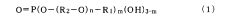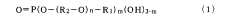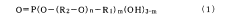Deep Eutectic Solvents Corrosion Behavior: Metals Compatibility, Films And Inhibitors
SEP 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DES Corrosion Background and Research Objectives
Deep Eutectic Solvents (DES) have emerged as a promising class of green solvents over the past two decades, offering environmentally friendly alternatives to conventional organic solvents and ionic liquids. These systems, formed through the complexation of hydrogen bond acceptors with hydrogen bond donors, exhibit remarkable properties including low volatility, biodegradability, and tunable physicochemical characteristics. The evolution of DES technology has progressed from fundamental discovery to application exploration across various industries including pharmaceuticals, electrochemistry, and materials processing.
The corrosion behavior of metals in DES environments represents a critical technological challenge that has gained increasing attention in recent years. Initially overlooked in early DES research, corrosion phenomena have become recognized as a significant factor limiting broader industrial adoption. The historical trajectory shows a shift from viewing DES as universally "green" to a more nuanced understanding that their corrosive properties vary substantially based on composition, temperature, and metal substrate.
Recent technological trends indicate growing interest in tailoring DES formulations specifically to minimize corrosion while maintaining desired solvent properties. This represents a departure from earlier approaches that focused primarily on functionality without considering material compatibility. The scientific community has begun systematically mapping corrosion mechanisms in various DES systems, establishing a foundation for more targeted research efforts.
The primary objectives of this technical research are multifaceted. First, to comprehensively characterize corrosion behaviors of industrially relevant metals (including carbon steel, stainless steel, aluminum alloys, copper, and titanium) when exposed to common DES formulations under varying conditions. Second, to elucidate fundamental corrosion mechanisms specific to DES environments, particularly focusing on how hydrogen bonding networks and charge delocalization influence electrochemical processes at metal surfaces.
Additionally, this research aims to develop and evaluate protective strategies, including the formation of passive films and the incorporation of corrosion inhibitors compatible with DES chemistry. The ultimate goal is to establish design principles for corrosion-resistant DES systems that maintain their green credentials while expanding their applicability in industrial processes requiring metal compatibility.
The technological trajectory suggests that successful resolution of DES corrosion challenges could unlock significant new applications in heat transfer systems, electroplating, metal processing, and energy storage technologies where metal-solvent interactions are unavoidable. This research therefore addresses a critical barrier to broader DES implementation while contributing to the larger goal of transitioning industrial processes toward more sustainable chemical technologies.
The corrosion behavior of metals in DES environments represents a critical technological challenge that has gained increasing attention in recent years. Initially overlooked in early DES research, corrosion phenomena have become recognized as a significant factor limiting broader industrial adoption. The historical trajectory shows a shift from viewing DES as universally "green" to a more nuanced understanding that their corrosive properties vary substantially based on composition, temperature, and metal substrate.
Recent technological trends indicate growing interest in tailoring DES formulations specifically to minimize corrosion while maintaining desired solvent properties. This represents a departure from earlier approaches that focused primarily on functionality without considering material compatibility. The scientific community has begun systematically mapping corrosion mechanisms in various DES systems, establishing a foundation for more targeted research efforts.
The primary objectives of this technical research are multifaceted. First, to comprehensively characterize corrosion behaviors of industrially relevant metals (including carbon steel, stainless steel, aluminum alloys, copper, and titanium) when exposed to common DES formulations under varying conditions. Second, to elucidate fundamental corrosion mechanisms specific to DES environments, particularly focusing on how hydrogen bonding networks and charge delocalization influence electrochemical processes at metal surfaces.
Additionally, this research aims to develop and evaluate protective strategies, including the formation of passive films and the incorporation of corrosion inhibitors compatible with DES chemistry. The ultimate goal is to establish design principles for corrosion-resistant DES systems that maintain their green credentials while expanding their applicability in industrial processes requiring metal compatibility.
The technological trajectory suggests that successful resolution of DES corrosion challenges could unlock significant new applications in heat transfer systems, electroplating, metal processing, and energy storage technologies where metal-solvent interactions are unavoidable. This research therefore addresses a critical barrier to broader DES implementation while contributing to the larger goal of transitioning industrial processes toward more sustainable chemical technologies.
Market Analysis of DES Applications
The Deep Eutectic Solvents (DES) market has witnessed significant growth in recent years, driven by increasing environmental concerns and stringent regulations against traditional volatile organic solvents. The global DES market was valued at approximately $28.6 million in 2022 and is projected to reach $51.7 million by 2028, growing at a CAGR of 10.3% during the forecast period.
The pharmaceutical industry represents the largest application segment for DES, accounting for about 32% of the market share. DES offers advantages in drug formulation, extraction, and purification processes, providing enhanced solubility for poorly water-soluble drugs. The pharmaceutical sector's demand is primarily driven by the need for greener extraction methods and improved bioavailability of active pharmaceutical ingredients.
Chemical processing follows as the second-largest application segment, holding approximately 27% of the market share. In this sector, DES serves as reaction media, catalysts, and extraction solvents. The ability of DES to dissolve metal oxides makes them particularly valuable in metal processing applications, despite corrosion concerns that require careful material selection and inhibitor development.
The electrochemical industry represents a rapidly growing application segment, with a projected CAGR of 12.8% through 2028. DES is increasingly utilized in electroplating, electropolishing, and energy storage applications. The unique conductivity properties of DES, combined with their wide electrochemical windows, make them attractive alternatives to conventional electrolytes.
Regionally, Europe dominates the DES market with approximately 38% share, followed by North America (29%) and Asia-Pacific (24%). The European dominance is attributed to stringent environmental regulations and substantial research funding. However, the Asia-Pacific region is expected to witness the fastest growth rate due to expanding industrial activities and increasing adoption of green technologies.
Key challenges limiting wider DES adoption include corrosion concerns, particularly with certain metal substrates, and the need for standardized corrosion testing protocols. The market also faces competition from other green solvents such as ionic liquids and supercritical CO2. Nevertheless, ongoing research into corrosion inhibitors and protective films is expected to address these limitations and expand application possibilities.
The DES market remains highly fragmented, with numerous small to medium-sized enterprises focusing on specialized applications. Strategic collaborations between academic institutions and industry players are driving innovation in corrosion-resistant DES formulations, potentially opening new market opportunities in sensitive applications like aerospace and medical devices.
The pharmaceutical industry represents the largest application segment for DES, accounting for about 32% of the market share. DES offers advantages in drug formulation, extraction, and purification processes, providing enhanced solubility for poorly water-soluble drugs. The pharmaceutical sector's demand is primarily driven by the need for greener extraction methods and improved bioavailability of active pharmaceutical ingredients.
Chemical processing follows as the second-largest application segment, holding approximately 27% of the market share. In this sector, DES serves as reaction media, catalysts, and extraction solvents. The ability of DES to dissolve metal oxides makes them particularly valuable in metal processing applications, despite corrosion concerns that require careful material selection and inhibitor development.
The electrochemical industry represents a rapidly growing application segment, with a projected CAGR of 12.8% through 2028. DES is increasingly utilized in electroplating, electropolishing, and energy storage applications. The unique conductivity properties of DES, combined with their wide electrochemical windows, make them attractive alternatives to conventional electrolytes.
Regionally, Europe dominates the DES market with approximately 38% share, followed by North America (29%) and Asia-Pacific (24%). The European dominance is attributed to stringent environmental regulations and substantial research funding. However, the Asia-Pacific region is expected to witness the fastest growth rate due to expanding industrial activities and increasing adoption of green technologies.
Key challenges limiting wider DES adoption include corrosion concerns, particularly with certain metal substrates, and the need for standardized corrosion testing protocols. The market also faces competition from other green solvents such as ionic liquids and supercritical CO2. Nevertheless, ongoing research into corrosion inhibitors and protective films is expected to address these limitations and expand application possibilities.
The DES market remains highly fragmented, with numerous small to medium-sized enterprises focusing on specialized applications. Strategic collaborations between academic institutions and industry players are driving innovation in corrosion-resistant DES formulations, potentially opening new market opportunities in sensitive applications like aerospace and medical devices.
Current Challenges in Metal-DES Compatibility
Despite the promising applications of Deep Eutectic Solvents (DES) across various industries, their widespread implementation faces significant challenges related to metal compatibility. The corrosive nature of many DES formulations presents a fundamental obstacle to their industrial adoption, particularly in processes involving metallic equipment and components. This corrosion behavior varies dramatically depending on the specific DES composition, operating conditions, and metal substrate.
One primary challenge is the lack of comprehensive understanding regarding corrosion mechanisms in DES environments. Unlike aqueous corrosion, which has been extensively studied for decades, the corrosion processes in DES systems involve complex interactions between hydrogen bond donors, hydrogen bond acceptors, and metal surfaces that remain inadequately characterized. The diversity of possible DES formulations further complicates this understanding, as each unique combination may exhibit distinct corrosion behaviors.
Temperature dependency represents another critical challenge, as many industrial applications require operation at elevated temperatures where corrosion rates typically accelerate. Research indicates that corrosion rates in DES systems often follow Arrhenius behavior, with significant increases observed at higher temperatures. This temperature sensitivity necessitates careful consideration when designing DES-based processes for high-temperature applications.
Water content in DES systems presents a paradoxical challenge. While DES are often promoted as "green" alternatives to conventional solvents partly due to their water tolerance, the presence of water can dramatically alter their corrosion behavior. Even small amounts of absorbed atmospheric moisture can transform a relatively benign DES into a highly corrosive medium for certain metals, complicating both laboratory studies and industrial implementations.
The development of effective corrosion inhibition strategies specifically tailored for DES environments remains underdeveloped. Traditional corrosion inhibitors designed for aqueous or organic solvent systems often demonstrate limited effectiveness in DES media due to differences in solubility, adsorption mechanisms, and electrochemical behavior at the metal-DES interface.
Long-term stability of protective films formed in DES environments represents another significant challenge. While some metals may initially form passive layers in certain DES formulations, the stability of these protective films over extended periods remains questionable, particularly under dynamic operating conditions involving temperature fluctuations, mechanical stress, or composition changes.
Standardized testing protocols for evaluating metal-DES compatibility are notably absent, hindering comparative assessments across different research groups and industrial settings. This lack of standardization complicates material selection decisions and risk assessments for DES implementation in commercial applications.
One primary challenge is the lack of comprehensive understanding regarding corrosion mechanisms in DES environments. Unlike aqueous corrosion, which has been extensively studied for decades, the corrosion processes in DES systems involve complex interactions between hydrogen bond donors, hydrogen bond acceptors, and metal surfaces that remain inadequately characterized. The diversity of possible DES formulations further complicates this understanding, as each unique combination may exhibit distinct corrosion behaviors.
Temperature dependency represents another critical challenge, as many industrial applications require operation at elevated temperatures where corrosion rates typically accelerate. Research indicates that corrosion rates in DES systems often follow Arrhenius behavior, with significant increases observed at higher temperatures. This temperature sensitivity necessitates careful consideration when designing DES-based processes for high-temperature applications.
Water content in DES systems presents a paradoxical challenge. While DES are often promoted as "green" alternatives to conventional solvents partly due to their water tolerance, the presence of water can dramatically alter their corrosion behavior. Even small amounts of absorbed atmospheric moisture can transform a relatively benign DES into a highly corrosive medium for certain metals, complicating both laboratory studies and industrial implementations.
The development of effective corrosion inhibition strategies specifically tailored for DES environments remains underdeveloped. Traditional corrosion inhibitors designed for aqueous or organic solvent systems often demonstrate limited effectiveness in DES media due to differences in solubility, adsorption mechanisms, and electrochemical behavior at the metal-DES interface.
Long-term stability of protective films formed in DES environments represents another significant challenge. While some metals may initially form passive layers in certain DES formulations, the stability of these protective films over extended periods remains questionable, particularly under dynamic operating conditions involving temperature fluctuations, mechanical stress, or composition changes.
Standardized testing protocols for evaluating metal-DES compatibility are notably absent, hindering comparative assessments across different research groups and industrial settings. This lack of standardization complicates material selection decisions and risk assessments for DES implementation in commercial applications.
Existing Corrosion Protection Solutions for DES
01 Corrosion inhibition properties of deep eutectic solvents
Deep eutectic solvents (DES) can be formulated to exhibit corrosion inhibition properties for various metal surfaces. These formulations typically contain hydrogen bond donors and acceptors that create a protective layer on metal surfaces, preventing direct contact with corrosive agents. The corrosion inhibition efficiency depends on the specific components of the DES, their concentration, and the metal substrate being protected. These solvents offer environmentally friendly alternatives to traditional corrosion inhibitors.- Corrosion inhibition properties of deep eutectic solvents: Deep eutectic solvents (DES) can be formulated to exhibit corrosion inhibition properties for various metal surfaces. These formulations typically contain hydrogen bond donors and acceptors that create a protective film on metal surfaces, preventing direct contact with corrosive agents. The corrosion inhibition efficiency depends on the specific components of the DES, their concentration, and the metal substrate being protected.
- Composition modifications to improve corrosion resistance: The corrosion behavior of deep eutectic solvents can be modified by adjusting their composition. Adding specific additives, such as quaternary ammonium salts, metal halides, or organic acids, can significantly improve their corrosion resistance properties. These modifications alter the physical and chemical properties of the DES, including viscosity, conductivity, and surface interaction, which directly influence their corrosion behavior when in contact with different materials.
- Temperature and environmental effects on DES corrosion behavior: The corrosion behavior of deep eutectic solvents is significantly influenced by temperature and environmental conditions. Higher temperatures typically accelerate corrosion processes, while specific environmental factors like humidity, oxygen content, and pH can either enhance or mitigate corrosive effects. Understanding these relationships is crucial for predicting the long-term performance of DES in various industrial applications and developing appropriate corrosion management strategies.
- Metal-specific corrosion behavior with deep eutectic solvents: Different metals and alloys exhibit varying corrosion behaviors when exposed to deep eutectic solvents. Factors such as the electrochemical potential of the metal, surface properties, and the specific composition of the DES determine the corrosion rate and mechanism. Some metals form passive layers in contact with certain DES formulations, providing inherent protection, while others may experience accelerated corrosion. This metal-specific behavior is important for material selection in DES applications.
- Electrochemical analysis of DES corrosion mechanisms: Electrochemical techniques are employed to analyze and understand the corrosion mechanisms of deep eutectic solvents on various materials. Methods such as electrochemical impedance spectroscopy, polarization studies, and surface analysis provide insights into the kinetics and thermodynamics of corrosion processes. These analyses help in developing models to predict corrosion behavior and in designing DES formulations with optimized corrosion properties for specific applications.
02 Composition modifications to improve corrosion resistance
The corrosion behavior of deep eutectic solvents can be modified by adjusting their composition. Adding specific additives such as quaternary ammonium salts, metal halides, or organic acids can significantly improve their corrosion resistance properties. These modified DES formulations create more stable protective films on metal surfaces and can withstand more aggressive environments. The synergistic effect between different components in the DES plays a crucial role in determining their overall corrosion behavior.Expand Specific Solutions03 Temperature and environmental effects on DES corrosion behavior
The corrosion behavior of deep eutectic solvents is significantly influenced by temperature and environmental conditions. At elevated temperatures, some DES formulations may become more corrosive due to increased ionic mobility and chemical reactivity. Humidity, oxygen content, and pH also affect the corrosion properties of these solvents. Understanding these environmental factors is essential for predicting the long-term corrosion performance of DES in various applications and developing appropriate mitigation strategies.Expand Specific Solutions04 Metal-specific corrosion behavior with deep eutectic solvents
Different metals and alloys exhibit varying corrosion behaviors when exposed to deep eutectic solvents. Ferrous metals, aluminum alloys, copper, and their alloys each interact differently with specific DES compositions. Some metals may form passive layers that provide protection, while others may experience accelerated corrosion in certain DES formulations. The electrochemical properties of both the metal substrate and the DES components determine the corrosion mechanisms and rates, which is crucial for material selection in DES applications.Expand Specific Solutions05 Analytical methods for evaluating DES corrosion behavior
Various analytical techniques are employed to evaluate the corrosion behavior of deep eutectic solvents on different materials. Electrochemical impedance spectroscopy, potentiodynamic polarization, weight loss measurements, and surface analysis techniques such as SEM and XPS provide comprehensive insights into corrosion mechanisms and rates. These methods help in quantifying corrosion parameters, understanding degradation pathways, and developing more effective corrosion-resistant DES formulations for industrial applications.Expand Specific Solutions
Key Industry Players in DES Technology
Deep Eutectic Solvents (DES) corrosion behavior represents an emerging field at the intersection of green chemistry and materials science, currently in its growth phase. The global market for DES applications is expanding rapidly, projected to reach significant scale as industries seek sustainable alternatives to conventional solvents. Technical maturity varies across applications, with leading companies developing specialized solutions. BASF, ExxonMobil, and Henkel are pioneering industrial applications, while Halliburton and ChampionX focus on energy sector implementations. Academic institutions like King Fahd University and Max Planck Society are advancing fundamental research. Corrosion inhibition technologies are being developed by specialty chemical manufacturers including Croda, Dorf-Ketal, and Afton Chemical, with protective coating innovations coming from PPG Industries and Dunn-Edwards. The competitive landscape reflects a balance between established chemical corporations and specialized solution providers.
BASF Corp.
Technical Solution: BASF has developed proprietary deep eutectic solvent (DES) formulations specifically engineered to minimize metal corrosion in industrial applications. Their approach combines choline chloride-based DESs with carefully selected hydrogen bond donors to create environmentally friendly solvents with controlled corrosivity. BASF's research has demonstrated that incorporating specific corrosion inhibitors, such as benzotriazole derivatives and imidazolium compounds, into their DES formulations can significantly reduce corrosion rates on various metal substrates including steel, aluminum, and copper alloys. Their technology includes surface-active components that form protective films on metal surfaces, creating a barrier against corrosive species while maintaining the desirable properties of the DES.
Strengths: Extensive chemical expertise allows for precise formulation control; global research capabilities enable comprehensive testing across diverse metal types; strong integration with existing industrial processes. Weaknesses: Some formulations may have limited temperature stability ranges; higher production costs compared to conventional solvents; potential compatibility issues with certain specialized alloys.
Ecolab USA, Inc.
Technical Solution: Ecolab has pioneered advanced DES-based cleaning and processing solutions with integrated corrosion protection systems. Their technology utilizes custom-designed DES formulations containing quaternary ammonium compounds combined with specific hydrogen bond donors that minimize aggressive interactions with metal surfaces. Ecolab's approach incorporates proprietary film-forming corrosion inhibitors that adsorb onto metal surfaces, creating nanoscale protective layers that prevent direct contact between the DES and the metal substrate. Their research has demonstrated that these protective films remain stable even under flow conditions and elevated temperatures, providing long-term protection in industrial environments. Ecolab has also developed monitoring systems to track corrosion rates in real-time, allowing for adaptive dosing of inhibitors based on changing process conditions.
Strengths: Extensive field testing in real industrial environments provides practical validation; solutions designed for specific industry applications; integrated monitoring capabilities allow for proactive corrosion management. Weaknesses: Some formulations may require frequent inhibitor replenishment; higher initial implementation costs; potential performance variations across different metal alloy compositions.
Critical Patents in DES Corrosion Inhibition
Lubricants leading to better equipment cleanliness
PatentInactiveUS20180355270A1
Innovation
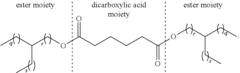
- Incorporating a synthetic ester with an Iodine value lower than 10 g I/100 g measured according to DGF C-V 11b, selected from diesters of dicarboxylic acids or polyol esters, into lubricant compositions to reduce deposit formation without altering the amount of detergents and dispersants, thereby improving cleanliness and oxidative stability.
Corrosion inhibitor for metal, metal part of electronic component treated with the corrosion inhibitor, copper foil or copper alloy foil, and corrosion inhibition method for metal
PatentInactiveJP2012132040A
Innovation
- A thin organic compound film with a contact angle of 90 degrees or more and a thickness of 0.1 to 10 nm is applied to metal surfaces, comprising fluorohydrocarbon groups and phosphate esters, which enhances salt water corrosion resistance without significantly reducing solder wettability.
Environmental Impact of DES Corrosion Inhibitors
The environmental implications of Deep Eutectic Solvents (DES) corrosion inhibitors represent a critical aspect of their industrial application. As green solvents, DES have emerged as promising alternatives to conventional volatile organic compounds and ionic liquids due to their biodegradability, low toxicity, and sustainable preparation methods. However, the environmental impact of DES corrosion inhibitors requires comprehensive assessment across their entire lifecycle.
The biodegradability of DES corrosion inhibitors significantly outperforms traditional petroleum-based inhibitors. Research indicates that many DES formulations, particularly those derived from natural components like choline chloride combined with hydrogen bond donors such as glycerol or urea, demonstrate enhanced biodegradation rates in environmental conditions. This characteristic substantially reduces their persistence in aquatic ecosystems following accidental release or disposal.
Ecotoxicological studies reveal that DES corrosion inhibitors generally exhibit lower aquatic toxicity compared to conventional alternatives. Investigations using standard test organisms including Daphnia magna and various fish species have demonstrated reduced acute and chronic toxicity profiles. However, the environmental impact varies considerably depending on the specific DES composition, with some hydrogen bond donors potentially causing more significant ecological effects than others.
The production processes for DES corrosion inhibitors typically require less energy and generate fewer emissions than conventional inhibitor manufacturing. Life cycle assessments indicate reduced carbon footprints, particularly when utilizing bio-based or waste-derived components. This advantage extends to their application phase, where lower volatility reduces atmospheric emissions and occupational exposure risks.
Waste management considerations for DES systems present both opportunities and challenges. While their biodegradability facilitates natural attenuation in the environment, proper treatment protocols for DES-containing waste streams remain underdeveloped in many industrial settings. The potential for metal ion complexation by spent DES inhibitors may require specialized treatment approaches to prevent secondary contamination of water resources.
Regulatory frameworks governing DES corrosion inhibitors continue to evolve globally. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar programs elsewhere increasingly recognize the environmental advantages of DES-based technologies, potentially accelerating their industrial adoption through favorable regulatory treatment.
Future research directions should focus on optimizing DES formulations specifically for enhanced environmental performance without compromising corrosion inhibition efficiency. Developing standardized ecotoxicological testing protocols specifically for DES systems would enable more accurate environmental impact assessments and facilitate regulatory approval processes.
The biodegradability of DES corrosion inhibitors significantly outperforms traditional petroleum-based inhibitors. Research indicates that many DES formulations, particularly those derived from natural components like choline chloride combined with hydrogen bond donors such as glycerol or urea, demonstrate enhanced biodegradation rates in environmental conditions. This characteristic substantially reduces their persistence in aquatic ecosystems following accidental release or disposal.
Ecotoxicological studies reveal that DES corrosion inhibitors generally exhibit lower aquatic toxicity compared to conventional alternatives. Investigations using standard test organisms including Daphnia magna and various fish species have demonstrated reduced acute and chronic toxicity profiles. However, the environmental impact varies considerably depending on the specific DES composition, with some hydrogen bond donors potentially causing more significant ecological effects than others.
The production processes for DES corrosion inhibitors typically require less energy and generate fewer emissions than conventional inhibitor manufacturing. Life cycle assessments indicate reduced carbon footprints, particularly when utilizing bio-based or waste-derived components. This advantage extends to their application phase, where lower volatility reduces atmospheric emissions and occupational exposure risks.
Waste management considerations for DES systems present both opportunities and challenges. While their biodegradability facilitates natural attenuation in the environment, proper treatment protocols for DES-containing waste streams remain underdeveloped in many industrial settings. The potential for metal ion complexation by spent DES inhibitors may require specialized treatment approaches to prevent secondary contamination of water resources.
Regulatory frameworks governing DES corrosion inhibitors continue to evolve globally. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar programs elsewhere increasingly recognize the environmental advantages of DES-based technologies, potentially accelerating their industrial adoption through favorable regulatory treatment.
Future research directions should focus on optimizing DES formulations specifically for enhanced environmental performance without compromising corrosion inhibition efficiency. Developing standardized ecotoxicological testing protocols specifically for DES systems would enable more accurate environmental impact assessments and facilitate regulatory approval processes.
Standardization and Testing Protocols for DES Corrosion
The standardization of testing protocols for Deep Eutectic Solvents (DES) corrosion represents a critical gap in current research and industrial applications. Despite the growing interest in DES as green alternatives to conventional solvents, there exists significant variability in how corrosion testing is conducted, making cross-study comparisons challenging and potentially hindering widespread adoption.
Current testing methodologies for DES corrosion vary widely across research groups and industries, with inconsistencies in sample preparation, exposure conditions, and evaluation metrics. This lack of standardization creates difficulties in establishing reliable corrosion rates and mechanisms, ultimately impeding the development of effective corrosion mitigation strategies for specific metal-DES combinations.
Electrochemical testing protocols require particular attention, as techniques such as potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and linear polarization resistance (LPR) are commonly employed but with varying parameters. Standardizing scan rates, frequency ranges, and electrode configurations would significantly enhance data reliability and reproducibility across different laboratories.
Immersion testing, another fundamental approach, suffers from inconsistencies in duration, temperature control, and surface area-to-volume ratios. Establishing standardized protocols for these parameters, along with uniform methods for cleaning and weighing specimens before and after exposure, would provide more comparable corrosion rate data.
Temperature effects present additional challenges, as DES properties can change dramatically with temperature variations. Standardized protocols should include specific temperature ranges for testing, with clear reporting requirements for thermal history and stability during experiments. This is particularly important given that many DES applications involve elevated temperatures.
Surface analysis techniques such as SEM, XPS, and FTIR require standardized sample preparation and analysis protocols to ensure consistent characterization of corrosion products and protective films. Establishing reference spectra and imaging parameters would facilitate more meaningful comparisons between different studies.
The development of reference materials and control samples represents another critical aspect of standardization. Certified reference materials with known corrosion behaviors in specific DES formulations would provide benchmarks against which new materials or inhibitor formulations could be evaluated, enhancing reliability and accelerating development cycles.
International collaboration between academic institutions, industry partners, and standards organizations will be essential to establish widely accepted testing protocols. Organizations such as ASTM International, NACE International, and ISO could play pivotal roles in developing and disseminating these standards, ensuring their adoption across diverse sectors utilizing DES technologies.
Current testing methodologies for DES corrosion vary widely across research groups and industries, with inconsistencies in sample preparation, exposure conditions, and evaluation metrics. This lack of standardization creates difficulties in establishing reliable corrosion rates and mechanisms, ultimately impeding the development of effective corrosion mitigation strategies for specific metal-DES combinations.
Electrochemical testing protocols require particular attention, as techniques such as potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and linear polarization resistance (LPR) are commonly employed but with varying parameters. Standardizing scan rates, frequency ranges, and electrode configurations would significantly enhance data reliability and reproducibility across different laboratories.
Immersion testing, another fundamental approach, suffers from inconsistencies in duration, temperature control, and surface area-to-volume ratios. Establishing standardized protocols for these parameters, along with uniform methods for cleaning and weighing specimens before and after exposure, would provide more comparable corrosion rate data.
Temperature effects present additional challenges, as DES properties can change dramatically with temperature variations. Standardized protocols should include specific temperature ranges for testing, with clear reporting requirements for thermal history and stability during experiments. This is particularly important given that many DES applications involve elevated temperatures.
Surface analysis techniques such as SEM, XPS, and FTIR require standardized sample preparation and analysis protocols to ensure consistent characterization of corrosion products and protective films. Establishing reference spectra and imaging parameters would facilitate more meaningful comparisons between different studies.
The development of reference materials and control samples represents another critical aspect of standardization. Certified reference materials with known corrosion behaviors in specific DES formulations would provide benchmarks against which new materials or inhibitor formulations could be evaluated, enhancing reliability and accelerating development cycles.
International collaboration between academic institutions, industry partners, and standards organizations will be essential to establish widely accepted testing protocols. Organizations such as ASTM International, NACE International, and ISO could play pivotal roles in developing and disseminating these standards, ensuring their adoption across diverse sectors utilizing DES technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!