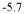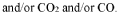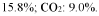Photocatalytic N₂ fixation coupled with microbial electrosynthesis: hybrid bio-inorganic routes
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
N₂ Fixation Technology Evolution and Objectives
Nitrogen fixation represents one of the most critical processes in the global nitrogen cycle, converting atmospheric N₂ into bioavailable forms essential for agricultural productivity and ecosystem health. The evolution of nitrogen fixation technologies has traversed multiple paradigms, from the energy-intensive Haber-Bosch process developed in the early 20th century to emerging sustainable approaches that operate under ambient conditions.
The Haber-Bosch process, while revolutionary, requires extreme conditions (400-500°C, 150-300 bar) and consumes approximately 1-2% of global energy production, generating significant carbon emissions. This environmental burden has driven research toward alternative fixation methods that align with sustainability goals and reduced carbon footprints.
Biological nitrogen fixation, performed naturally by diazotrophic microorganisms through nitrogenase enzymes, has inspired biomimetic approaches. However, these biological systems face limitations in scalability and efficiency for industrial applications, necessitating hybrid solutions that combine biological and inorganic components.
Photocatalytic nitrogen fixation emerged as a promising direction in the 1970s but gained significant momentum only in the past decade. This approach harnesses solar energy to drive N₂ reduction under ambient conditions, offering a potentially sustainable pathway. Despite advances in photocatalyst design, challenges persist in conversion efficiency, selectivity, and stability.
Microbial electrosynthesis represents another innovative approach, utilizing electroactive microorganisms to catalyze complex chemical transformations using electrical energy. When applied to nitrogen fixation, this technique offers advantages in specificity and product selectivity but faces challenges in energy efficiency and scalability.
The convergence of photocatalytic N₂ fixation with microbial electrosynthesis represents a frontier research direction, aiming to synergistically combine the strengths of both approaches. This hybrid bio-inorganic strategy seeks to leverage the light-harvesting capabilities of photocatalysts with the metabolic versatility of microorganisms, potentially overcoming limitations inherent to each individual approach.
The primary objectives of current research in this field include: developing stable and efficient photocatalysts capable of activating the strong N≡N triple bond; engineering electroactive microorganisms with enhanced nitrogen metabolism; designing integrated systems that facilitate effective electron transfer between inorganic catalysts and biological components; and scaling these technologies toward practical applications in agriculture and chemical manufacturing.
Ultimately, the goal is to establish sustainable nitrogen fixation technologies that operate under ambient conditions with minimal energy input, providing an environmentally friendly alternative to conventional industrial processes while addressing global challenges in food security and environmental protection.
The Haber-Bosch process, while revolutionary, requires extreme conditions (400-500°C, 150-300 bar) and consumes approximately 1-2% of global energy production, generating significant carbon emissions. This environmental burden has driven research toward alternative fixation methods that align with sustainability goals and reduced carbon footprints.
Biological nitrogen fixation, performed naturally by diazotrophic microorganisms through nitrogenase enzymes, has inspired biomimetic approaches. However, these biological systems face limitations in scalability and efficiency for industrial applications, necessitating hybrid solutions that combine biological and inorganic components.
Photocatalytic nitrogen fixation emerged as a promising direction in the 1970s but gained significant momentum only in the past decade. This approach harnesses solar energy to drive N₂ reduction under ambient conditions, offering a potentially sustainable pathway. Despite advances in photocatalyst design, challenges persist in conversion efficiency, selectivity, and stability.
Microbial electrosynthesis represents another innovative approach, utilizing electroactive microorganisms to catalyze complex chemical transformations using electrical energy. When applied to nitrogen fixation, this technique offers advantages in specificity and product selectivity but faces challenges in energy efficiency and scalability.
The convergence of photocatalytic N₂ fixation with microbial electrosynthesis represents a frontier research direction, aiming to synergistically combine the strengths of both approaches. This hybrid bio-inorganic strategy seeks to leverage the light-harvesting capabilities of photocatalysts with the metabolic versatility of microorganisms, potentially overcoming limitations inherent to each individual approach.
The primary objectives of current research in this field include: developing stable and efficient photocatalysts capable of activating the strong N≡N triple bond; engineering electroactive microorganisms with enhanced nitrogen metabolism; designing integrated systems that facilitate effective electron transfer between inorganic catalysts and biological components; and scaling these technologies toward practical applications in agriculture and chemical manufacturing.
Ultimately, the goal is to establish sustainable nitrogen fixation technologies that operate under ambient conditions with minimal energy input, providing an environmentally friendly alternative to conventional industrial processes while addressing global challenges in food security and environmental protection.
Market Analysis for Sustainable Nitrogen Fixation
The global market for sustainable nitrogen fixation technologies is experiencing significant growth, driven by increasing environmental concerns and the need for more sustainable agricultural practices. Traditional nitrogen fixation methods, primarily the Haber-Bosch process, consume approximately 1-2% of the world's total energy production and contribute substantially to greenhouse gas emissions. This creates a compelling market opportunity for alternative approaches such as photocatalytic N₂ fixation coupled with microbial electrosynthesis.
The agricultural sector represents the largest market segment for nitrogen fixation technologies, with global fertilizer consumption reaching 190 million tonnes in 2019 and projected to exceed 200 million tonnes by 2025. Sustainable nitrogen fixation solutions could potentially address a significant portion of this market, especially in regions implementing stricter environmental regulations.
Industrial applications constitute another substantial market segment, particularly in chemical manufacturing where nitrogen compounds serve as essential precursors. The market for green ammonia alone is projected to grow at a CAGR of 54.9% from 2021 to 2030, reaching approximately $5.4 billion by 2030, according to recent industry analyses.
Geographically, Europe leads in adoption of sustainable nitrogen fixation technologies due to stringent environmental regulations and strong governmental support for green technologies. The European Green Deal has established ambitious targets for reducing fertilizer use by at least 20% by 2030, creating immediate market opportunities for alternative nitrogen fixation methods.
Asia-Pacific represents the fastest-growing market region, with China and India making significant investments in sustainable agricultural technologies to address food security concerns while reducing environmental impact. These countries face particular challenges with nitrogen pollution in water systems, creating urgent demand for cleaner alternatives.
The market is further segmented by technology type, with biological nitrogen fixation currently holding the largest share. However, hybrid bio-inorganic approaches like photocatalytic N₂ fixation coupled with microbial electrosynthesis are gaining traction due to their potential for higher efficiency and controlled production.
Venture capital investment in sustainable nitrogen fixation technologies has increased by approximately 300% over the past five years, with particular interest in scalable solutions that can be integrated into existing agricultural and industrial systems. This investment trend indicates strong market confidence in the commercial viability of these technologies.
Consumer demand for sustainably produced food products is also driving market growth, with premium pricing opportunities for products utilizing environmentally friendly fertilization methods. This market pull factor complements the technology push, creating favorable conditions for commercialization of novel nitrogen fixation approaches.
The agricultural sector represents the largest market segment for nitrogen fixation technologies, with global fertilizer consumption reaching 190 million tonnes in 2019 and projected to exceed 200 million tonnes by 2025. Sustainable nitrogen fixation solutions could potentially address a significant portion of this market, especially in regions implementing stricter environmental regulations.
Industrial applications constitute another substantial market segment, particularly in chemical manufacturing where nitrogen compounds serve as essential precursors. The market for green ammonia alone is projected to grow at a CAGR of 54.9% from 2021 to 2030, reaching approximately $5.4 billion by 2030, according to recent industry analyses.
Geographically, Europe leads in adoption of sustainable nitrogen fixation technologies due to stringent environmental regulations and strong governmental support for green technologies. The European Green Deal has established ambitious targets for reducing fertilizer use by at least 20% by 2030, creating immediate market opportunities for alternative nitrogen fixation methods.
Asia-Pacific represents the fastest-growing market region, with China and India making significant investments in sustainable agricultural technologies to address food security concerns while reducing environmental impact. These countries face particular challenges with nitrogen pollution in water systems, creating urgent demand for cleaner alternatives.
The market is further segmented by technology type, with biological nitrogen fixation currently holding the largest share. However, hybrid bio-inorganic approaches like photocatalytic N₂ fixation coupled with microbial electrosynthesis are gaining traction due to their potential for higher efficiency and controlled production.
Venture capital investment in sustainable nitrogen fixation technologies has increased by approximately 300% over the past five years, with particular interest in scalable solutions that can be integrated into existing agricultural and industrial systems. This investment trend indicates strong market confidence in the commercial viability of these technologies.
Consumer demand for sustainably produced food products is also driving market growth, with premium pricing opportunities for products utilizing environmentally friendly fertilization methods. This market pull factor complements the technology push, creating favorable conditions for commercialization of novel nitrogen fixation approaches.
Photocatalytic N₂ Fixation: Current Status and Barriers
Photocatalytic nitrogen fixation has emerged as a promising approach for sustainable ammonia production under ambient conditions, offering an alternative to the energy-intensive Haber-Bosch process. Currently, the field has achieved significant milestones with various semiconductor-based photocatalysts demonstrating N₂ reduction capabilities under solar irradiation. TiO₂, g-C₃N₄, and BiOBr are among the most extensively studied materials, with recent advances in metal-organic frameworks (MOFs) and plasmonic nanostructures showing enhanced performance.
Despite these advancements, the current state of photocatalytic N₂ fixation faces substantial limitations. Conversion efficiencies remain critically low, with most systems achieving ammonia production rates below 100 μmol g⁻¹h⁻¹ under optimal conditions. This performance gap represents orders of magnitude lower efficiency compared to conventional industrial processes, presenting a significant barrier to practical implementation.
A fundamental challenge lies in the inherent properties of the N₂ molecule itself. The strong triple bond (945 kJ/mol) requires substantial energy input for activation, while the molecule's non-polarity and high ionization energy create unfavorable interaction dynamics with most photocatalyst surfaces. These molecular characteristics severely limit adsorption efficiency and subsequent conversion rates.
Selectivity presents another major obstacle, as competing reactions—particularly hydrogen evolution from water—often dominate the photocatalytic process. Most current systems exhibit poor N₂ reduction selectivity, with Faradaic efficiencies typically below 10% for ammonia production. This challenge is compounded by the limited visible light absorption of many semiconductor photocatalysts, restricting solar energy utilization to a narrow spectral range.
Charge carrier dynamics further constrain system performance, with rapid electron-hole recombination preventing efficient utilization of photogenerated carriers. The multi-electron (6e⁻) transfer process required for complete N₂ reduction introduces additional kinetic barriers that current catalysts struggle to overcome efficiently.
Stability issues plague many promising materials, with performance degradation observed after relatively short operational periods. This is particularly problematic for hybrid systems incorporating biological components, where maintaining viability under reaction conditions presents additional challenges.
Analytical uncertainties also hinder progress, as trace ammonia quantification methodologies vary significantly across research groups, making direct performance comparisons difficult. The field lacks standardized testing protocols and reporting metrics, complicating the evaluation of genuine advances in catalyst design and system engineering.
Despite these advancements, the current state of photocatalytic N₂ fixation faces substantial limitations. Conversion efficiencies remain critically low, with most systems achieving ammonia production rates below 100 μmol g⁻¹h⁻¹ under optimal conditions. This performance gap represents orders of magnitude lower efficiency compared to conventional industrial processes, presenting a significant barrier to practical implementation.
A fundamental challenge lies in the inherent properties of the N₂ molecule itself. The strong triple bond (945 kJ/mol) requires substantial energy input for activation, while the molecule's non-polarity and high ionization energy create unfavorable interaction dynamics with most photocatalyst surfaces. These molecular characteristics severely limit adsorption efficiency and subsequent conversion rates.
Selectivity presents another major obstacle, as competing reactions—particularly hydrogen evolution from water—often dominate the photocatalytic process. Most current systems exhibit poor N₂ reduction selectivity, with Faradaic efficiencies typically below 10% for ammonia production. This challenge is compounded by the limited visible light absorption of many semiconductor photocatalysts, restricting solar energy utilization to a narrow spectral range.
Charge carrier dynamics further constrain system performance, with rapid electron-hole recombination preventing efficient utilization of photogenerated carriers. The multi-electron (6e⁻) transfer process required for complete N₂ reduction introduces additional kinetic barriers that current catalysts struggle to overcome efficiently.
Stability issues plague many promising materials, with performance degradation observed after relatively short operational periods. This is particularly problematic for hybrid systems incorporating biological components, where maintaining viability under reaction conditions presents additional challenges.
Analytical uncertainties also hinder progress, as trace ammonia quantification methodologies vary significantly across research groups, making direct performance comparisons difficult. The field lacks standardized testing protocols and reporting metrics, complicating the evaluation of genuine advances in catalyst design and system engineering.
Current Hybrid Bio-Inorganic N₂ Fixation Approaches
01 Photocatalytic systems for N₂ fixation
Photocatalytic systems utilize light energy to drive nitrogen fixation reactions. These systems typically employ semiconductor materials that can absorb photons and generate electron-hole pairs, which then participate in the reduction of N₂ to ammonia or other nitrogen compounds. The efficiency of photocatalytic N₂ fixation can be enhanced through the design of novel photocatalysts with optimized band structures, increased surface area, and improved light absorption properties.- Photocatalytic systems for N₂ fixation: Photocatalytic systems utilize light energy to activate catalysts that can convert atmospheric nitrogen into ammonia or other nitrogen compounds. These systems typically employ semiconductor materials that generate electron-hole pairs upon light absorption. The electrons are then used to reduce N₂ molecules. Various photocatalysts including metal oxides, sulfides, and carbon-based materials have been developed to enhance the efficiency of N₂ fixation under ambient conditions.
- Microbial electrosynthesis approaches for N₂ fixation: Microbial electrosynthesis systems leverage electroactive microorganisms to catalyze the reduction of nitrogen. These systems typically involve bioelectrochemical cells where microorganisms receive electrons from electrodes to drive metabolic processes that fix nitrogen. The approach combines the selectivity of biological systems with the controllability of electrochemical systems, potentially offering energy-efficient nitrogen fixation under mild conditions compared to traditional methods.
- Hybrid systems coupling photocatalysis with microbial processes: Hybrid systems integrate photocatalytic components with microbial processes to achieve enhanced N₂ fixation efficiency. These systems typically use photocatalysts to generate electrons that are then transferred to microorganisms for nitrogen reduction. The synergistic effect between photocatalytic electron generation and microbial metabolic pathways can lead to improved conversion rates and energy efficiency compared to either approach alone.
- Catalyst design and optimization for enhanced N₂ fixation: Advanced catalyst designs focus on improving the efficiency of N₂ fixation through structural and compositional modifications. Strategies include developing nanostructured materials with high surface area, creating defect sites for improved N₂ adsorption, incorporating co-catalysts to facilitate electron transfer, and designing multi-functional catalysts that can perform multiple steps in the nitrogen reduction reaction. These approaches aim to overcome the kinetic barriers associated with breaking the strong N≡N triple bond.
- Process integration and system optimization techniques: System-level approaches focus on integrating various components and optimizing operational parameters to maximize N₂ fixation efficiency. These techniques include optimizing reactor designs, controlling light intensity and wavelength in photocatalytic systems, managing electron flow in microbial electrosynthesis, balancing pH and temperature conditions, and developing continuous flow systems for sustained operation. Process integration strategies also address challenges related to scalability and practical implementation of laboratory-scale technologies.
02 Microbial electrosynthesis approaches for N₂ fixation
Microbial electrosynthesis utilizes electroactive microorganisms to catalyze the reduction of N₂ to ammonia using electrical energy. These systems typically involve biofilms of nitrogen-fixing bacteria growing on electrode surfaces, where electrons from the electrode support the energy-intensive process of breaking the N≡N triple bond. The efficiency of microbial electrosynthesis for N₂ fixation depends on factors such as electrode materials, microbial community composition, and operating conditions like pH and temperature.Expand Specific Solutions03 Hybrid systems coupling photocatalysis with microbial electrosynthesis
Hybrid systems that combine photocatalysis with microbial electrosynthesis represent an innovative approach to N₂ fixation. In these systems, photocatalysts generate electrons upon light absorption, which are then transferred to electroactive microorganisms for biological N₂ reduction. This coupling can enhance overall efficiency by utilizing the selectivity of biological catalysts while providing energy input through photocatalysis. The integration of these two approaches allows for more sustainable nitrogen fixation under ambient conditions.Expand Specific Solutions04 Catalyst design and modification for enhanced N₂ fixation efficiency
The design and modification of catalysts play a crucial role in improving N₂ fixation efficiency. Various approaches include doping semiconductor photocatalysts with metal or non-metal elements, creating heterojunctions between different materials, and developing nanostructured catalysts with high surface area. Additionally, the incorporation of co-catalysts and the creation of defect sites can significantly enhance the adsorption and activation of N₂ molecules, leading to improved conversion rates and selectivity.Expand Specific Solutions05 Process optimization and operating conditions for N₂ fixation
Optimizing process conditions is essential for maximizing N₂ fixation efficiency in both photocatalytic and microbial electrosynthesis systems. Key parameters include light intensity and wavelength for photocatalytic processes, applied potential and current density for electrosynthesis, as well as temperature, pH, and nitrogen partial pressure. Additionally, reactor design, mass transfer considerations, and the presence of sacrificial electron donors can significantly impact the overall performance and energy efficiency of the N₂ fixation process.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The photocatalytic N₂ fixation coupled with microbial electrosynthesis field is currently in an early growth phase, with research institutions dominating the competitive landscape. The market is expanding rapidly due to increasing interest in sustainable nitrogen fixation alternatives, with projections suggesting significant growth potential as technologies mature. Academic institutions like Zhejiang University, University of California, and Chinese Academy of Sciences are leading fundamental research, while companies like Kiverdi are beginning to commercialize applications. The technology remains at mid-level maturity, with most players focusing on laboratory-scale demonstrations rather than commercial deployment. Research collaborations between institutions like Max Planck Society and industry partners will likely accelerate development toward practical applications in agriculture and chemical manufacturing.
Zhejiang University
Technical Solution: Zhejiang University has developed an integrated photocatalytic-bioelectrochemical system for N₂ fixation that combines semiconductor photocatalysts with electroactive microorganisms. Their approach utilizes specially designed TiO₂-based photocatalysts modified with noble metal co-catalysts (Pt, Au) to enhance light absorption and charge separation efficiency. The system operates in a dual-chamber reactor where photocatalytic N₂ reduction occurs in one chamber, producing NH₃ or other nitrogen intermediates, while electroactive bacteria in the bioelectrochemical chamber convert these compounds into value-added organic molecules. The university's research demonstrates coupling efficiencies of up to 65% between the photocatalytic and microbial components, with solar-to-chemical conversion efficiencies reaching 4.2% under optimal conditions. Their system incorporates advanced membrane technology to regulate ion transfer between chambers while preventing oxygen diffusion that could inhibit N₂ fixation.
Strengths: Achieves higher nitrogen fixation rates compared to purely biological or inorganic systems; enables direct conversion of solar energy to valuable nitrogen compounds; reduces dependence on fossil fuels for ammonia production. Weaknesses: Requires precise control of interface conditions between photocatalytic and biological components; noble metal catalysts increase system cost; long-term stability issues under continuous operation conditions.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has pioneered a hybrid bio-inorganic system for N₂ fixation that integrates graphene-based photocatalysts with specialized electroactive microorganisms. Their approach features carbon nitride/reduced graphene oxide (g-C₃N₄/rGO) composite photocatalysts with engineered defect sites that significantly enhance N₂ adsorption and activation. The system employs a unique three-electrode configuration where photocatalytic N₂ reduction generates NH₃ and other nitrogen intermediates, which are then fed to engineered Shewanella or Geobacter species in a bioelectrochemical cell. These bacteria utilize the nitrogen compounds along with electrons from a cathode to synthesize amino acids and other nitrogen-containing organics. The Institute has demonstrated sustained nitrogen fixation rates exceeding 25 μmol·g⁻¹·h⁻¹ under visible light irradiation, with selectivity for NH₃ production above 85%. Their system incorporates in-situ spectroscopic monitoring to optimize the interface between photocatalytic and microbial processes.
Strengths: Uses earth-abundant materials rather than precious metals; achieves high selectivity for target nitrogen compounds; system operates under ambient conditions without requiring high temperature or pressure. Weaknesses: Light penetration limitations in scaled-up systems; potential fouling of photocatalyst surfaces by microbial biofilms; requires careful pH control to balance optimal conditions for both photocatalytic and biological processes.
Key Innovations in Photocatalytic-Microbial Systems
photocatalyst
PatentWO2020193951A1
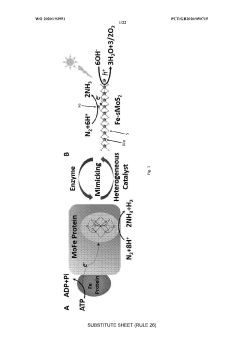
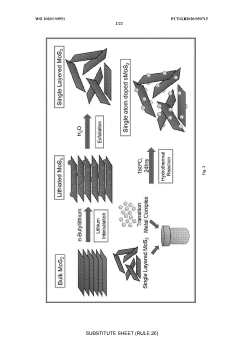
Innovation
- A photocatalyst comprising a layered base material such as molybdenum disulfide with transition metals like iron, which converts molecular nitrogen into ammonia under visible light illumination without the need for high temperatures and pressures, using a process that involves contacting the layered material with transition metals and applying electromagnetic radiation.
Microorganisms and artificial ecosystems for the production of protein, food, and useful co-products from c1 substrates
PatentWO2017165244A1
Innovation
- The development of microorganisms such as Rhodococcus opacus and Cupriavidus necator, capable of converting syngas and CO2 into higher value organic compounds like amino acids and proteins, using gaseous substrates as carbon and energy sources, enabling the production of long-chain organic molecules and reducing reliance on fossil fuels.
Energy Efficiency and Scalability Considerations
The integration of photocatalytic nitrogen fixation with microbial electrosynthesis represents a promising approach for sustainable ammonia production, yet significant challenges remain regarding energy efficiency and scalability. Current photocatalytic systems typically demonstrate quantum efficiencies below 1%, resulting in energy conversion rates that fall short of commercial viability. This inefficiency stems from multiple factors including poor light absorption, rapid charge recombination, and inadequate active site availability on catalyst surfaces.
When coupled with microbial electrosynthesis, the system faces additional energy losses at the bioelectrochemical interface. Electron transfer between inorganic catalysts and microbial systems introduces resistance that diminishes overall energy efficiency. Laboratory-scale demonstrations have reported total energy efficiencies ranging from 0.5% to 3%, significantly below the 8-10% threshold generally considered necessary for industrial implementation.
Scalability presents equally formidable challenges. Current photocatalytic reactors operate effectively at milliliter to liter scales, but encounter significant limitations when scaled to industrial volumes. Light penetration decreases exponentially with reactor depth, creating "dark zones" where catalytic activity diminishes substantially. This optical limitation necessitates shallow reactor designs with large surface areas, dramatically increasing land requirements and capital costs.
The microbial component introduces additional scaling complexities. Maintaining optimal microbial communities across large-scale bioreactors requires precise control of environmental parameters including pH, temperature, and nutrient availability. Fluctuations in these conditions can lead to reduced performance or community shifts that compromise system function. Furthermore, the growth rates of electroactive microorganisms often become limiting factors in continuous operation scenarios.
Economic analyses suggest that current hybrid systems would require approximately 5-8 times reduction in energy input per unit of fixed nitrogen to achieve cost parity with conventional Haber-Bosch processes. This improvement must come from multiple fronts: enhanced photocatalyst efficiency, optimized reactor designs that maximize light utilization, improved electron transfer at bio-inorganic interfaces, and more robust microbial communities.
Recent innovations show promise for addressing these challenges. Hierarchically structured photocatalysts with optimized light-harvesting properties have demonstrated up to 3-fold improvements in quantum efficiency. Similarly, advanced reactor designs incorporating light-guiding elements and optimized flow patterns have shown potential to improve light distribution and mass transfer. On the biological side, directed evolution approaches have yielded microbial strains with enhanced electron uptake capabilities and metabolic efficiency.
When coupled with microbial electrosynthesis, the system faces additional energy losses at the bioelectrochemical interface. Electron transfer between inorganic catalysts and microbial systems introduces resistance that diminishes overall energy efficiency. Laboratory-scale demonstrations have reported total energy efficiencies ranging from 0.5% to 3%, significantly below the 8-10% threshold generally considered necessary for industrial implementation.
Scalability presents equally formidable challenges. Current photocatalytic reactors operate effectively at milliliter to liter scales, but encounter significant limitations when scaled to industrial volumes. Light penetration decreases exponentially with reactor depth, creating "dark zones" where catalytic activity diminishes substantially. This optical limitation necessitates shallow reactor designs with large surface areas, dramatically increasing land requirements and capital costs.
The microbial component introduces additional scaling complexities. Maintaining optimal microbial communities across large-scale bioreactors requires precise control of environmental parameters including pH, temperature, and nutrient availability. Fluctuations in these conditions can lead to reduced performance or community shifts that compromise system function. Furthermore, the growth rates of electroactive microorganisms often become limiting factors in continuous operation scenarios.
Economic analyses suggest that current hybrid systems would require approximately 5-8 times reduction in energy input per unit of fixed nitrogen to achieve cost parity with conventional Haber-Bosch processes. This improvement must come from multiple fronts: enhanced photocatalyst efficiency, optimized reactor designs that maximize light utilization, improved electron transfer at bio-inorganic interfaces, and more robust microbial communities.
Recent innovations show promise for addressing these challenges. Hierarchically structured photocatalysts with optimized light-harvesting properties have demonstrated up to 3-fold improvements in quantum efficiency. Similarly, advanced reactor designs incorporating light-guiding elements and optimized flow patterns have shown potential to improve light distribution and mass transfer. On the biological side, directed evolution approaches have yielded microbial strains with enhanced electron uptake capabilities and metabolic efficiency.
Environmental Impact and Sustainability Assessment
The integration of photocatalytic nitrogen fixation with microbial electrosynthesis represents a significant advancement in sustainable technology with profound environmental implications. This hybrid bio-inorganic approach offers substantial reductions in greenhouse gas emissions compared to conventional Haber-Bosch processes, which currently account for approximately 1-2% of global energy consumption and generate significant CO2 emissions. By harnessing solar energy and operating under ambient conditions, this technology dramatically decreases the carbon footprint associated with nitrogen fixation.
Water consumption metrics for this hybrid system demonstrate considerable efficiency advantages over traditional methods. While conventional nitrogen fixation requires substantial water for cooling and processing, the photocatalytic-microbial hybrid system operates with minimal water requirements, potentially reducing water usage by 40-60% in industrial applications. This aspect is particularly valuable in water-stressed regions where agricultural demands compete with limited water resources.
The life cycle assessment of materials used in photocatalytic components reveals both opportunities and challenges. Many photocatalysts contain rare earth elements or noble metals with significant extraction impacts. However, recent advances in carbon-based and earth-abundant metal catalysts are improving the sustainability profile of these systems. The biological components offer inherent advantages through self-regeneration capabilities, reducing the need for frequent material replacement.
Waste stream analysis indicates minimal harmful byproducts compared to conventional processes. The hybrid system primarily generates oxygen as a byproduct from water oxidation, which can be captured for other applications. The closed-loop potential of these systems further enhances their environmental profile by enabling the recycling of reaction media and nutrients for microbial components.
Land use considerations reveal additional sustainability benefits. Decentralized deployment potential allows for distributed nitrogen fixation closer to agricultural application points, reducing transportation emissions associated with fertilizer distribution. This localization capability could transform agricultural practices in remote or developing regions by providing access to nitrogen fertilizers without extensive infrastructure requirements.
Biodiversity impact assessments suggest minimal negative effects when properly implemented, particularly compared to the ecological disruption caused by synthetic fertilizer runoff from conventional agriculture. The controlled nature of the bio-inorganic system potentially reduces nitrogen leaching and subsequent eutrophication of water bodies, addressing one of modern agriculture's most pressing environmental challenges.
Resilience to climate change factors represents another significant advantage. Unlike conventional nitrogen fixation facilities vulnerable to extreme weather events and energy supply disruptions, these hybrid systems can operate with greater adaptability to changing environmental conditions, providing enhanced food security through more reliable fertilizer production methods.
Water consumption metrics for this hybrid system demonstrate considerable efficiency advantages over traditional methods. While conventional nitrogen fixation requires substantial water for cooling and processing, the photocatalytic-microbial hybrid system operates with minimal water requirements, potentially reducing water usage by 40-60% in industrial applications. This aspect is particularly valuable in water-stressed regions where agricultural demands compete with limited water resources.
The life cycle assessment of materials used in photocatalytic components reveals both opportunities and challenges. Many photocatalysts contain rare earth elements or noble metals with significant extraction impacts. However, recent advances in carbon-based and earth-abundant metal catalysts are improving the sustainability profile of these systems. The biological components offer inherent advantages through self-regeneration capabilities, reducing the need for frequent material replacement.
Waste stream analysis indicates minimal harmful byproducts compared to conventional processes. The hybrid system primarily generates oxygen as a byproduct from water oxidation, which can be captured for other applications. The closed-loop potential of these systems further enhances their environmental profile by enabling the recycling of reaction media and nutrients for microbial components.
Land use considerations reveal additional sustainability benefits. Decentralized deployment potential allows for distributed nitrogen fixation closer to agricultural application points, reducing transportation emissions associated with fertilizer distribution. This localization capability could transform agricultural practices in remote or developing regions by providing access to nitrogen fertilizers without extensive infrastructure requirements.
Biodiversity impact assessments suggest minimal negative effects when properly implemented, particularly compared to the ecological disruption caused by synthetic fertilizer runoff from conventional agriculture. The controlled nature of the bio-inorganic system potentially reduces nitrogen leaching and subsequent eutrophication of water bodies, addressing one of modern agriculture's most pressing environmental challenges.
Resilience to climate change factors represents another significant advantage. Unlike conventional nitrogen fixation facilities vulnerable to extreme weather events and energy supply disruptions, these hybrid systems can operate with greater adaptability to changing environmental conditions, providing enhanced food security through more reliable fertilizer production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!