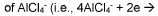Deep Eutectic Solvents In Battery Electrolytes: Ionic Transport, SEI Formation And Stability
SEP 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DES Battery Electrolytes Background and Objectives
Deep Eutectic Solvents (DES) have emerged as a revolutionary class of materials in the field of battery electrolytes, representing a significant shift from conventional electrolyte systems. The development of DES can be traced back to the early 2000s, initially as alternatives to ionic liquids in various chemical processes. Their application in battery technologies gained momentum around 2010-2015, when researchers began exploring their potential to address critical limitations in existing battery electrolytes.
The evolution of battery electrolyte technology has progressed from traditional liquid electrolytes based on organic carbonates to more advanced systems incorporating ionic liquids, polymer electrolytes, and now DES. This progression has been driven by the persistent challenges of safety, performance, and sustainability in battery technologies. DES represent a promising advancement in this evolutionary chain due to their unique physicochemical properties.
DES are formed through the complexation of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), resulting in a significant depression of the freezing point compared to the individual components. This characteristic enables them to remain liquid at room temperature despite being composed of solid precursors. The versatility in component selection allows for tailored properties, making DES highly adaptable for specific battery requirements.
The technical objectives for DES in battery electrolytes center around enhancing three critical aspects: ionic transport efficiency, solid electrolyte interphase (SEI) formation, and long-term stability. Improving ionic conductivity is paramount for high-rate capability batteries, while controlled SEI formation directly impacts battery cycling efficiency and longevity. Stability under various operational conditions remains a fundamental requirement for practical applications.
Current research aims to develop DES formulations that can achieve ionic conductivities exceeding 10^-3 S/cm at room temperature, form stable and ion-conductive SEI layers, and maintain performance over extended cycling (>1000 cycles). Additionally, there is significant interest in DES systems that can operate safely across wider temperature ranges (-20°C to 60°C) than conventional electrolytes.
The trajectory of DES development is increasingly focused on understanding the fundamental mechanisms of ion transport and interfacial chemistry. This knowledge is essential for designing next-generation DES electrolytes that can enable higher energy density batteries while maintaining safety and extending operational lifetimes. The ultimate goal is to establish DES as a viable, sustainable alternative to conventional electrolytes in commercial battery applications.
The evolution of battery electrolyte technology has progressed from traditional liquid electrolytes based on organic carbonates to more advanced systems incorporating ionic liquids, polymer electrolytes, and now DES. This progression has been driven by the persistent challenges of safety, performance, and sustainability in battery technologies. DES represent a promising advancement in this evolutionary chain due to their unique physicochemical properties.
DES are formed through the complexation of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), resulting in a significant depression of the freezing point compared to the individual components. This characteristic enables them to remain liquid at room temperature despite being composed of solid precursors. The versatility in component selection allows for tailored properties, making DES highly adaptable for specific battery requirements.
The technical objectives for DES in battery electrolytes center around enhancing three critical aspects: ionic transport efficiency, solid electrolyte interphase (SEI) formation, and long-term stability. Improving ionic conductivity is paramount for high-rate capability batteries, while controlled SEI formation directly impacts battery cycling efficiency and longevity. Stability under various operational conditions remains a fundamental requirement for practical applications.
Current research aims to develop DES formulations that can achieve ionic conductivities exceeding 10^-3 S/cm at room temperature, form stable and ion-conductive SEI layers, and maintain performance over extended cycling (>1000 cycles). Additionally, there is significant interest in DES systems that can operate safely across wider temperature ranges (-20°C to 60°C) than conventional electrolytes.
The trajectory of DES development is increasingly focused on understanding the fundamental mechanisms of ion transport and interfacial chemistry. This knowledge is essential for designing next-generation DES electrolytes that can enable higher energy density batteries while maintaining safety and extending operational lifetimes. The ultimate goal is to establish DES as a viable, sustainable alternative to conventional electrolytes in commercial battery applications.
Market Analysis for Advanced Battery Electrolyte Solutions
The global market for advanced battery electrolytes is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. The compound annual growth rate (CAGR) for advanced battery electrolytes is projected to exceed 8% between 2023 and 2030, with the market value expected to reach approximately $7.5 billion by 2030.
Deep Eutectic Solvents (DES) represent an emerging segment within this market, offering significant advantages over conventional electrolyte solutions. Their potential to enhance battery performance while addressing safety concerns positions them as a disruptive technology in the electrolyte landscape. Current market penetration of DES-based electrolytes remains limited, accounting for less than 2% of the total electrolyte market, indicating substantial growth potential.
The EV sector constitutes the largest demand driver, representing nearly 60% of the advanced electrolyte market. As automotive manufacturers commit to electrification targets, the demand for high-performance, safe, and sustainable battery technologies continues to accelerate. Tesla, Volkswagen Group, and BYD have all announced research initiatives exploring next-generation electrolyte solutions, including DES-based systems.
Energy storage systems represent the second-largest market segment, with grid-scale applications showing particular interest in electrolyte solutions that offer enhanced safety profiles and operational longevity. The renewable energy sector's growth directly correlates with increased demand for advanced battery technologies, creating a symbiotic relationship between these markets.
Consumer electronics manufacturers are increasingly seeking differentiation through battery performance, creating another significant market opportunity for novel electrolyte solutions. Apple, Samsung, and other major OEMs have filed patents related to advanced electrolyte formulations, signaling industry-wide interest in this technology.
Regional analysis reveals Asia-Pacific as the dominant market for advanced battery electrolytes, accounting for approximately 65% of global production and consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-performance electrolyte innovation. North America and Europe are rapidly expanding their domestic battery supply chains, creating new market opportunities for advanced electrolyte technologies.
Market barriers include cost considerations, with DES-based electrolytes currently commanding a premium of 30-40% over conventional solutions. However, economies of scale and manufacturing optimization are expected to reduce this gap significantly within the next 3-5 years. Regulatory frameworks regarding battery safety and environmental impact are evolving favorably for DES technologies, as they typically offer reduced flammability and improved sustainability profiles compared to traditional electrolytes.
Deep Eutectic Solvents (DES) represent an emerging segment within this market, offering significant advantages over conventional electrolyte solutions. Their potential to enhance battery performance while addressing safety concerns positions them as a disruptive technology in the electrolyte landscape. Current market penetration of DES-based electrolytes remains limited, accounting for less than 2% of the total electrolyte market, indicating substantial growth potential.
The EV sector constitutes the largest demand driver, representing nearly 60% of the advanced electrolyte market. As automotive manufacturers commit to electrification targets, the demand for high-performance, safe, and sustainable battery technologies continues to accelerate. Tesla, Volkswagen Group, and BYD have all announced research initiatives exploring next-generation electrolyte solutions, including DES-based systems.
Energy storage systems represent the second-largest market segment, with grid-scale applications showing particular interest in electrolyte solutions that offer enhanced safety profiles and operational longevity. The renewable energy sector's growth directly correlates with increased demand for advanced battery technologies, creating a symbiotic relationship between these markets.
Consumer electronics manufacturers are increasingly seeking differentiation through battery performance, creating another significant market opportunity for novel electrolyte solutions. Apple, Samsung, and other major OEMs have filed patents related to advanced electrolyte formulations, signaling industry-wide interest in this technology.
Regional analysis reveals Asia-Pacific as the dominant market for advanced battery electrolytes, accounting for approximately 65% of global production and consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-performance electrolyte innovation. North America and Europe are rapidly expanding their domestic battery supply chains, creating new market opportunities for advanced electrolyte technologies.
Market barriers include cost considerations, with DES-based electrolytes currently commanding a premium of 30-40% over conventional solutions. However, economies of scale and manufacturing optimization are expected to reduce this gap significantly within the next 3-5 years. Regulatory frameworks regarding battery safety and environmental impact are evolving favorably for DES technologies, as they typically offer reduced flammability and improved sustainability profiles compared to traditional electrolytes.
Current Challenges in DES Ionic Transport Mechanisms
Despite significant advancements in Deep Eutectic Solvents (DES) for battery electrolytes, several critical challenges persist in understanding and optimizing their ionic transport mechanisms. The fundamental issue lies in the complex molecular interactions within DES systems, which create unique solvation environments that differ substantially from conventional electrolytes. These interactions directly impact ion mobility and conductivity, yet remain insufficiently characterized at the molecular level.
One primary challenge is the high viscosity inherent to many DES formulations, which significantly impedes ionic mobility and results in lower conductivity compared to traditional carbonate-based electrolytes. This viscosity-conductivity relationship becomes particularly problematic at lower operating temperatures, where some DES systems experience dramatic increases in viscosity that render them practically unusable for energy storage applications.
The temperature dependence of ionic transport in DES electrolytes presents another substantial challenge. Unlike conventional electrolytes that follow predictable Arrhenius behavior, many DES systems exhibit non-linear conductivity responses across operating temperature ranges. This anomalous behavior complicates the development of comprehensive transport models and makes performance prediction difficult across diverse environmental conditions.
Ion speciation and solvation structure within DES environments remain poorly understood despite their critical importance. The formation of complex ion aggregates and the presence of diverse hydrogen bonding networks significantly affect charge carrier concentration and mobility. Current analytical techniques struggle to fully characterize these dynamic structures in situ, creating a knowledge gap that hinders rational electrolyte design.
The interface between DES electrolytes and electrode materials introduces additional transport challenges. Ion desolvation energetics at electrode surfaces differ markedly from conventional systems, affecting charge transfer kinetics and ultimately battery performance. The mechanisms governing these interfacial processes remain largely unresolved, particularly regarding how the unique hydrogen bonding networks in DES influence ion transfer across the electrolyte-electrode boundary.
Computational modeling of DES ionic transport faces significant hurdles due to the complex, non-ideal interactions that characterize these systems. Current molecular dynamics simulations often fail to accurately capture the full range of intermolecular forces present in DES, limiting their predictive capability for transport properties. The development of more sophisticated force fields and multi-scale modeling approaches represents an ongoing challenge for the field.
One primary challenge is the high viscosity inherent to many DES formulations, which significantly impedes ionic mobility and results in lower conductivity compared to traditional carbonate-based electrolytes. This viscosity-conductivity relationship becomes particularly problematic at lower operating temperatures, where some DES systems experience dramatic increases in viscosity that render them practically unusable for energy storage applications.
The temperature dependence of ionic transport in DES electrolytes presents another substantial challenge. Unlike conventional electrolytes that follow predictable Arrhenius behavior, many DES systems exhibit non-linear conductivity responses across operating temperature ranges. This anomalous behavior complicates the development of comprehensive transport models and makes performance prediction difficult across diverse environmental conditions.
Ion speciation and solvation structure within DES environments remain poorly understood despite their critical importance. The formation of complex ion aggregates and the presence of diverse hydrogen bonding networks significantly affect charge carrier concentration and mobility. Current analytical techniques struggle to fully characterize these dynamic structures in situ, creating a knowledge gap that hinders rational electrolyte design.
The interface between DES electrolytes and electrode materials introduces additional transport challenges. Ion desolvation energetics at electrode surfaces differ markedly from conventional systems, affecting charge transfer kinetics and ultimately battery performance. The mechanisms governing these interfacial processes remain largely unresolved, particularly regarding how the unique hydrogen bonding networks in DES influence ion transfer across the electrolyte-electrode boundary.
Computational modeling of DES ionic transport faces significant hurdles due to the complex, non-ideal interactions that characterize these systems. Current molecular dynamics simulations often fail to accurately capture the full range of intermolecular forces present in DES, limiting their predictive capability for transport properties. The development of more sophisticated force fields and multi-scale modeling approaches represents an ongoing challenge for the field.
Current DES Formulations for Enhanced Ionic Conductivity
01 Composition and formulation of DES electrolytes
Deep Eutectic Solvents (DES) can be formulated with various hydrogen bond donors and acceptors to create effective battery electrolytes. These compositions typically include combinations of quaternary ammonium salts with hydrogen bond donors like urea, glycerol, or carboxylic acids. The specific ratio of components affects the melting point, viscosity, and conductivity of the resulting electrolyte. Additives can be incorporated to further enhance performance characteristics such as ionic conductivity and electrochemical stability.- Composition and formulation of DES electrolytes: Deep Eutectic Solvents (DES) can be formulated with various hydrogen bond donors and acceptors to create electrolytes with tailored properties for battery applications. These formulations typically involve combining quaternary ammonium salts with hydrogen bond donors like urea, glycerol, or carboxylic acids. The specific composition affects the melting point, viscosity, and conductivity of the electrolyte, which are crucial parameters for battery performance. Additives can be incorporated to further enhance the electrochemical stability and ionic conductivity of DES-based electrolytes.
- Ionic transport mechanisms in DES electrolytes: The ionic transport in DES electrolytes occurs through a complex mechanism involving the formation and breaking of hydrogen bonds. The viscosity of DES significantly influences ionic mobility, with lower viscosity generally leading to higher ionic conductivity. Temperature also plays a crucial role in enhancing ionic transport by reducing viscosity and increasing ion dissociation. The unique solvation environment in DES affects the coordination of lithium ions, which impacts their transport number and overall battery performance. Understanding these transport mechanisms is essential for optimizing DES electrolytes for high-performance batteries.
- SEI formation and interfacial stability: The solid electrolyte interphase (SEI) formed when using DES electrolytes has unique characteristics compared to conventional electrolytes. DES components can participate in the formation of a more stable and uniform SEI layer on electrode surfaces, which is crucial for long-term cycling stability. The hydrogen bonding network in DES can contribute to a more flexible and self-healing SEI that accommodates volume changes during cycling. Additionally, the reduced flammability of many DES formulations enhances the safety profile of batteries by minimizing risks associated with thermal runaway and electrolyte decomposition.
- Thermal and electrochemical stability of DES: DES electrolytes often exhibit superior thermal stability compared to conventional organic electrolytes, allowing for safer operation at elevated temperatures. The electrochemical stability window of DES can be tuned by selecting appropriate hydrogen bond donors and acceptors, enabling compatibility with high-voltage cathode materials. The strong hydrogen bonding network in DES contributes to reduced volatility and flammability, enhancing overall battery safety. Some DES formulations demonstrate remarkable stability against oxidation at the cathode and reduction at the anode, which is essential for preventing electrolyte decomposition during cycling.
- Performance enhancement strategies for DES-based batteries: Various strategies can be employed to enhance the performance of DES-based battery electrolytes. These include incorporating lithium salts to improve ionic conductivity, adding nanoparticles to create composite electrolytes with enhanced transport properties, and developing hybrid systems that combine DES with other electrolyte types. Molecular design approaches focus on reducing viscosity while maintaining the beneficial properties of DES. Additionally, surface modification of electrodes can improve compatibility with DES electrolytes, leading to better interfacial kinetics and cycling performance.
02 Ionic transport mechanisms in DES electrolytes
Ionic transport in Deep Eutectic Solvent electrolytes occurs through complex mechanisms involving hydrogen bond networks. The mobility of ions depends on the viscosity of the DES, which is influenced by temperature and composition. Charge carriers move through the formation and breaking of hydrogen bonds, with transport properties being affected by the strength of these interactions. The addition of lithium salts or other ionic species can modify the transport pathways and enhance conductivity for battery applications.Expand Specific Solutions03 SEI formation and interface stability
The Solid Electrolyte Interphase (SEI) formed when using Deep Eutectic Solvents as battery electrolytes has unique characteristics compared to conventional electrolytes. DES components contribute to forming protective layers on electrode surfaces that can prevent continuous electrolyte decomposition. The chemical composition of the SEI influences the cycling stability and coulombic efficiency of batteries. Controlling SEI formation through electrolyte design and additives is crucial for improving battery performance and longevity.Expand Specific Solutions04 Thermal and electrochemical stability of DES electrolytes
Deep Eutectic Solvents offer enhanced thermal stability compared to conventional organic electrolytes, allowing for safer battery operation across wider temperature ranges. The electrochemical stability window of DES electrolytes can be tuned through careful selection of hydrogen bond donors and acceptors. This stability is critical for preventing electrolyte decomposition during charging and discharging cycles. The non-flammable nature of many DES formulations provides additional safety benefits for battery applications.Expand Specific Solutions05 Novel DES systems and performance enhancements
Recent innovations in Deep Eutectic Solvent electrolytes include bio-based formulations, hybrid systems combining DES with conventional electrolytes, and nanocomposite approaches. These novel systems aim to address limitations of traditional DES such as viscosity and conductivity challenges. Performance enhancements include the incorporation of nanomaterials to improve ionic conductivity, polymer matrices for mechanical stability, and functional additives that suppress dendrite formation. These advancements contribute to developing next-generation batteries with improved energy density and cycle life.Expand Specific Solutions
Key Industry Players in DES Electrolyte Development
Deep Eutectic Solvents (DES) in battery electrolytes represent an emerging technology in the energy storage sector, currently transitioning from early research to commercial development. The market for advanced battery electrolytes is expanding rapidly, driven by the growing electric vehicle and energy storage sectors, with projections exceeding $7 billion by 2027. Major players like LG Energy Solution, Samsung SDI, and StoreDot are investing heavily in this technology, while research institutions such as Monash University and Shanghai Institute of Ceramics are advancing fundamental understanding of ionic transport mechanisms. Companies including Wildcat Discovery Technologies and Sila Nanotechnologies are developing proprietary DES formulations to enhance battery performance, focusing on improved SEI formation and long-term stability. The technology remains in early-to-mid maturity, with significant R&D efforts addressing challenges in scalability and integration with existing battery manufacturing processes.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a proprietary DES electrolyte system based on choline chloride and trifluoroacetamide with lithium salt additives, specifically engineered for high-voltage cathode materials. Their approach focuses on creating a robust SEI layer through controlled decomposition of the DES components, resulting in a fluorine-rich protective film that prevents continuous electrolyte degradation. The company's research shows that their DES formulation achieves ionic conductivities of approximately 2.8 mS/cm at 25°C while maintaining stability at voltages up to 4.6V vs. Li/Li+. A key innovation in their technology is the incorporation of lithium bis(fluorosulfonyl)imide (LiFSI) at optimized concentrations, which works synergistically with the DES to enhance both ionic conductivity and interfacial stability. LG Energy Solution has successfully demonstrated this technology in prototype cells with NMC811 cathodes, achieving energy densities comparable to conventional electrolytes while significantly improving thermal runaway resistance and cycle life at elevated temperatures (45°C).
Strengths: Excellent oxidative stability at high voltages, enabling compatibility with high-energy cathode materials. Superior thermal safety characteristics with self-extinguishing properties. Weaknesses: Higher cost compared to conventional electrolytes due to specialty chemicals required for formulation. Potential challenges with low-temperature performance, particularly below 0°C.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has leveraged their high-throughput screening platform to develop optimized DES electrolyte formulations for lithium-ion batteries. Their approach combines computational modeling with rapid experimental validation to identify novel DES compositions with superior properties. Wildcat's leading formulation utilizes a combination of quaternary phosphonium salts with carefully selected hydrogen bond donors, achieving ionic conductivities of 3.2 mS/cm at room temperature while forming highly stable SEI layers. Their proprietary additive package includes fluorinated compounds that contribute to SEI formation and stability, particularly on high-capacity silicon-containing anodes. The company has demonstrated that their DES electrolytes can effectively suppress lithium dendrite formation, potentially enabling safer high-energy batteries with lithium metal anodes. Testing in full cells with graphite anodes and NMC622 cathodes has shown over 90% capacity retention after 500 cycles at 1C rates, with significantly improved performance at elevated temperatures compared to conventional carbonate electrolytes.
Strengths: Highly customizable formulations that can be tailored to specific electrode chemistries and operating conditions. Excellent compatibility with silicon-containing anodes, addressing a key challenge for next-generation batteries. Weaknesses: Complex formulations may present manufacturing challenges for large-scale production. Potential intellectual property constraints due to the proprietary nature of their additive packages.
Critical Patents and Research on SEI Formation with DES
Battery formation protocols
PatentWO2024000043A1
Innovation
- A super-concentrated sodium salt containing ionic liquid electrolyte with a sodium salt concentration of 75% or greater is used to form a SEI on hard carbon anodes through high current density polarisation cycles, resulting in a thinner, more conductive SEI with reduced interfacial resistance.
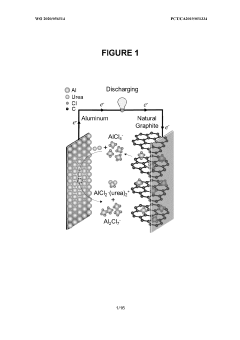
Aluminum-ion battery using aluminum chloride/amide-based deep eutectic solvents
PatentWO2020056514A1
Innovation
- The development of aluminum-ion batteries using aluminum chloride/amide-based deep eutectic solvents as electrolytes, combined with inexpensive graphitic materials and other cathode active materials, such as pyrolytic and natural graphite, to create a cost-effective and safer battery technology.
Environmental Impact and Sustainability of DES Electrolytes
The environmental impact and sustainability of Deep Eutectic Solvents (DES) as battery electrolytes represent a significant advantage over conventional electrolyte systems. DES formulations typically consist of naturally occurring, biodegradable components that can be derived from renewable resources, offering a substantially reduced environmental footprint compared to traditional organic solvent-based electrolytes.
Life cycle assessments of DES electrolytes demonstrate considerably lower ecotoxicity profiles and reduced carbon emissions during production. The synthesis of DES typically requires minimal energy input, with many formulations achievable through simple mixing procedures at moderate temperatures without additional purification steps, resulting in process energy savings of up to 60-70% compared to conventional electrolyte manufacturing.
The biodegradability of many DES components addresses end-of-life concerns for battery systems. Studies indicate that certain choline chloride-based DES formulations can achieve over 80% biodegradation within 28 days under standard testing conditions, compared to less than 20% for conventional carbonate-based electrolytes. This characteristic significantly reduces the environmental persistence of battery waste.
From a safety perspective, DES electrolytes offer reduced flammability and volatility compared to conventional organic electrolytes. Their negligible vapor pressure and higher flash points translate to decreased risk of thermal runaway events and associated environmental contamination through fires or explosions. Toxicological studies further confirm that many DES formulations exhibit minimal acute toxicity to aquatic organisms and mammalian cell lines.
The sustainability advantages extend to resource conservation. Many DES components can be derived from agricultural byproducts or waste streams, creating potential circular economy opportunities. For instance, glycerol-based DES systems utilize a major byproduct of biodiesel production, while certain organic acid components can be sourced from biomass processing waste.
Regulatory frameworks increasingly favor these environmentally benign alternatives. The European Union's Battery Directive and similar regulations worldwide are progressively restricting hazardous substances in battery components, positioning DES electrolytes advantageously for future compliance. Several DES components already meet REACH requirements without special exemptions.
However, challenges remain in scaling up sustainable production methods and establishing comprehensive recycling protocols specific to DES-based battery systems. Current research focuses on optimizing recovery processes for DES components and valuable metals from spent batteries, with preliminary studies suggesting recovery rates exceeding 90% for certain formulations under laboratory conditions.
Life cycle assessments of DES electrolytes demonstrate considerably lower ecotoxicity profiles and reduced carbon emissions during production. The synthesis of DES typically requires minimal energy input, with many formulations achievable through simple mixing procedures at moderate temperatures without additional purification steps, resulting in process energy savings of up to 60-70% compared to conventional electrolyte manufacturing.
The biodegradability of many DES components addresses end-of-life concerns for battery systems. Studies indicate that certain choline chloride-based DES formulations can achieve over 80% biodegradation within 28 days under standard testing conditions, compared to less than 20% for conventional carbonate-based electrolytes. This characteristic significantly reduces the environmental persistence of battery waste.
From a safety perspective, DES electrolytes offer reduced flammability and volatility compared to conventional organic electrolytes. Their negligible vapor pressure and higher flash points translate to decreased risk of thermal runaway events and associated environmental contamination through fires or explosions. Toxicological studies further confirm that many DES formulations exhibit minimal acute toxicity to aquatic organisms and mammalian cell lines.
The sustainability advantages extend to resource conservation. Many DES components can be derived from agricultural byproducts or waste streams, creating potential circular economy opportunities. For instance, glycerol-based DES systems utilize a major byproduct of biodiesel production, while certain organic acid components can be sourced from biomass processing waste.
Regulatory frameworks increasingly favor these environmentally benign alternatives. The European Union's Battery Directive and similar regulations worldwide are progressively restricting hazardous substances in battery components, positioning DES electrolytes advantageously for future compliance. Several DES components already meet REACH requirements without special exemptions.
However, challenges remain in scaling up sustainable production methods and establishing comprehensive recycling protocols specific to DES-based battery systems. Current research focuses on optimizing recovery processes for DES components and valuable metals from spent batteries, with preliminary studies suggesting recovery rates exceeding 90% for certain formulations under laboratory conditions.
Safety Considerations and Performance Benchmarking
Safety considerations for Deep Eutectic Solvents (DES) in battery applications represent a critical aspect of their commercial viability. Unlike conventional organic electrolytes, DES systems generally exhibit significantly lower flammability and volatility, addressing one of the primary safety concerns in lithium-ion battery technology. Thermal stability tests demonstrate that many DES formulations maintain structural integrity at temperatures exceeding 100°C, compared to conventional carbonate electrolytes that decompose at lower temperatures.
Toxicity profiles of DES electrolytes show promising results, with many formulations utilizing bio-derived hydrogen bond donors that present reduced environmental and health hazards. This characteristic aligns with growing regulatory pressure for greener battery technologies, potentially simplifying transportation regulations and reducing safety equipment requirements during manufacturing.
Performance benchmarking against conventional electrolytes reveals both advantages and limitations. Ionic conductivity measurements indicate that optimized DES formulations can achieve 10^-3 to 10^-4 S/cm at room temperature, approaching but generally not exceeding the performance of traditional lithium salt/carbonate solvent systems. However, the conductivity gap narrows significantly at elevated temperatures, suggesting particular advantages for high-temperature applications.
Cycling stability tests demonstrate that DES-based batteries typically achieve 80-90% capacity retention after 500 cycles, comparable to mid-tier commercial electrolytes. The rate capability, however, often lags behind conventional systems, with performance drops becoming more pronounced at high C-rates (>2C), indicating limitations in power-intensive applications.
SEI formation characteristics in DES systems show distinctive advantages, with electrochemical impedance spectroscopy revealing more stable interfacial resistance over extended cycling. This translates to improved long-term performance stability, particularly in elevated temperature conditions where conventional electrolytes typically experience accelerated degradation.
Standardized safety tests including nail penetration, crush testing, and thermal runaway evaluations demonstrate superior safety metrics for DES-based cells. Notably, thermal runaway onset temperatures are typically elevated by 20-50°C compared to conventional electrolytes, providing a significantly expanded safety margin.
Economic analysis indicates that while some DES components currently carry higher costs than traditional electrolytes, their simplified synthesis routes and potential for large-scale production could achieve cost parity in high-volume manufacturing scenarios. Additionally, reduced safety infrastructure requirements may offset initial material cost disadvantages in production environments.
Toxicity profiles of DES electrolytes show promising results, with many formulations utilizing bio-derived hydrogen bond donors that present reduced environmental and health hazards. This characteristic aligns with growing regulatory pressure for greener battery technologies, potentially simplifying transportation regulations and reducing safety equipment requirements during manufacturing.
Performance benchmarking against conventional electrolytes reveals both advantages and limitations. Ionic conductivity measurements indicate that optimized DES formulations can achieve 10^-3 to 10^-4 S/cm at room temperature, approaching but generally not exceeding the performance of traditional lithium salt/carbonate solvent systems. However, the conductivity gap narrows significantly at elevated temperatures, suggesting particular advantages for high-temperature applications.
Cycling stability tests demonstrate that DES-based batteries typically achieve 80-90% capacity retention after 500 cycles, comparable to mid-tier commercial electrolytes. The rate capability, however, often lags behind conventional systems, with performance drops becoming more pronounced at high C-rates (>2C), indicating limitations in power-intensive applications.
SEI formation characteristics in DES systems show distinctive advantages, with electrochemical impedance spectroscopy revealing more stable interfacial resistance over extended cycling. This translates to improved long-term performance stability, particularly in elevated temperature conditions where conventional electrolytes typically experience accelerated degradation.
Standardized safety tests including nail penetration, crush testing, and thermal runaway evaluations demonstrate superior safety metrics for DES-based cells. Notably, thermal runaway onset temperatures are typically elevated by 20-50°C compared to conventional electrolytes, providing a significantly expanded safety margin.
Economic analysis indicates that while some DES components currently carry higher costs than traditional electrolytes, their simplified synthesis routes and potential for large-scale production could achieve cost parity in high-volume manufacturing scenarios. Additionally, reduced safety infrastructure requirements may offset initial material cost disadvantages in production environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!