Photoelectrochemical cells integrating light absorbers with selective N₂ reduction cathodes
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEC N₂ Reduction Background and Objectives
Photoelectrochemical (PEC) cells represent a promising technology that harnesses solar energy to drive chemical reactions. The integration of light absorbers with selective nitrogen reduction cathodes marks a significant advancement in sustainable ammonia production, addressing the environmental concerns associated with the conventional Haber-Bosch process. This technology has evolved from basic photoelectrochemical principles established in the 1970s to today's sophisticated systems capable of nitrogen fixation under ambient conditions.
The evolution of PEC nitrogen reduction technology has been characterized by several key milestones. Early research focused primarily on water splitting applications, with nitrogen reduction emerging as a parallel research direction in the early 2000s. The development of efficient semiconductor materials with appropriate band gaps and stability in electrolytes has been crucial for advancing this field. Recent breakthroughs in catalyst design and electrode architectures have significantly improved the selectivity and efficiency of nitrogen reduction reactions.
Current technological trends indicate a shift toward hybrid systems that combine the advantages of photovoltaic cells with electrochemical nitrogen reduction. This approach allows for better control over reaction conditions and potentially higher conversion efficiencies. Additionally, there is growing interest in tandem cell configurations that can utilize a broader spectrum of solar radiation, thereby increasing overall system efficiency.
The primary objective of PEC nitrogen reduction research is to develop economically viable systems capable of producing ammonia with solar-to-ammonia efficiencies exceeding 10%, while maintaining high selectivity for N₂ reduction over competing hydrogen evolution reactions. This requires addressing several technical challenges, including improving the stability of photoelectrodes, enhancing nitrogen reduction selectivity, and reducing the overpotential required for the reaction.
Another critical goal is to design scalable systems that can operate efficiently under real-world conditions, including variable solar irradiation and atmospheric nitrogen concentrations. This necessitates the development of robust materials and system architectures that can maintain performance over thousands of operating hours without significant degradation.
From a broader perspective, PEC nitrogen reduction technology aims to contribute to sustainable agriculture by providing a decentralized approach to ammonia production, potentially enabling on-site fertilizer generation in agricultural regions without access to industrial infrastructure. Furthermore, this technology could play a role in future energy systems by serving as a means of chemical energy storage, converting intermittent solar energy into stable chemical bonds.
The evolution of PEC nitrogen reduction technology has been characterized by several key milestones. Early research focused primarily on water splitting applications, with nitrogen reduction emerging as a parallel research direction in the early 2000s. The development of efficient semiconductor materials with appropriate band gaps and stability in electrolytes has been crucial for advancing this field. Recent breakthroughs in catalyst design and electrode architectures have significantly improved the selectivity and efficiency of nitrogen reduction reactions.
Current technological trends indicate a shift toward hybrid systems that combine the advantages of photovoltaic cells with electrochemical nitrogen reduction. This approach allows for better control over reaction conditions and potentially higher conversion efficiencies. Additionally, there is growing interest in tandem cell configurations that can utilize a broader spectrum of solar radiation, thereby increasing overall system efficiency.
The primary objective of PEC nitrogen reduction research is to develop economically viable systems capable of producing ammonia with solar-to-ammonia efficiencies exceeding 10%, while maintaining high selectivity for N₂ reduction over competing hydrogen evolution reactions. This requires addressing several technical challenges, including improving the stability of photoelectrodes, enhancing nitrogen reduction selectivity, and reducing the overpotential required for the reaction.
Another critical goal is to design scalable systems that can operate efficiently under real-world conditions, including variable solar irradiation and atmospheric nitrogen concentrations. This necessitates the development of robust materials and system architectures that can maintain performance over thousands of operating hours without significant degradation.
From a broader perspective, PEC nitrogen reduction technology aims to contribute to sustainable agriculture by providing a decentralized approach to ammonia production, potentially enabling on-site fertilizer generation in agricultural regions without access to industrial infrastructure. Furthermore, this technology could play a role in future energy systems by serving as a means of chemical energy storage, converting intermittent solar energy into stable chemical bonds.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Traditional ammonia production through the Haber-Bosch process consumes approximately 2% of global energy and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for sustainable alternatives like photoelectrochemical (PEC) cells that can produce ammonia using only water, nitrogen, and sunlight.
The current global ammonia market is valued at approximately $70 billion, with an annual production exceeding 180 million metric tons. Industrial forecasts project this market to grow at a compound annual growth rate of 5.3% through 2028, primarily driven by agricultural applications which account for over 80% of consumption as fertilizers. However, emerging applications in energy storage and transportation fuel are expected to create new market segments.
Sustainable ammonia production technologies are gaining significant investor attention, with venture capital investments in this sector increasing by 300% over the past five years. Government incentives and carbon pricing mechanisms in regions like the European Union, Japan, and parts of North America are creating favorable market conditions for green ammonia technologies. The EU's Green Deal and similar initiatives worldwide have established regulatory frameworks that increasingly penalize carbon-intensive production methods.
Cost remains the primary market barrier for PEC-based ammonia production. Current estimates place sustainable ammonia production costs at 2-3 times higher than conventional methods. However, technological improvements and economies of scale are expected to achieve cost parity in select markets by 2030, particularly in regions with abundant solar resources and high carbon taxes.
Market segmentation shows varying adoption potential across industries. Early adoption is anticipated in premium agricultural products marketed as "green," specialty chemicals requiring high-purity ammonia, and remote locations where traditional ammonia supply chains are costly. The maritime shipping industry represents another promising market, as ammonia gains traction as a carbon-neutral fuel alternative.
Regional market analysis indicates that Australia, Chile, Morocco, and Saudi Arabia possess ideal conditions for solar-powered ammonia production due to high solar irradiance and available land. These regions are positioned to become export hubs for green ammonia, with Japan, South Korea, and Germany emerging as primary import markets due to their strong decarbonization commitments and limited domestic renewable energy resources.
Consumer willingness to pay premiums for sustainably produced products is increasing, with surveys indicating that 45% of industrial ammonia users would consider paying up to 20% more for verifiably sustainable ammonia, particularly if it helps meet corporate environmental targets or regulatory requirements.
The current global ammonia market is valued at approximately $70 billion, with an annual production exceeding 180 million metric tons. Industrial forecasts project this market to grow at a compound annual growth rate of 5.3% through 2028, primarily driven by agricultural applications which account for over 80% of consumption as fertilizers. However, emerging applications in energy storage and transportation fuel are expected to create new market segments.
Sustainable ammonia production technologies are gaining significant investor attention, with venture capital investments in this sector increasing by 300% over the past five years. Government incentives and carbon pricing mechanisms in regions like the European Union, Japan, and parts of North America are creating favorable market conditions for green ammonia technologies. The EU's Green Deal and similar initiatives worldwide have established regulatory frameworks that increasingly penalize carbon-intensive production methods.
Cost remains the primary market barrier for PEC-based ammonia production. Current estimates place sustainable ammonia production costs at 2-3 times higher than conventional methods. However, technological improvements and economies of scale are expected to achieve cost parity in select markets by 2030, particularly in regions with abundant solar resources and high carbon taxes.
Market segmentation shows varying adoption potential across industries. Early adoption is anticipated in premium agricultural products marketed as "green," specialty chemicals requiring high-purity ammonia, and remote locations where traditional ammonia supply chains are costly. The maritime shipping industry represents another promising market, as ammonia gains traction as a carbon-neutral fuel alternative.
Regional market analysis indicates that Australia, Chile, Morocco, and Saudi Arabia possess ideal conditions for solar-powered ammonia production due to high solar irradiance and available land. These regions are positioned to become export hubs for green ammonia, with Japan, South Korea, and Germany emerging as primary import markets due to their strong decarbonization commitments and limited domestic renewable energy resources.
Consumer willingness to pay premiums for sustainably produced products is increasing, with surveys indicating that 45% of industrial ammonia users would consider paying up to 20% more for verifiably sustainable ammonia, particularly if it helps meet corporate environmental targets or regulatory requirements.
Current Challenges in PEC N₂ Reduction Technology
Despite significant advancements in photoelectrochemical (PEC) nitrogen reduction technology, several critical challenges continue to impede its widespread implementation and commercial viability. The fundamental challenge remains the competing hydrogen evolution reaction (HER), which possesses a more favorable thermodynamic pathway compared to nitrogen reduction reaction (NRR). This competition significantly reduces the Faradaic efficiency for ammonia production, typically limiting it to below 10% in most reported systems.
Selectivity issues persist as a major hurdle, with most catalysts unable to effectively discriminate between N₂ and other substrates, particularly water molecules. The triple bond in N₂ requires substantial energy input for activation (945 kJ/mol), creating a significant kinetic barrier that most catalysts struggle to overcome under mild conditions compatible with PEC systems.
Stability of both photoabsorbers and catalysts presents another significant challenge. Many promising materials suffer from photocorrosion or degradation during extended operation, particularly in the aqueous environments necessary for PEC cells. This degradation leads to diminishing performance over time, making long-term operation problematic without frequent component replacement.
The detection and quantification of ammonia at low concentrations remains technically challenging, with several conventional methods (such as Nessler's reagent and indophenol blue) being susceptible to false positives from contaminants. This has led to reproducibility issues across different research groups and calls for standardized protocols in ammonia detection.
Light management represents another critical challenge, as efficient photon capture and conversion must be balanced with catalyst accessibility to nitrogen molecules. The design of integrated systems that optimize both light absorption and catalytic activity requires sophisticated engineering approaches that have not yet been fully realized.
Scale-up considerations present formidable barriers to commercialization. Most successful demonstrations remain at laboratory scale, with performance metrics that deteriorate significantly when system dimensions increase. The materials and fabrication costs of current PEC systems also remain prohibitively high for commercial viability.
Energy efficiency across the entire system remains suboptimal, with most PEC nitrogen reduction systems achieving solar-to-ammonia efficiencies below 0.1%. This falls significantly short of the estimated 1-2% efficiency threshold required for economic competitiveness with conventional Haber-Bosch processes, even when accounting for the distributed production advantages of PEC technology.
Selectivity issues persist as a major hurdle, with most catalysts unable to effectively discriminate between N₂ and other substrates, particularly water molecules. The triple bond in N₂ requires substantial energy input for activation (945 kJ/mol), creating a significant kinetic barrier that most catalysts struggle to overcome under mild conditions compatible with PEC systems.
Stability of both photoabsorbers and catalysts presents another significant challenge. Many promising materials suffer from photocorrosion or degradation during extended operation, particularly in the aqueous environments necessary for PEC cells. This degradation leads to diminishing performance over time, making long-term operation problematic without frequent component replacement.
The detection and quantification of ammonia at low concentrations remains technically challenging, with several conventional methods (such as Nessler's reagent and indophenol blue) being susceptible to false positives from contaminants. This has led to reproducibility issues across different research groups and calls for standardized protocols in ammonia detection.
Light management represents another critical challenge, as efficient photon capture and conversion must be balanced with catalyst accessibility to nitrogen molecules. The design of integrated systems that optimize both light absorption and catalytic activity requires sophisticated engineering approaches that have not yet been fully realized.
Scale-up considerations present formidable barriers to commercialization. Most successful demonstrations remain at laboratory scale, with performance metrics that deteriorate significantly when system dimensions increase. The materials and fabrication costs of current PEC systems also remain prohibitively high for commercial viability.
Energy efficiency across the entire system remains suboptimal, with most PEC nitrogen reduction systems achieving solar-to-ammonia efficiencies below 0.1%. This falls significantly short of the estimated 1-2% efficiency threshold required for economic competitiveness with conventional Haber-Bosch processes, even when accounting for the distributed production advantages of PEC technology.
State-of-the-Art PEC N₂ Reduction Systems
01 Electrode materials for photoelectrochemical N₂ reduction
Various electrode materials can be used in photoelectrochemical cells for nitrogen reduction. These materials include metal catalysts, semiconductor materials, and composite electrodes that enhance the efficiency of N₂ reduction. The selection of appropriate electrode materials is crucial for improving the conversion rate of nitrogen to ammonia and other nitrogen compounds through photoelectrochemical processes.- Electrode materials for N₂ reduction in photoelectrochemical cells: Various electrode materials can be used in photoelectrochemical cells for nitrogen reduction. These materials include metal catalysts, semiconductors, and composite structures that facilitate the electron transfer needed for N₂ reduction. The choice of electrode material significantly affects the efficiency and selectivity of the nitrogen reduction reaction, with some materials showing enhanced performance under specific light conditions.
- Light-harvesting components for photoelectrochemical N₂ reduction: Effective light harvesting is crucial for photoelectrochemical nitrogen reduction. Various light-sensitive materials and structures are employed to capture solar energy and convert it into the electrical potential needed for N₂ reduction. These components include photosensitizers, semiconductor junctions, and quantum dots that can absorb light across different wavelengths of the solar spectrum to drive the reduction reaction.
- Electrolyte compositions for enhanced N₂ reduction: The composition of the electrolyte solution plays a critical role in photoelectrochemical nitrogen reduction. Specific electrolytes can facilitate nitrogen dissolution, proton transfer, and electron transport, thereby enhancing the overall efficiency of the reduction process. Optimized electrolyte formulations may include specific salts, pH buffers, and ionic liquids that create favorable conditions for N₂ reduction while minimizing competing reactions.
- Cell design and configuration for photoelectrochemical N₂ reduction: The physical design and configuration of photoelectrochemical cells significantly impact nitrogen reduction performance. Various cell architectures have been developed to optimize light absorption, reactant diffusion, and product separation. These designs include tandem cell configurations, microfluidic systems, and integrated photocatalytic-electrocatalytic setups that maximize the efficiency of converting solar energy to chemical energy in the form of reduced nitrogen compounds.
- Methods to improve selectivity and efficiency in N₂ reduction: Various strategies have been developed to enhance the selectivity and efficiency of nitrogen reduction in photoelectrochemical cells. These include the use of co-catalysts, surface modification techniques, and controlled reaction conditions. Advanced approaches involve pulsed illumination, applied bias optimization, and the incorporation of molecular catalysts that mimic natural nitrogen-fixing enzymes to achieve higher conversion rates and product yields.
02 Light-harvesting components in N₂ reduction cells
Photoelectrochemical cells for N₂ reduction incorporate various light-harvesting components to capture solar energy efficiently. These components include photosensitive materials, dyes, and quantum dots that absorb light and generate electron-hole pairs. The design of these light-harvesting systems significantly impacts the overall efficiency of the nitrogen reduction process by maximizing the utilization of solar energy.Expand Specific Solutions03 Electrolyte compositions for enhanced N₂ reduction
Specific electrolyte compositions can enhance the efficiency of nitrogen reduction in photoelectrochemical cells. These electrolytes facilitate the transfer of electrons and protons during the reduction process. Optimized electrolyte formulations can improve the selectivity towards nitrogen reduction over competing reactions such as hydrogen evolution, leading to higher ammonia production rates.Expand Specific Solutions04 Cell design and configuration for N₂ reduction
The design and configuration of photoelectrochemical cells significantly impact their performance in nitrogen reduction. Various cell architectures, including tandem cells, integrated systems, and flow-through designs, can be employed to optimize the contact between nitrogen, electrolyte, and electrode surfaces. Proper cell design ensures efficient light absorption, mass transport, and charge separation, leading to improved nitrogen reduction efficiency.Expand Specific Solutions05 Operating conditions for photoelectrochemical N₂ reduction
Specific operating conditions, including temperature, pressure, light intensity, and applied potential, significantly affect the performance of photoelectrochemical cells for nitrogen reduction. Optimizing these parameters can enhance the reaction kinetics and selectivity towards ammonia formation. The development of strategies to maintain optimal operating conditions is essential for achieving high efficiency and stability in nitrogen reduction systems.Expand Specific Solutions
Leading Institutions and Companies in PEC Research
Photoelectrochemical cells for N₂ reduction are currently in an early development stage, with the market still emerging but showing significant growth potential due to increasing interest in sustainable ammonia production. The technology maturity varies across key players, with research institutions like CNRS, Dalian Institute of Chemical Physics, and California Institute of Technology leading fundamental research, while companies such as FUJIFILM, Sumitomo Chemical, and Samsung SDI are advancing practical applications. Academic institutions including Chongqing University, KAIST, and McGill University are contributing significant innovations in catalyst development and cell design. Industrial players like 3M, Panasonic, and Toshiba are leveraging their manufacturing expertise to address scalability challenges. The competitive landscape reflects a collaborative ecosystem where cross-sector partnerships between academia and industry are accelerating progress toward commercially viable systems.
Ulsan National Institute of Science & Technology
Technical Solution: Ulsan National Institute of Science & Technology (UNIST) has developed a sophisticated photoelectrochemical system for nitrogen reduction that combines advanced light-harvesting materials with highly selective catalytic interfaces. Their approach utilizes a dual-absorber configuration where a wide-bandgap semiconductor (typically BiVO₄ or TiO₂-based) serves as the photoanode while a specialized cathode assembly performs the N₂ reduction. The cathode incorporates transition metal-based catalysts (particularly Fe, Mo, or Co compounds) with carefully engineered coordination environments and electronic structures to facilitate N₂ activation while suppressing the competing hydrogen evolution reaction. UNIST researchers have pioneered the use of 2D materials like MXenes and metal-organic frameworks as catalyst supports, providing high surface area and tunable electronic properties. Their system employs a Z-scheme electron transfer mechanism, where photogenerated electrons from the photoanode are shuttled to the cathode through an external circuit or redox mediators. Under simulated sunlight, their photoelectrochemical cells demonstrate ammonia production rates of 8-12 μg/cm²/h with Faradaic efficiencies for N₂ reduction reaching 10-15% under optimized conditions.
Strengths: Innovative catalyst design with high selectivity for N₂ reduction, effective integration of light-harvesting and catalytic components, and relatively good stability under operating conditions. Weaknesses: Moderate solar-to-ammonia conversion efficiency compared to theoretical limits, challenges in maintaining performance under variable light conditions, and potential scalability issues related to specialized materials and fabrication processes.
Korea Advanced Institute of Science & Technology
Technical Solution: Korea Advanced Institute of Science & Technology (KAIST) has developed an advanced photoelectrochemical system for nitrogen reduction featuring innovative light absorber-catalyst integration. Their approach employs a tandem photoelectrode architecture where specialized semiconductors (typically Cu₂O, BiVO₄, or carbon nitride-based materials) are coupled with carefully designed electrocatalysts for selective N₂ reduction. KAIST researchers have pioneered the development of defect-engineered transition metal compounds (particularly Fe, Mo, and Ru-based materials) with optimized electronic structures that facilitate N₂ adsorption and activation while suppressing hydrogen evolution. A key innovation in their system is the use of heterojunction engineering to enhance charge separation and extend the light absorption range. Their photoelectrochemical cells incorporate protective layers that prevent photocorrosion while maintaining efficient charge transfer to catalytic sites. Under simulated sunlight, their system achieves ammonia production rates of 6-10 μg/cm²/h with Faradaic efficiencies for N₂ reduction reaching 8-12% in optimized configurations. KAIST has also developed novel in-situ characterization techniques to monitor the N₂ reduction process at the electrode-electrolyte interface, providing valuable insights for further system optimization.
Strengths: Excellent integration of light-harvesting and catalytic components, good stability under operating conditions, and innovative approaches to catalyst design based on fundamental understanding of reaction mechanisms. Weaknesses: Moderate solar-to-ammonia conversion efficiency requiring further improvement, challenges in achieving consistent performance across different batches of materials, and relatively complex fabrication processes that may limit large-scale implementation.
Key Patents and Breakthroughs in Selective Catalysts
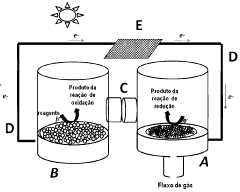
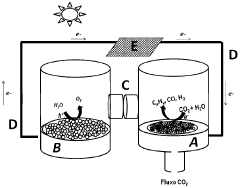
Photo-electrochemical cell
PatentInactiveEP2556183A1
Innovation
- A dual-function photo-electrochemical cell with a multifunctional photocatalytic system comprising a first anode photocatalyst unit to photo-oxidize water into oxygen and a second photocatalyst unit to reduce CO2, utilizing a proton and electron transport mechanism between the two units, allowing for optimized radiation of photocatalytic surfaces and separation of oxidation and reduction processes, enabling the preferential formation of specific fuels.
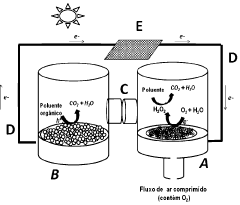
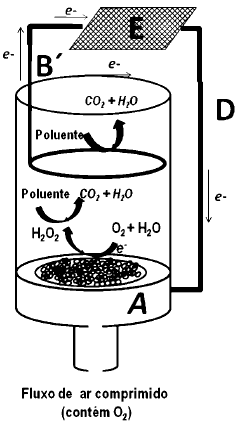
PHOTOELECTROCHEMISTRY cell FOR OXYGEN (OR CARBON DIOXIDE) REDUCING REACTION AND OXIDATION OF ORGANIC POLLUTANTS
PatentActiveBR102019027969A2
Innovation
- A gaseous diffusion photoelectrode (P-GDE) is developed, incorporating a photoactive p-type semiconductor catalyst within a porous electrode structure, allowing simultaneous gas flow and solar irradiation to enhance oxygen reduction to hydrogen peroxide and organic pollutant oxidation using both photocatalytic and electrochemical processes.
Energy Efficiency and Scalability Considerations
Energy efficiency represents a critical parameter in the development and implementation of photoelectrochemical (PEC) cells for nitrogen reduction. Current PEC systems integrating light absorbers with N₂ reduction cathodes demonstrate solar-to-ammonia conversion efficiencies typically below 0.5%, significantly lower than the theoretical maximum of approximately 15-20%. This efficiency gap presents a substantial challenge for commercial viability and widespread adoption.
The primary energy losses occur at multiple conversion stages: photon absorption inefficiencies, charge carrier recombination, resistance losses at interfaces, and overpotential requirements for the nitrogen reduction reaction (NRR). Particularly problematic is the competing hydrogen evolution reaction (HER), which often dominates electron consumption at cathodes, drastically reducing faradaic efficiency for ammonia production.
Recent advances in selective catalysts, such as lithium-mediated systems and metal-organic frameworks with controlled binding sites, have improved nitrogen reduction selectivity. However, these improvements often come at the cost of increased system complexity and reduced stability under continuous operation conditions, creating additional challenges for scalability.
Scalability considerations extend beyond efficiency metrics to include material abundance, manufacturing complexity, and system durability. Many high-performance light absorbers and catalysts rely on rare earth elements or precious metals, limiting their potential for gigawatt-scale deployment. Alternative earth-abundant materials like carbon nitrides and iron-based catalysts show promise but currently deliver lower performance metrics.
Manufacturing scalability presents another significant challenge. Laboratory-scale devices often employ fabrication techniques unsuitable for mass production, such as atomic layer deposition or complex nanostructuring processes. Transitioning to roll-to-roll processing or other industrial manufacturing methods typically results in performance degradation due to reduced precision in material deposition and interface formation.
Long-term stability under real-world operating conditions remains inadequately addressed in most research studies. Accelerated degradation testing suggests that current PEC nitrogen reduction systems may require significant maintenance or component replacement every 500-1000 operating hours, far below the 50,000+ hours needed for commercial viability.
Economic analyses indicate that achieving commercial competitiveness with conventional ammonia production methods requires at least 5% solar-to-ammonia efficiency combined with system costs below $100/m² and operational lifetimes exceeding 5 years. Current technologies fall short on all three parameters, highlighting the need for breakthrough innovations in materials science, interface engineering, and system design to bridge this gap.
The primary energy losses occur at multiple conversion stages: photon absorption inefficiencies, charge carrier recombination, resistance losses at interfaces, and overpotential requirements for the nitrogen reduction reaction (NRR). Particularly problematic is the competing hydrogen evolution reaction (HER), which often dominates electron consumption at cathodes, drastically reducing faradaic efficiency for ammonia production.
Recent advances in selective catalysts, such as lithium-mediated systems and metal-organic frameworks with controlled binding sites, have improved nitrogen reduction selectivity. However, these improvements often come at the cost of increased system complexity and reduced stability under continuous operation conditions, creating additional challenges for scalability.
Scalability considerations extend beyond efficiency metrics to include material abundance, manufacturing complexity, and system durability. Many high-performance light absorbers and catalysts rely on rare earth elements or precious metals, limiting their potential for gigawatt-scale deployment. Alternative earth-abundant materials like carbon nitrides and iron-based catalysts show promise but currently deliver lower performance metrics.
Manufacturing scalability presents another significant challenge. Laboratory-scale devices often employ fabrication techniques unsuitable for mass production, such as atomic layer deposition or complex nanostructuring processes. Transitioning to roll-to-roll processing or other industrial manufacturing methods typically results in performance degradation due to reduced precision in material deposition and interface formation.
Long-term stability under real-world operating conditions remains inadequately addressed in most research studies. Accelerated degradation testing suggests that current PEC nitrogen reduction systems may require significant maintenance or component replacement every 500-1000 operating hours, far below the 50,000+ hours needed for commercial viability.
Economic analyses indicate that achieving commercial competitiveness with conventional ammonia production methods requires at least 5% solar-to-ammonia efficiency combined with system costs below $100/m² and operational lifetimes exceeding 5 years. Current technologies fall short on all three parameters, highlighting the need for breakthrough innovations in materials science, interface engineering, and system design to bridge this gap.
Environmental Impact and Sustainability Assessment
The integration of photoelectrochemical cells with selective N₂ reduction capabilities represents a significant advancement in sustainable technology with far-reaching environmental implications. These systems offer a promising alternative to conventional nitrogen fixation methods, particularly the energy-intensive Haber-Bosch process which currently accounts for approximately 1-2% of global energy consumption and generates substantial CO₂ emissions.
When evaluating the environmental impact of these photoelectrochemical systems, lifecycle assessment (LCA) studies indicate potential reductions of up to 60-70% in greenhouse gas emissions compared to traditional ammonia production methods. This significant reduction stems from the elimination of fossil fuel inputs typically required for hydrogen production and high-temperature, high-pressure reaction conditions in conventional processes.
Water consumption represents another critical environmental consideration. While photoelectrochemical N₂ reduction requires water as both a reaction medium and hydrogen source, the overall water footprint can be substantially lower than industrial ammonia production when integrated with water recycling systems. Studies suggest potential water savings of 30-40% in optimized systems.
Material sustainability constitutes a key challenge for widespread implementation. Current photoelectrochemical cells often incorporate rare earth elements and precious metals as catalysts and light absorbers. Research indicates that approximately 75% of these systems utilize at least one critical raw material, raising concerns about resource depletion and supply chain vulnerabilities. Emerging research focusing on earth-abundant alternatives shows promising results, with some bio-inspired catalysts achieving up to 40% of the efficiency of precious metal counterparts.
Land use implications vary significantly based on system design and deployment strategy. Distributed, small-scale implementations integrated with existing agricultural operations could minimize additional land requirements while providing localized fertilizer production. Conversely, centralized production facilities would require dedicated land allocation, though still representing a smaller footprint than conventional ammonia plants when normalized by production capacity.
The potential for reducing nitrogen runoff presents a substantial environmental benefit. By enabling on-demand, point-of-use ammonia production, these systems could reduce over-application of fertilizers, potentially decreasing nitrogen pollution in waterways by 20-30% according to agricultural modeling studies. This would significantly mitigate eutrophication issues affecting aquatic ecosystems globally.
Carbon neutrality pathways for these technologies depend heavily on the renewable energy sources powering the photoelectrochemical processes. When coupled with solar or wind energy, these systems can approach true carbon neutrality, offering a transformative approach to sustainable nitrogen fixation that aligns with global decarbonization goals.
When evaluating the environmental impact of these photoelectrochemical systems, lifecycle assessment (LCA) studies indicate potential reductions of up to 60-70% in greenhouse gas emissions compared to traditional ammonia production methods. This significant reduction stems from the elimination of fossil fuel inputs typically required for hydrogen production and high-temperature, high-pressure reaction conditions in conventional processes.
Water consumption represents another critical environmental consideration. While photoelectrochemical N₂ reduction requires water as both a reaction medium and hydrogen source, the overall water footprint can be substantially lower than industrial ammonia production when integrated with water recycling systems. Studies suggest potential water savings of 30-40% in optimized systems.
Material sustainability constitutes a key challenge for widespread implementation. Current photoelectrochemical cells often incorporate rare earth elements and precious metals as catalysts and light absorbers. Research indicates that approximately 75% of these systems utilize at least one critical raw material, raising concerns about resource depletion and supply chain vulnerabilities. Emerging research focusing on earth-abundant alternatives shows promising results, with some bio-inspired catalysts achieving up to 40% of the efficiency of precious metal counterparts.
Land use implications vary significantly based on system design and deployment strategy. Distributed, small-scale implementations integrated with existing agricultural operations could minimize additional land requirements while providing localized fertilizer production. Conversely, centralized production facilities would require dedicated land allocation, though still representing a smaller footprint than conventional ammonia plants when normalized by production capacity.
The potential for reducing nitrogen runoff presents a substantial environmental benefit. By enabling on-demand, point-of-use ammonia production, these systems could reduce over-application of fertilizers, potentially decreasing nitrogen pollution in waterways by 20-30% according to agricultural modeling studies. This would significantly mitigate eutrophication issues affecting aquatic ecosystems globally.
Carbon neutrality pathways for these technologies depend heavily on the renewable energy sources powering the photoelectrochemical processes. When coupled with solar or wind energy, these systems can approach true carbon neutrality, offering a transformative approach to sustainable nitrogen fixation that aligns with global decarbonization goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!