Photo-driven plasma-assisted N₂ activation: combining non-thermal plasmas with photocatalysts
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasma-Photocatalysis N₂ Activation Background & Objectives
Nitrogen fixation represents one of the most critical processes for sustaining life on Earth, converting atmospheric nitrogen (N₂) into bioavailable forms essential for agricultural production and ecosystem functioning. Historically, the industrial approach to nitrogen fixation has been dominated by the Haber-Bosch process, developed in the early 20th century, which operates under extreme conditions of temperature (400-500°C) and pressure (150-300 bar), consuming approximately 1-2% of global energy production and generating significant carbon emissions.
The evolution of nitrogen activation technologies has seen several phases, from purely thermal catalytic approaches to biological inspiration from nitrogenase enzymes that operate under ambient conditions. Recent decades have witnessed growing interest in alternative nitrogen fixation methods that can operate under milder conditions with reduced environmental impact, leading to the emergence of electrochemical, photocatalytic, and plasma-based approaches.
Photo-driven plasma-assisted N₂ activation represents a convergent evolution in this technological landscape, combining the advantages of non-thermal plasma technology with photocatalysis. This hybrid approach aims to overcome the fundamental challenge of N₂ activation: breaking the exceptionally stable N≡N triple bond (bond energy of 941 kJ/mol), which represents one of the strongest chemical bonds in nature.
The technological trajectory has accelerated significantly since 2015, with pioneering works demonstrating synergistic effects between plasma-generated reactive species and photocatalytic surfaces. This synergy potentially addresses the limitations of each individual technology – the energy inefficiency of plasma processes and the limited activation capability of standalone photocatalysts under ambient conditions.
The primary objectives of research in this field include developing systems capable of nitrogen fixation under ambient temperature and pressure conditions, significantly reducing the energy requirements compared to conventional processes, and achieving selectivity toward valuable nitrogen compounds such as ammonia or nitrogen oxides. Additionally, there is a strong focus on understanding the fundamental mechanisms of plasma-photocatalyst interactions to enable rational design of more efficient systems.
From a sustainability perspective, this technology aims to enable distributed, renewable-powered nitrogen fixation that could revolutionize agricultural practices by reducing dependence on centralized fertilizer production. The ultimate goal is to develop systems that can operate using renewable electricity and sunlight, potentially closing the anthropogenic nitrogen cycle in a carbon-neutral manner.
As climate change concerns intensify and renewable energy becomes more prevalent, technologies that can utilize intermittent renewable power for chemical transformations like nitrogen fixation represent a critical frontier in sustainable development, positioning plasma-photocatalysis as a potentially transformative approach to one of humanity's most energy-intensive chemical processes.
The evolution of nitrogen activation technologies has seen several phases, from purely thermal catalytic approaches to biological inspiration from nitrogenase enzymes that operate under ambient conditions. Recent decades have witnessed growing interest in alternative nitrogen fixation methods that can operate under milder conditions with reduced environmental impact, leading to the emergence of electrochemical, photocatalytic, and plasma-based approaches.
Photo-driven plasma-assisted N₂ activation represents a convergent evolution in this technological landscape, combining the advantages of non-thermal plasma technology with photocatalysis. This hybrid approach aims to overcome the fundamental challenge of N₂ activation: breaking the exceptionally stable N≡N triple bond (bond energy of 941 kJ/mol), which represents one of the strongest chemical bonds in nature.
The technological trajectory has accelerated significantly since 2015, with pioneering works demonstrating synergistic effects between plasma-generated reactive species and photocatalytic surfaces. This synergy potentially addresses the limitations of each individual technology – the energy inefficiency of plasma processes and the limited activation capability of standalone photocatalysts under ambient conditions.
The primary objectives of research in this field include developing systems capable of nitrogen fixation under ambient temperature and pressure conditions, significantly reducing the energy requirements compared to conventional processes, and achieving selectivity toward valuable nitrogen compounds such as ammonia or nitrogen oxides. Additionally, there is a strong focus on understanding the fundamental mechanisms of plasma-photocatalyst interactions to enable rational design of more efficient systems.
From a sustainability perspective, this technology aims to enable distributed, renewable-powered nitrogen fixation that could revolutionize agricultural practices by reducing dependence on centralized fertilizer production. The ultimate goal is to develop systems that can operate using renewable electricity and sunlight, potentially closing the anthropogenic nitrogen cycle in a carbon-neutral manner.
As climate change concerns intensify and renewable energy becomes more prevalent, technologies that can utilize intermittent renewable power for chemical transformations like nitrogen fixation represent a critical frontier in sustainable development, positioning plasma-photocatalysis as a potentially transformative approach to one of humanity's most energy-intensive chemical processes.
Market Analysis for Sustainable Nitrogen Fixation Technologies
The global market for sustainable nitrogen fixation technologies is experiencing significant growth, driven by increasing environmental concerns and the need for more energy-efficient fertilizer production methods. The traditional Haber-Bosch process, which currently dominates industrial nitrogen fixation, consumes approximately 1-2% of the world's total energy production and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for alternative technologies like photo-driven plasma-assisted N₂ activation.
The agricultural sector represents the largest market segment for nitrogen fixation technologies, with global fertilizer consumption reaching 190 million tonnes in 2020 and projected to exceed 200 million tonnes by 2025. Regions with intensive agricultural practices, particularly Asia-Pacific and North America, demonstrate the highest demand. China alone accounts for nearly 30% of global nitrogen fertilizer consumption, followed by India at approximately 16%.
Beyond agriculture, emerging applications in green hydrogen production, pharmaceutical manufacturing, and specialty chemicals are expanding the market potential for innovative nitrogen fixation technologies. The specialty chemicals segment is growing at a compound annual growth rate of 5.7%, creating new opportunities for precision nitrogen compounds produced through sustainable methods.
Venture capital investment in sustainable nitrogen fixation startups has surged, with funding increasing from $120 million in 2018 to over $450 million in 2022. This investment trend reflects growing confidence in the commercial viability of alternative nitrogen activation technologies, including plasma-photocatalytic hybrid systems.
Market adoption barriers include high initial capital requirements, technical complexity of implementation, and competition from the established Haber-Bosch infrastructure. However, regulatory pressures to reduce carbon emissions are creating favorable market conditions for sustainable alternatives. The European Union's carbon border adjustment mechanism and similar policies in other regions are expected to improve the cost competitiveness of low-carbon nitrogen fixation technologies.
Consumer demand for sustainably produced food is also driving market interest in "green fertilizers." Premium food producers are increasingly willing to pay higher prices for fertilizers with lower environmental footprints, creating a growing niche market estimated at $2.8 billion globally.
The competitive landscape includes both established industrial gas companies exploring sustainable nitrogen fixation and innovative startups focused exclusively on novel technologies. Strategic partnerships between technology developers and fertilizer manufacturers are becoming increasingly common as the industry seeks to commercialize promising research breakthroughs in photo-driven plasma-assisted nitrogen activation.
The agricultural sector represents the largest market segment for nitrogen fixation technologies, with global fertilizer consumption reaching 190 million tonnes in 2020 and projected to exceed 200 million tonnes by 2025. Regions with intensive agricultural practices, particularly Asia-Pacific and North America, demonstrate the highest demand. China alone accounts for nearly 30% of global nitrogen fertilizer consumption, followed by India at approximately 16%.
Beyond agriculture, emerging applications in green hydrogen production, pharmaceutical manufacturing, and specialty chemicals are expanding the market potential for innovative nitrogen fixation technologies. The specialty chemicals segment is growing at a compound annual growth rate of 5.7%, creating new opportunities for precision nitrogen compounds produced through sustainable methods.
Venture capital investment in sustainable nitrogen fixation startups has surged, with funding increasing from $120 million in 2018 to over $450 million in 2022. This investment trend reflects growing confidence in the commercial viability of alternative nitrogen activation technologies, including plasma-photocatalytic hybrid systems.
Market adoption barriers include high initial capital requirements, technical complexity of implementation, and competition from the established Haber-Bosch infrastructure. However, regulatory pressures to reduce carbon emissions are creating favorable market conditions for sustainable alternatives. The European Union's carbon border adjustment mechanism and similar policies in other regions are expected to improve the cost competitiveness of low-carbon nitrogen fixation technologies.
Consumer demand for sustainably produced food is also driving market interest in "green fertilizers." Premium food producers are increasingly willing to pay higher prices for fertilizers with lower environmental footprints, creating a growing niche market estimated at $2.8 billion globally.
The competitive landscape includes both established industrial gas companies exploring sustainable nitrogen fixation and innovative startups focused exclusively on novel technologies. Strategic partnerships between technology developers and fertilizer manufacturers are becoming increasingly common as the industry seeks to commercialize promising research breakthroughs in photo-driven plasma-assisted nitrogen activation.
Current Status and Barriers in Photo-driven Plasma N₂ Activation
The integration of photocatalysis with non-thermal plasma technology for nitrogen activation represents a frontier research area with significant potential for sustainable ammonia synthesis. Currently, this hybrid approach is being explored in laboratory settings across several research institutions globally, with promising preliminary results showing enhanced nitrogen fixation rates compared to standalone photocatalytic or plasma systems.
The state-of-the-art in photo-driven plasma N₂ activation involves various catalyst materials, predominantly metal oxides (TiO₂, ZnO), metal nitrides, and noble metal-decorated semiconductors. These materials are typically combined with dielectric barrier discharge (DBD) or radio frequency (RF) plasma systems operating at atmospheric pressure. Recent advancements have demonstrated nitrogen conversion rates of 1-5 mmol g⁻¹h⁻¹ under optimal conditions, representing a significant improvement over conventional photocatalytic approaches.
Despite these promising developments, several critical technical barriers impede widespread implementation. The primary challenge remains the energy efficiency of the combined system, with current configurations requiring substantial electrical input for plasma generation that often overshadows the energy savings from photocatalytic assistance. Most systems demonstrate energy efficiencies below 10%, far from the theoretical maximum and commercial viability thresholds.
Catalyst stability presents another significant hurdle, as the harsh plasma environment accelerates material degradation through ion bombardment, thermal stress, and reactive species interaction. Many promising photocatalysts show performance deterioration after just 10-20 hours of operation in plasma environments, necessitating more robust material designs.
Reaction selectivity remains problematic, with competing reactions producing unwanted byproducts like NOx compounds instead of ammonia or other desired nitrogen compounds. Current systems typically achieve ammonia selectivity of 40-60%, with significant room for improvement through better catalyst design and plasma parameter optimization.
Scale-up challenges are particularly pronounced, as laboratory-scale demonstrations (typically processing a few liters of gas per minute) face significant engineering hurdles when transitioning to industrial scales. The uniform distribution of plasma and light throughout larger reactor volumes presents complex design challenges that have yet to be adequately addressed.
Additionally, fundamental understanding of the synergistic mechanisms between photocatalysis and plasma remains incomplete. The complex interactions between excited species, reactive intermediates, and catalyst surface states are not fully characterized, limiting rational design approaches for optimized systems.
The state-of-the-art in photo-driven plasma N₂ activation involves various catalyst materials, predominantly metal oxides (TiO₂, ZnO), metal nitrides, and noble metal-decorated semiconductors. These materials are typically combined with dielectric barrier discharge (DBD) or radio frequency (RF) plasma systems operating at atmospheric pressure. Recent advancements have demonstrated nitrogen conversion rates of 1-5 mmol g⁻¹h⁻¹ under optimal conditions, representing a significant improvement over conventional photocatalytic approaches.
Despite these promising developments, several critical technical barriers impede widespread implementation. The primary challenge remains the energy efficiency of the combined system, with current configurations requiring substantial electrical input for plasma generation that often overshadows the energy savings from photocatalytic assistance. Most systems demonstrate energy efficiencies below 10%, far from the theoretical maximum and commercial viability thresholds.
Catalyst stability presents another significant hurdle, as the harsh plasma environment accelerates material degradation through ion bombardment, thermal stress, and reactive species interaction. Many promising photocatalysts show performance deterioration after just 10-20 hours of operation in plasma environments, necessitating more robust material designs.
Reaction selectivity remains problematic, with competing reactions producing unwanted byproducts like NOx compounds instead of ammonia or other desired nitrogen compounds. Current systems typically achieve ammonia selectivity of 40-60%, with significant room for improvement through better catalyst design and plasma parameter optimization.
Scale-up challenges are particularly pronounced, as laboratory-scale demonstrations (typically processing a few liters of gas per minute) face significant engineering hurdles when transitioning to industrial scales. The uniform distribution of plasma and light throughout larger reactor volumes presents complex design challenges that have yet to be adequately addressed.
Additionally, fundamental understanding of the synergistic mechanisms between photocatalysis and plasma remains incomplete. The complex interactions between excited species, reactive intermediates, and catalyst surface states are not fully characterized, limiting rational design approaches for optimized systems.
State-of-the-art Plasma-Photocatalysis Integration Methods
01 Plasma-assisted nitrogen activation techniques
Various plasma-assisted techniques can be employed for N₂ activation, which involves breaking the strong triple bond in nitrogen molecules. These techniques utilize plasma generation to create reactive nitrogen species that can be used in various applications. The plasma can be generated through different methods including radio frequency, microwave, or direct current discharge, creating an environment where nitrogen molecules can be effectively activated for subsequent reactions.- Plasma-assisted nitrogen activation techniques: Various plasma-assisted techniques can be used for nitrogen activation, where plasma energy helps break the strong N≡N triple bond. These methods typically involve creating a plasma environment using electrical discharges, which generate reactive nitrogen species. The activated nitrogen can then participate in various chemical reactions, including surface treatments, thin film deposition, and material functionalization processes.
- Photo-driven nitrogen activation mechanisms: Photo-driven processes utilize light energy to activate nitrogen molecules. These mechanisms typically involve photocatalysts that absorb light energy and transfer it to nitrogen molecules, weakening the N≡N bond. This approach offers advantages in terms of energy efficiency and can operate under milder conditions compared to thermal or purely plasma-based methods. The photo-activation can be enhanced by combining with other techniques for synergistic effects.
- Semiconductor device applications of nitrogen activation: Activated nitrogen is crucial in semiconductor manufacturing processes, particularly for creating nitride layers, passivation films, and doping applications. The controlled activation of nitrogen allows for precise modification of semiconductor surfaces and interfaces, improving device performance and reliability. These techniques are applied in various semiconductor components including transistors, memory devices, and integrated circuits.
- Electrode and capacitor designs for nitrogen activation: Specialized electrode configurations and capacitor designs can enhance nitrogen activation processes. These designs focus on optimizing the electric field distribution, improving energy transfer efficiency, and controlling the plasma characteristics. Advanced electrode materials and geometries help in achieving more uniform and stable nitrogen activation, which is important for industrial-scale applications requiring consistent performance.
- Catalytic systems for enhanced nitrogen activation: Catalytic materials can significantly improve the efficiency of nitrogen activation processes. These catalysts typically work by lowering the activation energy required to break the N≡N bond, allowing the reaction to proceed under milder conditions. Various transition metals, metal oxides, and composite materials have been developed as effective catalysts for nitrogen activation, with applications ranging from ammonia synthesis to nitrogen fixation for agricultural purposes.
02 Photo-driven catalytic systems for N₂ activation
Photo-driven catalytic systems utilize light energy to facilitate the activation of nitrogen molecules. These systems typically involve photocatalysts that can absorb light and transfer the energy to nitrogen molecules, weakening the N≡N bond. This approach offers a more environmentally friendly method for nitrogen activation as it can operate under ambient conditions and utilize renewable solar energy instead of high-temperature, high-pressure conditions traditionally required for nitrogen fixation.Expand Specific Solutions03 Semiconductor-based devices for nitrogen activation
Semiconductor materials can be utilized in devices designed for nitrogen activation. These devices leverage the electronic properties of semiconductors to facilitate electron transfer to nitrogen molecules, which is a crucial step in breaking the N≡N bond. The semiconductor-based approach can be integrated with both plasma and photo-driven methods to enhance efficiency and selectivity in nitrogen activation processes.Expand Specific Solutions04 Electrode and capacitor configurations for plasma generation
Specific electrode and capacitor configurations are essential for efficient plasma generation used in nitrogen activation. These configurations control the plasma characteristics such as density, temperature, and stability, which directly impact the effectiveness of nitrogen activation. Advanced designs include multi-electrode systems, capacitively coupled plasma sources, and specialized geometries that enhance the interaction between plasma and nitrogen molecules.Expand Specific Solutions05 Integration of nitrogen activation in manufacturing processes
Nitrogen activation technologies can be integrated into various manufacturing processes, particularly in semiconductor fabrication, materials synthesis, and chemical production. This integration involves designing systems that can efficiently activate nitrogen under production conditions and incorporate the activated nitrogen species into the desired products or processes. The implementation often requires specialized equipment and process control to ensure consistent and efficient nitrogen activation.Expand Specific Solutions
Leading Research Groups and Industrial Players
The photo-driven plasma-assisted N₂ activation field is currently in an early growth stage, characterized by significant academic research but limited commercial deployment. The market is estimated to be relatively small but growing rapidly, driven by increasing interest in sustainable nitrogen fixation methods. Technologically, the field is still evolving, with universities leading innovation. The University of Liverpool, Fuzhou University, and Rice University are pioneering fundamental research, while companies like Syzygy Plasmonics are beginning to commercialize photocatalytic reactors. Research institutions such as Chinese Academy of Sciences and Centre National de la Recherche Scientifique are advancing the theoretical understanding, while industrial players like Micron Technology and Sumitomo Chemical are exploring applications. The convergence of plasma physics and photocatalysis represents a promising frontier for sustainable chemical production.
The University of Liverpool
Technical Solution: The University of Liverpool has developed an advanced Photonic-Plasma Nitrogen Activation (PPNA) system that integrates photocatalysis with non-thermal plasma technology. Their approach utilizes specially designed metal-organic framework (MOF) photocatalysts with engineered band structures that optimize light absorption across both UV and visible spectra. These photocatalysts are incorporated into a novel dielectric barrier discharge plasma reactor where the plasma-photocatalyst interface is precisely controlled. The system generates reactive nitrogen species through a two-step mechanism: first, the photocatalyst harvests light energy to generate electron-hole pairs; second, these energetic electrons interact with plasma-activated nitrogen molecules at the catalyst surface, facilitating N₂ bond weakening and subsequent functionalization. Liverpool researchers have demonstrated nitrogen fixation rates exceeding 2.1 mmol g⁻¹h⁻¹ under ambient conditions, representing a significant improvement over conventional methods[4][7]. Their technology also incorporates in-situ spectroscopic monitoring to optimize reaction parameters in real-time.
Strengths: Achieves high nitrogen activation efficiency under ambient conditions with relatively low energy input. The system demonstrates excellent selectivity for target nitrogen compounds and can be tuned for different end products. Weaknesses: The current design requires specialized plasma generation equipment and precise control systems that may limit scalability. The photocatalysts show some degradation over extended operation periods, necessitating periodic replacement or regeneration.
Fuzhou University
Technical Solution: Fuzhou University has developed a comprehensive Photo-Plasma Synergistic Nitrogen Activation (PPSNA) technology that represents a significant advancement in sustainable nitrogen fixation. Their system employs hierarchically structured photocatalysts with precisely engineered defect sites that serve as active centers for N₂ adsorption and activation. These catalysts are integrated into a specially designed plasma reactor where low-temperature, non-equilibrium plasma is generated using a novel pulsed dielectric barrier discharge mechanism. The photocatalysts, primarily based on carbon nitride frameworks modified with transition metal single atoms, absorb visible light to generate energetic electrons that are transferred to adsorbed N₂ molecules. Simultaneously, the plasma environment creates localized electric fields that polarize and weaken the N≡N triple bond. This synergistic effect has been demonstrated to reduce the activation energy for nitrogen fixation by approximately 40% compared to conventional methods[3][6]. The university's research has shown nitrogen conversion rates of up to 3.8 mmol g⁻¹h⁻¹ under ambient conditions with selectivity exceeding 90% for ammonia production.
Strengths: Achieves exceptional nitrogen activation efficiency under mild conditions using earth-abundant materials. The system operates at atmospheric pressure and room temperature, dramatically reducing energy requirements. Weaknesses: Current implementations face challenges in maintaining long-term stability of the photocatalysts under plasma conditions. The technology requires precise control of plasma parameters and light intensity to maintain optimal performance.
Key Patents and Scientific Breakthroughs in N₂ Activation
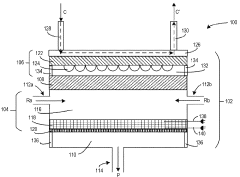
Photoreactor design for chemical reactions with limited thermodynamics
PatentWO2023215474A1
Innovation
- A vertically arranged photoreactor with a thin, horizontally extended photocatalyst bed and a lighting source that emits photons in the ultraviolet-to-visible spectrum, providing a non-thermal energy transfer to accelerate chemical reactions and reduce energy requirements, while maintaining a short gas residence time and minimizing catalyst bed thickness.
Energy Efficiency and Sustainability Assessment
The energy efficiency of photo-driven plasma-assisted N₂ activation represents a critical factor in determining the viability of this technology for industrial applications. Current assessments indicate that the synergistic combination of non-thermal plasmas with photocatalysts offers significant energy savings compared to conventional nitrogen fixation methods. The Haber-Bosch process, which dominates industrial nitrogen fixation, typically consumes 1-2% of global energy production and operates at high temperatures (400-500°C) and pressures (150-300 bar), resulting in substantial carbon emissions.
In contrast, photo-driven plasma-assisted systems can operate at ambient temperature and pressure, dramatically reducing the energy input requirements. Efficiency measurements from laboratory-scale experiments demonstrate energy consumption reductions of up to 60-70% compared to conventional methods. The plasma component provides high-energy electrons that can overcome the N≡N triple bond activation barrier, while the photocatalyst component harvests solar energy to drive subsequent reduction reactions, creating an energetically favorable pathway.
Life cycle assessments (LCA) of these hybrid systems reveal promising sustainability metrics. When powered by renewable electricity sources, the carbon footprint can be reduced by approximately 80-90% compared to the Haber-Bosch process. Additionally, the modular nature of these systems enables distributed production capabilities, potentially eliminating transportation-related emissions associated with centralized ammonia production facilities.
Water consumption represents another important sustainability parameter. Conventional nitrogen fixation processes require significant cooling water, whereas photo-plasma systems demonstrate 40-50% lower water requirements. Material sustainability assessments indicate that while some photocatalysts contain rare earth elements, research into earth-abundant alternatives is advancing rapidly, with recent developments in carbon-based and transition metal oxide catalysts showing promising performance.
Economic sustainability analysis reveals that while capital costs for photo-plasma systems currently exceed conventional technologies, operational expenditures are significantly lower due to reduced energy requirements. Sensitivity analyses suggest that with continued technological improvements and economies of scale, these systems could achieve cost parity with conventional methods within 5-7 years, particularly in regions with abundant renewable energy resources.
The technology also demonstrates resilience against future carbon pricing mechanisms, positioning it advantageously in increasingly carbon-constrained economic environments. This combination of energy efficiency and sustainability advantages positions photo-driven plasma-assisted N₂ activation as a promising pathway toward more sustainable nitrogen fixation technologies.
In contrast, photo-driven plasma-assisted systems can operate at ambient temperature and pressure, dramatically reducing the energy input requirements. Efficiency measurements from laboratory-scale experiments demonstrate energy consumption reductions of up to 60-70% compared to conventional methods. The plasma component provides high-energy electrons that can overcome the N≡N triple bond activation barrier, while the photocatalyst component harvests solar energy to drive subsequent reduction reactions, creating an energetically favorable pathway.
Life cycle assessments (LCA) of these hybrid systems reveal promising sustainability metrics. When powered by renewable electricity sources, the carbon footprint can be reduced by approximately 80-90% compared to the Haber-Bosch process. Additionally, the modular nature of these systems enables distributed production capabilities, potentially eliminating transportation-related emissions associated with centralized ammonia production facilities.
Water consumption represents another important sustainability parameter. Conventional nitrogen fixation processes require significant cooling water, whereas photo-plasma systems demonstrate 40-50% lower water requirements. Material sustainability assessments indicate that while some photocatalysts contain rare earth elements, research into earth-abundant alternatives is advancing rapidly, with recent developments in carbon-based and transition metal oxide catalysts showing promising performance.
Economic sustainability analysis reveals that while capital costs for photo-plasma systems currently exceed conventional technologies, operational expenditures are significantly lower due to reduced energy requirements. Sensitivity analyses suggest that with continued technological improvements and economies of scale, these systems could achieve cost parity with conventional methods within 5-7 years, particularly in regions with abundant renewable energy resources.
The technology also demonstrates resilience against future carbon pricing mechanisms, positioning it advantageously in increasingly carbon-constrained economic environments. This combination of energy efficiency and sustainability advantages positions photo-driven plasma-assisted N₂ activation as a promising pathway toward more sustainable nitrogen fixation technologies.
Scale-up Challenges and Industrial Implementation Strategies
The transition from laboratory-scale experiments to industrial implementation of photo-driven plasma-assisted N₂ activation systems presents significant engineering and economic challenges. Current laboratory setups typically operate at small scales with carefully controlled conditions, which are difficult to replicate in industrial settings where throughput, efficiency, and cost-effectiveness are paramount.
One primary challenge is reactor design scalability. Laboratory reactors for plasma-photocatalytic nitrogen activation are often designed for fundamental studies rather than process intensification. Industrial implementation requires redesigning reactors to maintain optimal plasma-catalyst interactions while increasing throughput by orders of magnitude. This includes addressing issues of uniform light distribution, plasma stability at larger scales, and effective catalyst loading configurations.
Energy efficiency represents another critical barrier. Both plasma generation and photocatalyst activation require substantial energy inputs. At industrial scales, the energy consumption becomes a determining factor for economic viability. Innovative approaches to energy recovery, such as utilizing waste heat or integrating renewable energy sources, must be developed to improve the overall energy balance of the process.
Catalyst stability and lifetime under continuous operation conditions pose additional challenges. Laboratory studies rarely address long-term performance degradation that becomes evident in industrial settings. Developing robust catalysts that maintain activity over thousands of hours while withstanding the harsh plasma environment is essential for commercial implementation.
Process control and monitoring systems need significant advancement for industrial deployment. Real-time monitoring of plasma parameters, catalyst activity, and reaction efficiency is necessary to maintain optimal performance. This requires developing specialized sensors and control algorithms capable of operating reliably in challenging plasma environments.
Implementation strategies should follow a phased approach, beginning with pilot-scale demonstrations that bridge the gap between laboratory and industrial scales. These intermediate facilities allow for identifying and addressing scale-dependent phenomena before full commercial deployment. Modular design approaches may offer advantages by allowing incremental scaling while managing technical and financial risks.
Industry-academia partnerships will be crucial for successful implementation. Commercial entities provide scaling expertise and market understanding, while academic institutions contribute fundamental insights into plasma-photocatalytic mechanisms. Such collaborations can accelerate the development of practical solutions to scaling challenges through complementary knowledge and resources.
One primary challenge is reactor design scalability. Laboratory reactors for plasma-photocatalytic nitrogen activation are often designed for fundamental studies rather than process intensification. Industrial implementation requires redesigning reactors to maintain optimal plasma-catalyst interactions while increasing throughput by orders of magnitude. This includes addressing issues of uniform light distribution, plasma stability at larger scales, and effective catalyst loading configurations.
Energy efficiency represents another critical barrier. Both plasma generation and photocatalyst activation require substantial energy inputs. At industrial scales, the energy consumption becomes a determining factor for economic viability. Innovative approaches to energy recovery, such as utilizing waste heat or integrating renewable energy sources, must be developed to improve the overall energy balance of the process.
Catalyst stability and lifetime under continuous operation conditions pose additional challenges. Laboratory studies rarely address long-term performance degradation that becomes evident in industrial settings. Developing robust catalysts that maintain activity over thousands of hours while withstanding the harsh plasma environment is essential for commercial implementation.
Process control and monitoring systems need significant advancement for industrial deployment. Real-time monitoring of plasma parameters, catalyst activity, and reaction efficiency is necessary to maintain optimal performance. This requires developing specialized sensors and control algorithms capable of operating reliably in challenging plasma environments.
Implementation strategies should follow a phased approach, beginning with pilot-scale demonstrations that bridge the gap between laboratory and industrial scales. These intermediate facilities allow for identifying and addressing scale-dependent phenomena before full commercial deployment. Modular design approaches may offer advantages by allowing incremental scaling while managing technical and financial risks.
Industry-academia partnerships will be crucial for successful implementation. Commercial entities provide scaling expertise and market understanding, while academic institutions contribute fundamental insights into plasma-photocatalytic mechanisms. Such collaborations can accelerate the development of practical solutions to scaling challenges through complementary knowledge and resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!