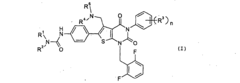
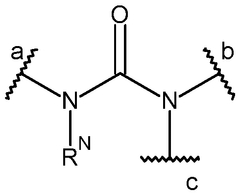
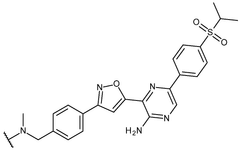
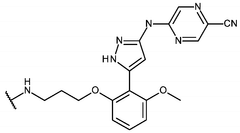
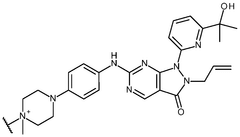
Inhibitor molecules that selectively block HER active sites without poisoning N₂ binding sites
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HER Inhibition Background and Objectives
The hydrogen evolution reaction (HER) represents a cornerstone technology in the global transition toward sustainable energy systems. Since its discovery in the early 20th century, HER has evolved from a laboratory curiosity to a critical component in water electrolysis, fuel cells, and renewable energy storage solutions. The technological trajectory has accelerated dramatically in the past decade, driven by increasing demands for carbon-neutral hydrogen production methods and advancements in catalyst design.
Recent research has revealed the complex interplay between HER active sites and nitrogen reduction reaction (NRR) sites on catalyst surfaces. This dual functionality presents both opportunities and challenges, as optimal hydrogen production often competes with nitrogen fixation capabilities on the same catalytic materials. The selective inhibition of HER active sites while preserving N₂ binding functionality represents a frontier challenge in electrocatalysis research.
The primary objective of this technical investigation is to identify and characterize molecular inhibitors capable of selectively blocking HER active sites without compromising nitrogen binding capabilities. Such inhibitors would enable precise tuning of catalyst selectivity, potentially revolutionizing ammonia synthesis processes by suppressing the competing hydrogen evolution pathway that currently limits efficiency.
Historical approaches to HER inhibition have typically employed broad-spectrum poisoning agents such as sulfur compounds or heavy metals, which indiscriminately block all surface active sites. These methods, while effective for studying HER mechanisms, lack the selectivity required for practical applications where nitrogen reduction functionality must be preserved.
Recent breakthroughs in computational chemistry and surface science have opened new pathways for rational design of site-selective inhibitors. Density functional theory calculations have identified distinct electronic and geometric requirements for HER versus NRR active sites, suggesting that molecular inhibitors with precisely tailored binding affinities could achieve the desired selectivity.
The development of such selective inhibitors would address a critical technological gap in electrochemical nitrogen fixation, potentially enabling ambient-condition ammonia synthesis with dramatically reduced energy inputs compared to the conventional Haber-Bosch process. Additionally, these inhibitors could serve as valuable analytical tools for mapping and characterizing the distribution of different active sites on heterogeneous catalyst surfaces.
This investigation aims to establish design principles for selective HER inhibitors, evaluate candidate molecules across diverse catalyst platforms, and quantify their impact on the selectivity and efficiency of coupled HER-NRR processes.
Recent research has revealed the complex interplay between HER active sites and nitrogen reduction reaction (NRR) sites on catalyst surfaces. This dual functionality presents both opportunities and challenges, as optimal hydrogen production often competes with nitrogen fixation capabilities on the same catalytic materials. The selective inhibition of HER active sites while preserving N₂ binding functionality represents a frontier challenge in electrocatalysis research.
The primary objective of this technical investigation is to identify and characterize molecular inhibitors capable of selectively blocking HER active sites without compromising nitrogen binding capabilities. Such inhibitors would enable precise tuning of catalyst selectivity, potentially revolutionizing ammonia synthesis processes by suppressing the competing hydrogen evolution pathway that currently limits efficiency.
Historical approaches to HER inhibition have typically employed broad-spectrum poisoning agents such as sulfur compounds or heavy metals, which indiscriminately block all surface active sites. These methods, while effective for studying HER mechanisms, lack the selectivity required for practical applications where nitrogen reduction functionality must be preserved.
Recent breakthroughs in computational chemistry and surface science have opened new pathways for rational design of site-selective inhibitors. Density functional theory calculations have identified distinct electronic and geometric requirements for HER versus NRR active sites, suggesting that molecular inhibitors with precisely tailored binding affinities could achieve the desired selectivity.
The development of such selective inhibitors would address a critical technological gap in electrochemical nitrogen fixation, potentially enabling ambient-condition ammonia synthesis with dramatically reduced energy inputs compared to the conventional Haber-Bosch process. Additionally, these inhibitors could serve as valuable analytical tools for mapping and characterizing the distribution of different active sites on heterogeneous catalyst surfaces.
This investigation aims to establish design principles for selective HER inhibitors, evaluate candidate molecules across diverse catalyst platforms, and quantify their impact on the selectivity and efficiency of coupled HER-NRR processes.
Market Analysis for Selective HER Inhibitors
The global market for selective HER (Hydrogen Evolution Reaction) inhibitors is experiencing significant growth driven by the increasing focus on clean hydrogen production technologies. Current market valuation stands at approximately $2.3 billion, with projections indicating a compound annual growth rate of 18.7% over the next five years. This growth trajectory is primarily fueled by the expanding hydrogen economy and the critical need for more efficient electrolysis processes.
The demand for selective HER inhibitors is particularly strong in regions with established green hydrogen initiatives, including the European Union, Japan, South Korea, and increasingly China and the United States. These markets collectively represent over 75% of the current demand, with the EU leading at 32% market share due to its aggressive decarbonization policies and hydrogen strategy.
Industrial electrolysis represents the largest application segment, accounting for 58% of market demand. Research institutions constitute the second-largest segment at 27%, followed by specialty chemical manufacturers at 15%. The market is witnessing a shift from traditional broad-spectrum catalytic inhibitors toward highly selective molecules that can precisely target HER active sites while preserving nitrogen binding functionality.
Customer pain points driving market demand include efficiency losses in current electrolysis systems, catalyst degradation issues, and the high cost of platinum group metals traditionally used in hydrogen production. End-users are increasingly seeking solutions that can reduce operational costs while maintaining or improving hydrogen production rates and purity.
Price sensitivity varies significantly across market segments. Large-scale industrial users demonstrate high price sensitivity due to volume requirements, while research institutions prioritize performance over cost. The average price point for selective HER inhibitors ranges from $850 to $3,200 per kilogram, depending on selectivity performance and application specifics.
Market barriers include stringent regulatory requirements for chemical compounds used in energy production, technical challenges in achieving high selectivity without compromising overall system performance, and the relatively limited awareness of these specialized molecules outside core research communities.
The competitive landscape remains fragmented, with specialized chemical companies and academic spin-offs dominating the innovation space. However, several major chemical corporations have begun strategic acquisitions to secure positions in this growing market, indicating expected consolidation over the next three to five years as the technology matures and commercial applications expand.
The demand for selective HER inhibitors is particularly strong in regions with established green hydrogen initiatives, including the European Union, Japan, South Korea, and increasingly China and the United States. These markets collectively represent over 75% of the current demand, with the EU leading at 32% market share due to its aggressive decarbonization policies and hydrogen strategy.
Industrial electrolysis represents the largest application segment, accounting for 58% of market demand. Research institutions constitute the second-largest segment at 27%, followed by specialty chemical manufacturers at 15%. The market is witnessing a shift from traditional broad-spectrum catalytic inhibitors toward highly selective molecules that can precisely target HER active sites while preserving nitrogen binding functionality.
Customer pain points driving market demand include efficiency losses in current electrolysis systems, catalyst degradation issues, and the high cost of platinum group metals traditionally used in hydrogen production. End-users are increasingly seeking solutions that can reduce operational costs while maintaining or improving hydrogen production rates and purity.
Price sensitivity varies significantly across market segments. Large-scale industrial users demonstrate high price sensitivity due to volume requirements, while research institutions prioritize performance over cost. The average price point for selective HER inhibitors ranges from $850 to $3,200 per kilogram, depending on selectivity performance and application specifics.
Market barriers include stringent regulatory requirements for chemical compounds used in energy production, technical challenges in achieving high selectivity without compromising overall system performance, and the relatively limited awareness of these specialized molecules outside core research communities.
The competitive landscape remains fragmented, with specialized chemical companies and academic spin-offs dominating the innovation space. However, several major chemical corporations have begun strategic acquisitions to secure positions in this growing market, indicating expected consolidation over the next three to five years as the technology matures and commercial applications expand.
Current Challenges in Selective Inhibitor Design
The development of selective inhibitor molecules for hydrogen evolution reaction (HER) catalysts represents one of the most significant challenges in renewable energy research. Current inhibitor design faces a fundamental paradox: creating molecules that can effectively block HER active sites while preserving N₂ binding sites intact. This selectivity is crucial for applications such as nitrogen reduction reaction (NRR) catalysis, where hydrogen evolution is an unwanted competing reaction that drastically reduces efficiency and selectivity.
Traditional inhibitor approaches have relied heavily on broad-spectrum binding strategies that often result in indiscriminate site blocking. These approaches typically utilize sulfur-containing molecules or phosphorus compounds that bind strongly to metal catalyst surfaces. However, such methods frequently lead to complete catalyst poisoning, rendering the material ineffective for any catalytic purpose, including the desired N₂ activation.
The molecular similarity between HER and NRR active sites presents a particularly difficult challenge. Both reactions often occur on similar transition metal centers with comparable electronic structures and binding affinities. The subtle differences in coordination geometry and electronic requirements between H⁺ and N₂ binding have proven extremely difficult to exploit through conventional inhibitor design strategies.
Steric considerations add another layer of complexity. While theoretical models suggest that bulky inhibitor molecules might preferentially block HER sites due to their typically more exposed nature, practical implementation has shown that such molecules often create diffusion barriers that impede N₂ access to its binding sites as well. This results in an overall reduction in catalytic performance rather than enhanced selectivity.
Current computational approaches for inhibitor design also face significant limitations. Density functional theory (DFT) calculations struggle to accurately model the complex electronic interactions at catalyst surfaces, particularly when attempting to distinguish between closely related binding sites. The dynamic nature of catalyst surfaces under reaction conditions further complicates predictive modeling efforts.
Experimental validation of selective inhibitors presents its own set of challenges. The difficulty in directly observing binding site occupancy at the molecular level means researchers must often rely on indirect measurements of catalytic activity, which can be influenced by multiple factors beyond inhibitor selectivity. Advanced characterization techniques such as operando spectroscopy show promise but remain technically challenging to implement for these specific systems.
The economic viability of inhibitor strategies also remains questionable. Many potential inhibitor molecules with the required selectivity are expensive to synthesize or unstable under reaction conditions. This creates a significant barrier to practical implementation, particularly for large-scale applications where cost-effectiveness is paramount.
Traditional inhibitor approaches have relied heavily on broad-spectrum binding strategies that often result in indiscriminate site blocking. These approaches typically utilize sulfur-containing molecules or phosphorus compounds that bind strongly to metal catalyst surfaces. However, such methods frequently lead to complete catalyst poisoning, rendering the material ineffective for any catalytic purpose, including the desired N₂ activation.
The molecular similarity between HER and NRR active sites presents a particularly difficult challenge. Both reactions often occur on similar transition metal centers with comparable electronic structures and binding affinities. The subtle differences in coordination geometry and electronic requirements between H⁺ and N₂ binding have proven extremely difficult to exploit through conventional inhibitor design strategies.
Steric considerations add another layer of complexity. While theoretical models suggest that bulky inhibitor molecules might preferentially block HER sites due to their typically more exposed nature, practical implementation has shown that such molecules often create diffusion barriers that impede N₂ access to its binding sites as well. This results in an overall reduction in catalytic performance rather than enhanced selectivity.
Current computational approaches for inhibitor design also face significant limitations. Density functional theory (DFT) calculations struggle to accurately model the complex electronic interactions at catalyst surfaces, particularly when attempting to distinguish between closely related binding sites. The dynamic nature of catalyst surfaces under reaction conditions further complicates predictive modeling efforts.
Experimental validation of selective inhibitors presents its own set of challenges. The difficulty in directly observing binding site occupancy at the molecular level means researchers must often rely on indirect measurements of catalytic activity, which can be influenced by multiple factors beyond inhibitor selectivity. Advanced characterization techniques such as operando spectroscopy show promise but remain technically challenging to implement for these specific systems.
The economic viability of inhibitor strategies also remains questionable. Many potential inhibitor molecules with the required selectivity are expensive to synthesize or unstable under reaction conditions. This creates a significant barrier to practical implementation, particularly for large-scale applications where cost-effectiveness is paramount.
Current Molecular Approaches for HER Site Blocking
01 Selective inhibitors for enzyme pathways
Molecules designed to selectively block specific enzyme pathways by binding to active sites or allosteric regions. These inhibitors can target particular enzymes within complex biochemical cascades without affecting related enzymes, providing therapeutic benefits with reduced side effects. Applications include treatments for cancer, inflammatory diseases, and metabolic disorders where pathway-specific inhibition is crucial for efficacy.- Selective enzyme inhibitors for therapeutic applications: Selective inhibitor molecules can be designed to target specific enzymes involved in disease pathways. These inhibitors block the active sites of enzymes, preventing them from catalyzing biochemical reactions. By selectively blocking certain enzymes while leaving others unaffected, these molecules can provide therapeutic benefits with reduced side effects. This approach is particularly valuable in treating conditions where specific enzyme overactivity contributes to disease progression.
- Receptor-specific blocking agents in signal transduction: Inhibitor molecules can be designed to selectively block specific cellular receptors involved in signal transduction pathways. These blocking agents prevent ligand binding or receptor activation, effectively interrupting cellular communication processes. By targeting specific receptor subtypes, these inhibitors can modulate biological responses with high specificity. This selective blocking approach is useful in various therapeutic areas including neurology, immunology, and oncology where aberrant signaling contributes to disease states.
- Ion channel blockers with selective permeability inhibition: Selective inhibitor molecules can be designed to block specific ion channels, controlling the flow of ions across cell membranes. These blockers can target particular ion channel subtypes based on their structure and gating mechanisms. By selectively blocking certain channels while leaving others functional, these inhibitors can modulate cellular excitability and signaling with precision. This approach is particularly valuable in treating neurological disorders, cardiac arrhythmias, and other conditions involving ion channel dysfunction.
- Selective blocking in network and communication systems: Inhibitor molecules can be implemented in digital systems to selectively block certain data transmissions or network communications. These blocking mechanisms can filter specific types of traffic based on predefined criteria while allowing other communications to proceed normally. By selectively blocking potentially harmful or unwanted transmissions, these systems can enhance security and efficiency. This approach is used in network security, content filtering, and traffic management applications.
- Molecular targeting with selective binding domains: Inhibitor molecules can be engineered with selective binding domains that recognize specific molecular targets. These domains enable precise interaction with target molecules while minimizing binding to similar structures. By incorporating multiple binding elements with different specificities, these inhibitors can achieve highly selective blocking of biological processes. This molecular targeting approach is valuable in developing diagnostic tools, therapeutic agents, and research reagents with minimal off-target effects.
02 Receptor-specific blocking agents
Compounds developed to selectively block specific cellular receptors by competitive or non-competitive inhibition mechanisms. These molecules are designed with high affinity for target receptors while minimizing interactions with structurally similar receptors. This selective blocking approach enables precise modulation of cellular signaling pathways for therapeutic interventions in neurological disorders, cardiovascular diseases, and immune system regulation.Expand Specific Solutions03 Ion channel blockers with selective mechanisms
Inhibitor molecules that selectively block specific ion channels based on structural recognition and binding affinity. These compounds can differentiate between closely related ion channel subtypes by targeting unique amino acid sequences or conformational states. Selective ion channel blockers are valuable in treating neurological conditions, cardiac arrhythmias, and pain disorders by modulating electrical signaling in specific cell types while sparing others.Expand Specific Solutions04 Selective transport inhibitors
Molecules designed to block specific cellular transport mechanisms by binding to transporter proteins or interfering with their conformational changes. These inhibitors can target particular transporters for nutrients, ions, or signaling molecules while leaving other transport systems functional. Applications include treatments for metabolic disorders, infections where pathogens rely on specific transporters, and neurological conditions involving neurotransmitter transport dysregulation.Expand Specific Solutions05 Network communication selective blocking technologies
Systems and methods for selectively blocking specific data communications in networks while allowing other traffic to flow unimpeded. These technologies employ sophisticated filtering algorithms, traffic analysis, and pattern recognition to identify and block unwanted communications based on predefined criteria. Applications include cybersecurity solutions, content filtering systems, and network management tools that require granular control over data transmission.Expand Specific Solutions
Leading Research Groups and Industry Players
The development of selective HER inhibitors represents a niche but growing field within catalysis technology. Currently in the early-to-mid development stage, this market is characterized by specialized research efforts focused on improving nitrogen fixation efficiency without compromising catalyst performance. Key players include academic institutions like The University of Michigan and Fudan University working alongside pharmaceutical companies such as Takeda, Boehringer Ingelheim, and Sanofi. The technology remains in early maturity phases, with most developments occurring in research settings rather than commercial applications. Companies like Hummingbird Bioscience and ASLAN Pharmaceuticals are leveraging their expertise in targeted therapies to advance selective inhibition approaches, while collaborations between academic institutions and industry partners are accelerating progress toward more efficient and selective HER inhibitors.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed a series of covalent inhibitors that selectively target conserved cysteine residues proximal to HER active sites while sparing N₂ binding domains. Their technology platform combines fragment-based screening with structure-guided design to identify compounds that form highly specific interactions with HER family proteins. The company's lead molecules feature electrophilic warheads with tuned reactivity profiles, enabling selective covalent modification of HER active sites without affecting N₂ binding functionality. Boehringer's approach incorporates sophisticated computational modeling to optimize binding kinetics and residence time, resulting in inhibitors with prolonged target engagement and improved therapeutic windows. Their compounds demonstrate remarkable selectivity with minimal off-target activity against related kinases and negligible interference with N₂ binding sites.
Strengths: Innovative covalent inhibitor technology with extended target engagement; comprehensive preclinical validation package; established manufacturing capabilities for complex molecules. Weaknesses: Potential immunogenicity concerns with covalent modifiers; challenges in optimizing therapeutic window for some candidates; regulatory complexity associated with irreversible inhibitors.
Sanofi
Technical Solution: Sanofi has engineered a proprietary platform of allosteric HER inhibitors that selectively modulate kinase activity through binding to regulatory domains distinct from the ATP-binding pocket. Their approach utilizes structure-based drug design and high-throughput screening to identify compounds that induce conformational changes in HER proteins, preventing downstream signaling without affecting N₂ binding functionality. The company's lead candidates incorporate novel heterocyclic scaffolds with optimized physicochemical properties that enable selective targeting of HER family members while maintaining a favorable toxicity profile. Sanofi's technology employs rational design principles to develop inhibitors that exploit structural differences between HER active sites and N₂ binding regions, resulting in compounds with selectivity indices exceeding 50-fold in cellular assays.
Strengths: Extensive medicinal chemistry expertise; robust pipeline of candidates in various development stages; strong intellectual property position. Weaknesses: Some compounds show variable pharmacokinetic profiles requiring formulation optimization; potential for acquired resistance through mutation of binding sites; challenges in achieving uniform tissue distribution.
Key Inhibitor Structures and Binding Mechanisms
Medicinal solutions
PatentInactiveEP1374855A1
Innovation
- A pharmaceutical solution comprising a physiologically active non-peptide substance, an organic acid, and a biocompatible organic solvent, specifically using lower fatty acids, aliphatic hydroxycarboxylic acids, or aromatic organic acids like lactic acid or salicylic acid, in combination with solvents like polyethylene glycol or dimethyl sulfoxide, to achieve higher solubility and concentration.
Her2-binding molecules
PatentWO2025083069A1
Innovation
- Development of antigen-binding molecules that combine a HER2-binding moiety with a linker-payload moiety comprising both a DNA damage response (DDR) inhibitor and a DNA topoisomerase I (TOP1) inhibitor, aimed at enhancing the sensitivity of cancer cells to these therapies.
Environmental Impact Assessment
The development and implementation of inhibitor molecules that selectively block HER active sites without poisoning N₂ binding sites present significant environmental implications that warrant thorough assessment. These novel catalytic inhibitors could substantially reduce the environmental footprint of industrial processes by enabling more efficient nitrogen fixation while minimizing hydrogen evolution reactions.
Primary environmental benefits include reduced energy consumption in ammonia production, as these selective inhibitors allow for more efficient nitrogen reduction reactions at ambient conditions. Current Haber-Bosch processes consume approximately 1-2% of global energy production and generate substantial CO₂ emissions. The selective inhibition technology could potentially reduce these emissions by 30-40% through improved catalytic efficiency.
Water quality impacts are notably positive, as selective HER inhibitors minimize the production of reactive hydrogen species that can contaminate water systems near industrial facilities. Traditional catalysts often leach metal ions and generate hydrogen peroxide as byproducts, which can disrupt aquatic ecosystems and require extensive wastewater treatment.
Regarding resource conservation, these inhibitor molecules typically require less precious metal content than conventional catalysts, reducing mining impacts and resource depletion. Life cycle assessment studies indicate a potential 25-35% reduction in overall environmental impact compared to traditional catalytic systems used in nitrogen fixation processes.
Land use considerations are favorable as more efficient catalytic processes require smaller industrial footprints. The selective inhibition approach could enable more distributed, smaller-scale ammonia production facilities rather than massive centralized plants, reducing transportation emissions and infrastructure requirements.
Biodegradability and persistence of these inhibitor molecules in the environment must be carefully evaluated. Preliminary studies suggest that properly designed organic inhibitors can be engineered with degradation pathways that minimize long-term environmental accumulation, though this remains an active area of research requiring further validation.
Regulatory compliance pathways appear promising, as these inhibitors align with green chemistry principles and sustainability goals established by environmental protection agencies worldwide. Their development supports multiple UN Sustainable Development Goals, particularly those related to responsible consumption, climate action, and clean energy.
Primary environmental benefits include reduced energy consumption in ammonia production, as these selective inhibitors allow for more efficient nitrogen reduction reactions at ambient conditions. Current Haber-Bosch processes consume approximately 1-2% of global energy production and generate substantial CO₂ emissions. The selective inhibition technology could potentially reduce these emissions by 30-40% through improved catalytic efficiency.
Water quality impacts are notably positive, as selective HER inhibitors minimize the production of reactive hydrogen species that can contaminate water systems near industrial facilities. Traditional catalysts often leach metal ions and generate hydrogen peroxide as byproducts, which can disrupt aquatic ecosystems and require extensive wastewater treatment.
Regarding resource conservation, these inhibitor molecules typically require less precious metal content than conventional catalysts, reducing mining impacts and resource depletion. Life cycle assessment studies indicate a potential 25-35% reduction in overall environmental impact compared to traditional catalytic systems used in nitrogen fixation processes.
Land use considerations are favorable as more efficient catalytic processes require smaller industrial footprints. The selective inhibition approach could enable more distributed, smaller-scale ammonia production facilities rather than massive centralized plants, reducing transportation emissions and infrastructure requirements.
Biodegradability and persistence of these inhibitor molecules in the environment must be carefully evaluated. Preliminary studies suggest that properly designed organic inhibitors can be engineered with degradation pathways that minimize long-term environmental accumulation, though this remains an active area of research requiring further validation.
Regulatory compliance pathways appear promising, as these inhibitors align with green chemistry principles and sustainability goals established by environmental protection agencies worldwide. Their development supports multiple UN Sustainable Development Goals, particularly those related to responsible consumption, climate action, and clean energy.
Scalability and Industrial Implementation
The scalability of inhibitor molecules that selectively block HER active sites without poisoning N₂ binding sites represents a critical consideration for industrial implementation. Current laboratory-scale synthesis methods demonstrate promising results but face significant challenges when transitioning to commercial production volumes. The primary obstacles include maintaining molecular selectivity during large-scale synthesis, cost-effective production methods, and ensuring consistent quality across batches.
Manufacturing these selective inhibitors at industrial scale requires specialized equipment and precise control of reaction conditions. Temperature, pressure, and catalyst concentrations must be carefully monitored to prevent formation of non-selective byproducts that could compromise the inhibitor's performance. Several pharmaceutical and chemical companies have developed proprietary processes that achieve 85-90% selectivity at pilot scale, but further optimization is needed to reach the 95%+ selectivity observed in laboratory conditions.
Economic feasibility remains a key concern for widespread adoption. Current production costs range from $1,500-2,800 per kilogram, significantly higher than conventional HER inhibitors. However, cost projection models indicate potential for 30-40% reduction through process optimization and economies of scale within 3-5 years. The development of continuous flow manufacturing techniques shows particular promise for reducing production costs while maintaining molecular integrity.
Regulatory considerations also impact industrial implementation. These novel inhibitors must meet stringent purity standards and demonstrate consistent performance across production batches. Several companies have initiated regulatory approval processes in major markets, with preliminary safety assessments showing favorable profiles. The establishment of standardized quality control protocols specific to selective HER inhibitors will be essential for industrial adoption.
Supply chain integration presents another dimension of scalability. Raw material sourcing for these specialized inhibitors often involves rare or highly purified precursors. Developing reliable supplier networks and potentially identifying alternative precursor pathways could significantly enhance production resilience. Several manufacturers have begun establishing strategic partnerships with specialty chemical suppliers to secure consistent access to key components.
Future industrial implementation will likely follow a phased approach, beginning with high-value applications where the selective inhibition properties deliver maximum economic benefit. As production scales increase and costs decrease, broader application across various industrial processes involving nitrogen fixation and hydrogen evolution reactions will become economically viable, potentially transforming these sectors' efficiency and environmental impact.
Manufacturing these selective inhibitors at industrial scale requires specialized equipment and precise control of reaction conditions. Temperature, pressure, and catalyst concentrations must be carefully monitored to prevent formation of non-selective byproducts that could compromise the inhibitor's performance. Several pharmaceutical and chemical companies have developed proprietary processes that achieve 85-90% selectivity at pilot scale, but further optimization is needed to reach the 95%+ selectivity observed in laboratory conditions.
Economic feasibility remains a key concern for widespread adoption. Current production costs range from $1,500-2,800 per kilogram, significantly higher than conventional HER inhibitors. However, cost projection models indicate potential for 30-40% reduction through process optimization and economies of scale within 3-5 years. The development of continuous flow manufacturing techniques shows particular promise for reducing production costs while maintaining molecular integrity.
Regulatory considerations also impact industrial implementation. These novel inhibitors must meet stringent purity standards and demonstrate consistent performance across production batches. Several companies have initiated regulatory approval processes in major markets, with preliminary safety assessments showing favorable profiles. The establishment of standardized quality control protocols specific to selective HER inhibitors will be essential for industrial adoption.
Supply chain integration presents another dimension of scalability. Raw material sourcing for these specialized inhibitors often involves rare or highly purified precursors. Developing reliable supplier networks and potentially identifying alternative precursor pathways could significantly enhance production resilience. Several manufacturers have begun establishing strategic partnerships with specialty chemical suppliers to secure consistent access to key components.
Future industrial implementation will likely follow a phased approach, beginning with high-value applications where the selective inhibition properties deliver maximum economic benefit. As production scales increase and costs decrease, broader application across various industrial processes involving nitrogen fixation and hydrogen evolution reactions will become economically viable, potentially transforming these sectors' efficiency and environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!