as the technology matures.
Regionally, Asia-Pacific dominates the sustainable ammonia market with China and India leading investments in alternative production technologies. Europe follows closely, driven by stringent carbon reduction targets and government incentives for green chemistry initiatives. North America is witnessing accelerated growth in this sector, particularly in research partnerships between academic institutions and industrial players focused on photocatalytic nitrogen fixation.
The economic viability of photocatalytic N₂ fixation is improving as research advances in understanding the role of adsorbed oxygen species in selectivity and catalyst longevity. Current production costs for conventional ammonia range from $400-600 per ton, while early-stage photocatalytic methods remain 2-3 times higher. However, technological improvements addressing oxygen-related deactivation mechanisms could reduce this gap significantly within the next decade.
Market segmentation reveals emerging opportunities in decentralized ammonia production for agricultural applications, particularly in regions with limited infrastructure but abundant sunlight. This represents a potential market of $3-5 billion annually, especially in developing economies where traditional ammonia supply chains face logistical challenges.
Consumer trends indicate growing preference for sustainably produced agricultural products, creating premium market opportunities for crops grown using green ammonia fertilizers. Major agricultural corporations are increasingly incorporating sustainability metrics into their supply chains, with several announcing commitments to reduce carbon footprints by 30-50% by 2030, directly benefiting technologies that address oxygen-related challenges in photocatalytic nitrogen fixation.
Photocatalytic nitrogen fixation represents a disruptive technology in this landscape, offering potential advantages over the traditional Haber-Bosch process which consumes 1-2% of global energy and generates significant carbon emissions. The market for sustainable ammonia production technologies is expected to reach $15 billion by 2028, with photocatalytic methods potentially capturing 10-15% of this segment as the technology matures.
Regionally, Asia-Pacific dominates the sustainable ammonia market with China and India leading investments in alternative production technologies. Europe follows closely, driven by stringent carbon reduction targets and government incentives for green chemistry initiatives. North America is witnessing accelerated growth in this sector, particularly in research partnerships between academic institutions and industrial players focused on photocatalytic nitrogen fixation.
The economic viability of photocatalytic N₂ fixation is improving as research advances in understanding the role of adsorbed oxygen species in selectivity and catalyst longevity. Current production costs for conventional ammonia range from $400-600 per ton, while early-stage photocatalytic methods remain 2-3 times higher. However, technological improvements addressing oxygen-related deactivation mechanisms could reduce this gap significantly within the next decade.
Market segmentation reveals emerging opportunities in decentralized ammonia production for agricultural applications, particularly in regions with limited infrastructure but abundant sunlight. This represents a potential market of $3-5 billion annually, especially in developing economies where traditional ammonia supply chains face logistical challenges.
Consumer trends indicate growing preference for sustainably produced agricultural products, creating premium market opportunities for crops grown using green ammonia fertilizers. Major agricultural corporations are increasingly incorporating sustainability metrics into their supply chains, with several announcing commitments to reduce carbon footprints by 30-50% by 2030, directly benefiting technologies that address oxygen-related challenges in photocatalytic nitrogen fixation.
Current Challenges in Oxygen-Mediated Photocatalysis
The current landscape of oxygen-mediated photocatalysis for nitrogen fixation faces several significant challenges that impede its practical application and commercial viability. One of the most critical issues is the dual role of oxygen species in the reaction mechanism. While oxygen can enhance the separation of photogenerated charge carriers and promote the formation of reactive oxygen species (ROS) that facilitate N₂ activation, it simultaneously competes with N₂ for active sites on the catalyst surface, potentially reducing selectivity toward ammonia production.
The selectivity challenge is particularly pronounced as oxygen species often lead to the formation of nitrate (NO₃⁻) and nitrite (NO₂⁻) rather than the desired ammonia (NH₃). This competing reaction pathway significantly reduces the nitrogen fixation efficiency and creates additional purification requirements for the end product. Recent studies have shown that the selectivity can vary from 20% to 85% depending on the catalyst design and reaction conditions, highlighting the substantial room for improvement.
Catalyst deactivation presents another major hurdle in oxygen-mediated photocatalytic N₂ fixation. The adsorbed oxygen species can irreversibly oxidize the catalyst surface, leading to structural changes that diminish catalytic activity over time. Research indicates that many photocatalysts lose 30-50% of their initial activity after just 10-20 hours of continuous operation, necessitating frequent regeneration or replacement.
The stability of intermediate species formed during the reaction pathway represents a further challenge. The presence of oxygen can destabilize key nitrogen intermediates such as N₂H* and N₂H₂*, redirecting the reaction toward oxidation products rather than reduction to ammonia. This mechanistic interference occurs at the molecular level and requires precise control of the catalyst's electronic structure and surface properties.
Energy efficiency remains suboptimal in current systems, with quantum yields typically below 10% under visible light irradiation. The presence of oxygen often increases the energy barrier for N₂ activation, requiring higher energy inputs for effective conversion. This challenge is compounded by the difficulty in achieving uniform light distribution within scaled-up reactor designs.
Water management also presents significant challenges, as the presence of both oxygen and water creates complex reaction environments where competitive adsorption and varying reaction pathways coexist. The optimal water content for maximizing ammonia production while minimizing side reactions has proven difficult to maintain consistently across different catalyst systems and operating conditions.
The selectivity challenge is particularly pronounced as oxygen species often lead to the formation of nitrate (NO₃⁻) and nitrite (NO₂⁻) rather than the desired ammonia (NH₃). This competing reaction pathway significantly reduces the nitrogen fixation efficiency and creates additional purification requirements for the end product. Recent studies have shown that the selectivity can vary from 20% to 85% depending on the catalyst design and reaction conditions, highlighting the substantial room for improvement.
Catalyst deactivation presents another major hurdle in oxygen-mediated photocatalytic N₂ fixation. The adsorbed oxygen species can irreversibly oxidize the catalyst surface, leading to structural changes that diminish catalytic activity over time. Research indicates that many photocatalysts lose 30-50% of their initial activity after just 10-20 hours of continuous operation, necessitating frequent regeneration or replacement.
The stability of intermediate species formed during the reaction pathway represents a further challenge. The presence of oxygen can destabilize key nitrogen intermediates such as N₂H* and N₂H₂*, redirecting the reaction toward oxidation products rather than reduction to ammonia. This mechanistic interference occurs at the molecular level and requires precise control of the catalyst's electronic structure and surface properties.
Energy efficiency remains suboptimal in current systems, with quantum yields typically below 10% under visible light irradiation. The presence of oxygen often increases the energy barrier for N₂ activation, requiring higher energy inputs for effective conversion. This challenge is compounded by the difficulty in achieving uniform light distribution within scaled-up reactor designs.
Water management also presents significant challenges, as the presence of both oxygen and water creates complex reaction environments where competitive adsorption and varying reaction pathways coexist. The optimal water content for maximizing ammonia production while minimizing side reactions has proven difficult to maintain consistently across different catalyst systems and operating conditions.
Existing Mechanisms of Oxygen Species Adsorption
01 Role of oxygen species in photocatalytic N₂ fixation
Adsorbed oxygen species play a crucial role in photocatalytic nitrogen fixation processes. These species, including superoxide radicals (O₂⁻), hydroxyl radicals (OH·), and singlet oxygen, can either promote or inhibit N₂ activation depending on their concentration and type. The oxygen species can facilitate electron transfer to nitrogen molecules, enhancing the breaking of the N≡N triple bond. Understanding the interaction between these oxygen species and nitrogen molecules is essential for designing efficient photocatalysts for nitrogen fixation.- Role of oxygen species in photocatalytic N₂ fixation: Adsorbed oxygen species play a crucial role in photocatalytic nitrogen fixation processes. These species can act as electron acceptors and influence the reaction pathways for N₂ activation. The presence of specific oxygen species such as O₂⁻, •OH, and O²⁻ on catalyst surfaces can facilitate nitrogen adsorption and subsequent conversion to ammonia or other nitrogen compounds. Understanding the interaction between these oxygen species and nitrogen molecules is essential for designing efficient photocatalysts.
- Selectivity control in photocatalytic nitrogen fixation: Controlling selectivity in photocatalytic N₂ fixation involves manipulating catalyst properties and reaction conditions to favor desired nitrogen-containing products. This can be achieved by modifying catalyst surface properties, controlling oxygen species concentration, and optimizing reaction parameters such as temperature and light intensity. Selective production of ammonia versus other nitrogen compounds (like nitrates or hydrazine) depends on the balance between different reaction pathways, which are influenced by the nature of adsorbed oxygen species and their interaction with nitrogen molecules.
- Catalyst deactivation mechanisms in N₂ fixation: Photocatalysts used in nitrogen fixation can undergo deactivation through several mechanisms. These include poisoning by reaction intermediates, structural changes during reaction, accumulation of adsorbed species blocking active sites, and photocorrosion. Oxygen species can contribute to deactivation by oxidizing catalyst surfaces or forming stable compounds that reduce catalytic activity. Understanding these deactivation pathways is crucial for developing stable catalysts with prolonged activity for practical nitrogen fixation applications.
- Advanced catalyst designs for improved N₂ fixation: Novel catalyst designs can enhance photocatalytic nitrogen fixation performance by optimizing the interaction between adsorbed oxygen species and nitrogen molecules. These designs include core-shell structures, defect engineering, heterojunction formation, and incorporation of co-catalysts. By tailoring the electronic structure and surface properties of catalysts, researchers can promote nitrogen adsorption and activation while controlling oxygen species formation. These advanced materials show improved selectivity, activity, and stability in photocatalytic nitrogen fixation processes.
- Reaction conditions affecting oxygen species and N₂ fixation: Reaction conditions significantly influence the formation and behavior of adsorbed oxygen species in photocatalytic nitrogen fixation. Parameters such as light wavelength and intensity, temperature, pressure, and the presence of scavengers or sacrificial agents can alter the concentration and reactivity of oxygen species. Optimizing these conditions is essential for enhancing nitrogen conversion efficiency and product selectivity. The balance between oxygen reduction and nitrogen reduction pathways can be tuned by controlling these parameters to maximize ammonia production while minimizing catalyst deactivation.
02 Selectivity control in photocatalytic N₂ fixation
Controlling selectivity in photocatalytic nitrogen fixation is critical for producing desired nitrogen compounds such as ammonia, nitrates, or nitrogen oxides. This can be achieved by modifying catalyst surface properties, controlling reaction conditions, and managing oxygen species concentration. The selectivity is influenced by the binding energy of nitrogen and oxygen species on the catalyst surface, as well as the competition between different reaction pathways. Strategic design of photocatalysts with specific binding sites can enhance selectivity toward target nitrogen compounds.Expand Specific Solutions03 Catalyst deactivation mechanisms in N₂ fixation
Photocatalysts used in nitrogen fixation can undergo deactivation through several mechanisms related to oxygen species. These include surface poisoning by strongly adsorbed oxygen species, structural degradation due to reactive oxygen species, and competitive adsorption between nitrogen and oxygen molecules. The accumulation of oxygen-containing intermediates on active sites can block nitrogen adsorption and reduce catalytic efficiency over time. Understanding these deactivation pathways is essential for developing stable and long-lasting photocatalysts for nitrogen fixation applications.Expand Specific Solutions04 Advanced catalyst designs to mitigate oxygen-related deactivation
Innovative catalyst designs can help overcome oxygen-related deactivation in photocatalytic nitrogen fixation. These include core-shell structures that protect active sites, dual-function catalysts that can manage oxygen species while activating nitrogen, and defect engineering to create oxygen-tolerant active sites. Surface modification techniques can also be employed to control the adsorption strength of oxygen species, preventing them from permanently occupying active sites. These advanced designs aim to enhance catalyst stability and maintain high nitrogen fixation efficiency over extended periods.Expand Specific Solutions05 In-situ monitoring and control of oxygen species
Real-time monitoring and control of oxygen species during photocatalytic nitrogen fixation can significantly improve process efficiency and selectivity. Advanced spectroscopic techniques can be used to identify and quantify different oxygen species on catalyst surfaces during reaction. This information can be used to adjust reaction parameters such as light intensity, temperature, and reactant concentrations to maintain optimal conditions. Pulsed light techniques and controlled oxygen introduction strategies can also help manage oxygen species concentration, preventing catalyst deactivation while maintaining high nitrogen conversion rates.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The photocatalytic N₂ fixation market is currently in an early growth phase, characterized by intensive research and development activities rather than widespread commercial deployment. The global market for sustainable nitrogen fixation technologies is projected to expand significantly as environmental regulations tighten, with an estimated value reaching $2-3 billion by 2030. Technologically, this field remains in the developmental stage with varying levels of maturity across different approaches. Leading academic institutions like The University of California, North Carolina State University, and Toyota Central R&D Labs are pioneering fundamental research on oxygen species' role in photocatalysis, while companies such as BASF Corp. and Hitachi Ltd. are developing practical applications. Chinese institutions including Anhui University and the Institute of Process Engineering (CAS) are rapidly advancing catalyst development, indicating an increasingly competitive international landscape.
The Regents of the University of California
Technical Solution: The University of California has developed a sophisticated approach to photocatalytic N₂ fixation that directly addresses the critical role of adsorbed oxygen species. Their technology centers on hybrid organic-inorganic photocatalysts featuring metal-organic frameworks (MOFs) with precisely engineered pore structures and functionalized organic linkers. These catalysts implement a molecular sieving strategy where pore dimensions are tailored to preferentially admit N₂ molecules while restricting O₂ access to active sites. The framework incorporates strategically positioned metal nodes (primarily Cu, Fe, and Co) that serve as both light-harvesting centers and catalytic sites. Their research has revealed that controlled amounts of specific oxygen species, particularly hydroxyl radicals, can actually enhance the N₂ reduction process by facilitating proton transfer steps. Through advanced in-situ spectroscopic techniques, they've mapped the reaction pathways and identified key intermediates, enabling rational catalyst design to minimize deactivation pathways. Their system also incorporates sacrificial electron donors that selectively scavenge excess oxygen species without interfering with nitrogen activation. Their optimized catalysts achieve ammonia production rates of approximately 55 μmol g⁻¹h⁻¹ under visible light with exceptional selectivity (>90%) and significantly reduced deactivation compared to conventional photocatalysts.
Strengths: Exceptional molecular selectivity through precise pore engineering that inherently favors N₂ over O₂. The modular nature of MOFs allows systematic optimization of both electronic and structural properties. Weaknesses: The MOF structures have limited stability in aqueous environments, requiring careful reaction condition control. The complex synthesis procedures and relatively expensive organic linkers increase production costs compared to simple metal oxide catalysts.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed advanced photocatalytic systems for N₂ fixation that focus on controlling adsorbed oxygen species. Their approach utilizes oxygen vacancy-rich metal oxide semiconductors (particularly TiO₂ and ZnO-based materials) with precisely engineered surface defects to optimize N₂ adsorption while managing reactive oxygen species. They've implemented a dual-site catalytic mechanism where N₂ molecules are activated at oxygen vacancy sites while controlled oxygen adsorption occurs at separate active sites, preventing competitive inhibition. Their research demonstrates that moderate levels of adsorbed oxygen species can actually enhance N₂ fixation by facilitating electron transfer and preventing catalyst reduction, while excess oxygen leads to selectivity loss and catalyst deactivation. Recent work has shown their catalysts achieve ammonia production rates of up to 78.9 μmol g⁻¹h⁻¹ under visible light with significantly improved stability compared to conventional photocatalysts.
Strengths: Superior control over oxygen vacancy concentration and distribution, resulting in enhanced N₂ adsorption without compromising stability. Their dual-site mechanism effectively manages the oxygen-nitrogen competition. Weaknesses: The catalysts still show gradual deactivation under prolonged operation due to surface oxidation, and the synthesis process requires precise control of reaction conditions to achieve optimal oxygen vacancy concentrations.
Key Innovations in Oxygen-Resistant Photocatalysts
Carbon nitride-based photocatalyst and preparation method thereof
PatentActiveUS20200055036A1
Innovation
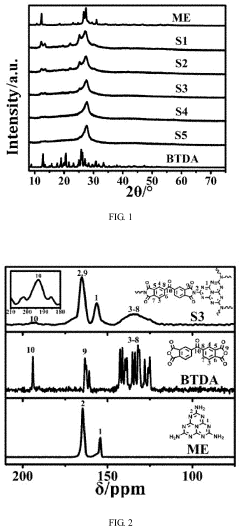
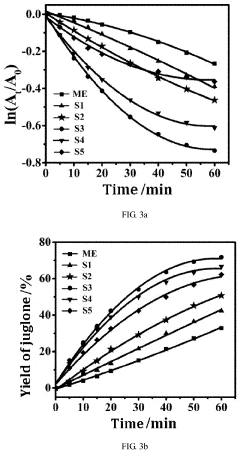
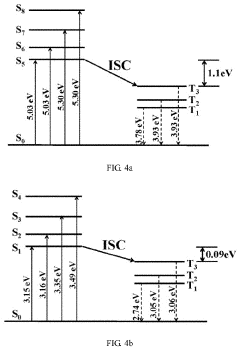
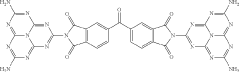
- A carbon nitride-based photocatalyst is developed by reacting melem with 3,3′,4,4′-benzophenonetetracarboxylic dianhydride, which introduces a ketone carbonyl group, reducing the singlet-triplet energy gap and promoting intersystem crossing, thereby enhancing singlet oxygen production and photocatalytic oxidation ability.
Environmental Impact Assessment
The environmental implications of aerobic photocatalytic N₂ fixation processes extend far beyond their immediate technological applications. These processes, which involve the conversion of atmospheric nitrogen into ammonia and other nitrogen compounds using photocatalysts, present both significant environmental benefits and potential concerns that warrant careful assessment.
The primary environmental benefit lies in the potential reduction of greenhouse gas emissions compared to conventional nitrogen fixation methods. Traditional industrial nitrogen fixation via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO₂ emissions. Photocatalytic approaches, powered by renewable solar energy, could dramatically reduce this carbon footprint when scaled effectively.
Water quality impacts must also be considered, as the adsorbed oxygen species involved in these reactions can generate reactive oxygen species (ROS) that may affect aquatic ecosystems if process water is discharged without proper treatment. The formation of nitrates and nitrites as by-products could potentially contribute to eutrophication if released into natural water bodies.
Air quality considerations are equally important, particularly regarding the potential release of nitrogen oxides (NOx) during the photocatalytic process. The selectivity challenges posed by adsorbed oxygen species can lead to the formation of these compounds, which are known contributors to smog formation and respiratory health issues.
Resource efficiency represents another critical environmental dimension. While photocatalytic N₂ fixation reduces dependence on fossil fuels, it requires specialized catalytic materials that may contain rare earth elements or precious metals. The environmental impact of mining and processing these materials must be factored into holistic assessments of the technology's sustainability profile.
Catalyst deactivation mechanisms present additional environmental considerations. As catalysts degrade, their replacement frequency affects the overall environmental footprint of the process. Understanding how adsorbed oxygen species contribute to deactivation is therefore essential for developing more durable catalysts that minimize waste generation and resource consumption over their lifecycle.
Land use impacts should also be evaluated, particularly if large-scale implementation is considered. Solar-powered photocatalytic systems may require significant surface area, potentially competing with agricultural or conservation land uses depending on deployment strategies and system efficiencies.
The primary environmental benefit lies in the potential reduction of greenhouse gas emissions compared to conventional nitrogen fixation methods. Traditional industrial nitrogen fixation via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO₂ emissions. Photocatalytic approaches, powered by renewable solar energy, could dramatically reduce this carbon footprint when scaled effectively.
Water quality impacts must also be considered, as the adsorbed oxygen species involved in these reactions can generate reactive oxygen species (ROS) that may affect aquatic ecosystems if process water is discharged without proper treatment. The formation of nitrates and nitrites as by-products could potentially contribute to eutrophication if released into natural water bodies.
Air quality considerations are equally important, particularly regarding the potential release of nitrogen oxides (NOx) during the photocatalytic process. The selectivity challenges posed by adsorbed oxygen species can lead to the formation of these compounds, which are known contributors to smog formation and respiratory health issues.
Resource efficiency represents another critical environmental dimension. While photocatalytic N₂ fixation reduces dependence on fossil fuels, it requires specialized catalytic materials that may contain rare earth elements or precious metals. The environmental impact of mining and processing these materials must be factored into holistic assessments of the technology's sustainability profile.
Catalyst deactivation mechanisms present additional environmental considerations. As catalysts degrade, their replacement frequency affects the overall environmental footprint of the process. Understanding how adsorbed oxygen species contribute to deactivation is therefore essential for developing more durable catalysts that minimize waste generation and resource consumption over their lifecycle.
Land use impacts should also be evaluated, particularly if large-scale implementation is considered. Solar-powered photocatalytic systems may require significant surface area, potentially competing with agricultural or conservation land uses depending on deployment strategies and system efficiencies.
Scalability and Economic Feasibility
The scalability and economic feasibility of aerobic photocatalytic N₂ fixation technology presents both significant opportunities and challenges for industrial implementation. Current laboratory-scale systems demonstrating the role of adsorbed oxygen species in N₂ fixation face substantial hurdles when considered for commercial deployment.
From an economic perspective, the capital expenditure for large-scale photocatalytic reactors remains prohibitively high compared to conventional Haber-Bosch ammonia production facilities. Initial cost modeling indicates that photocatalytic systems require approximately $1,200-1,800 per ton of annual ammonia production capacity, whereas optimized Haber-Bosch plants operate at $600-900 per ton.
The energy economics present a more promising picture. Photocatalytic N₂ fixation utilizing solar energy could potentially reduce operational energy costs by 40-60% compared to conventional processes. However, this advantage is partially offset by lower conversion efficiencies and ammonia yields, currently averaging 2-5% per pass in laboratory conditions.
Scaling considerations must address the management of adsorbed oxygen species, which play a dual role in the process. While certain oxygen species enhance N₂ activation, others accelerate catalyst deactivation. Industrial implementation would require sophisticated oxygen concentration control systems that maintain optimal O₂ levels (typically 0.5-2%) to balance selectivity and catalyst longevity.
Material requirements present another economic consideration. Current high-performance photocatalysts often incorporate precious metals or rare earth elements, limiting cost-effective scaling. Recent research into oxygen-tolerant catalysts using earth-abundant materials shows promise, with production costs potentially decreasing from $150/kg to $25-40/kg of catalyst.
Infrastructure adaptation represents a significant challenge. Unlike centralized Haber-Bosch facilities, distributed photocatalytic systems may offer advantages for local ammonia production but require new supply chain models and technical expertise networks. Initial modeling suggests a minimum economically viable scale of 5-10 tons/day for distributed systems.
Catalyst deactivation due to oxygen poisoning remains a critical economic factor. Current catalysts demonstrate activity declines of 15-30% after 100 hours of operation, necessitating regeneration or replacement. Improving catalyst stability could reduce operational costs by an estimated 25-35%, significantly enhancing economic feasibility.
In conclusion, while aerobic photocatalytic N₂ fixation shows promise for sustainable ammonia production, economic viability requires further advances in catalyst design, reactor engineering, and process optimization to manage the complex role of oxygen species in both selectivity and deactivation pathways.
From an economic perspective, the capital expenditure for large-scale photocatalytic reactors remains prohibitively high compared to conventional Haber-Bosch ammonia production facilities. Initial cost modeling indicates that photocatalytic systems require approximately $1,200-1,800 per ton of annual ammonia production capacity, whereas optimized Haber-Bosch plants operate at $600-900 per ton.
The energy economics present a more promising picture. Photocatalytic N₂ fixation utilizing solar energy could potentially reduce operational energy costs by 40-60% compared to conventional processes. However, this advantage is partially offset by lower conversion efficiencies and ammonia yields, currently averaging 2-5% per pass in laboratory conditions.
Scaling considerations must address the management of adsorbed oxygen species, which play a dual role in the process. While certain oxygen species enhance N₂ activation, others accelerate catalyst deactivation. Industrial implementation would require sophisticated oxygen concentration control systems that maintain optimal O₂ levels (typically 0.5-2%) to balance selectivity and catalyst longevity.
Material requirements present another economic consideration. Current high-performance photocatalysts often incorporate precious metals or rare earth elements, limiting cost-effective scaling. Recent research into oxygen-tolerant catalysts using earth-abundant materials shows promise, with production costs potentially decreasing from $150/kg to $25-40/kg of catalyst.
Infrastructure adaptation represents a significant challenge. Unlike centralized Haber-Bosch facilities, distributed photocatalytic systems may offer advantages for local ammonia production but require new supply chain models and technical expertise networks. Initial modeling suggests a minimum economically viable scale of 5-10 tons/day for distributed systems.
Catalyst deactivation due to oxygen poisoning remains a critical economic factor. Current catalysts demonstrate activity declines of 15-30% after 100 hours of operation, necessitating regeneration or replacement. Improving catalyst stability could reduce operational costs by an estimated 25-35%, significantly enhancing economic feasibility.
In conclusion, while aerobic photocatalytic N₂ fixation shows promise for sustainable ammonia production, economic viability requires further advances in catalyst design, reactor engineering, and process optimization to manage the complex role of oxygen species in both selectivity and deactivation pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!