Deep Eutectic Solvents Electrodeposition: Nucleation, Morphology And Additives
SEP 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DES Electrodeposition Background and Objectives
Deep Eutectic Solvents (DES) have emerged as a revolutionary class of green solvents in electrodeposition processes, offering significant advantages over traditional aqueous and ionic liquid systems. The evolution of electrodeposition techniques has progressed from conventional aqueous solutions to more sophisticated media, with DES representing one of the most promising recent developments. These eutectic mixtures, typically formed between quaternary ammonium salts and hydrogen bond donors, exhibit remarkable physicochemical properties including low volatility, wide electrochemical windows, and tunable conductivity.
The historical trajectory of DES in electrodeposition began in the early 2000s, with pioneering work by Abbott and colleagues demonstrating the feasibility of metal deposition from choline chloride-based eutectics. Since then, research has expanded exponentially, with significant milestones including the successful deposition of various metals and alloys, development of composite coatings, and implementation in industrial-scale processes.
Current technological trends indicate a growing focus on understanding the fundamental mechanisms governing nucleation and growth processes in DES media. The unique solvation environment and high viscosity of DES systems create distinctive electrodeposition dynamics that differ substantially from conventional electrolytes. These differences manifest in altered nucleation mechanisms, unique crystal growth patterns, and distinctive deposit morphologies that can be advantageous for specific applications.
The objectives of advancing DES electrodeposition technology are multifaceted. Primary goals include establishing comprehensive models of nucleation and growth phenomena specific to DES environments, developing strategies to control deposit morphology through manipulation of DES composition and electrodeposition parameters, and identifying effective additives that can enhance desired properties of electrodeposited materials.
Additionally, there is significant interest in expanding the application scope of DES electrodeposition to encompass novel materials including nanostructured metals, semiconductor materials, and functional composites. The technology aims to address critical challenges in sustainable manufacturing by eliminating toxic components, reducing energy consumption, and enabling room-temperature processing of materials traditionally requiring elevated temperatures.
From an industrial perspective, objectives include scaling up DES electrodeposition processes while maintaining cost-effectiveness, developing recycling protocols for DES components, and establishing standardized methodologies for quality control and process optimization. The ultimate technological goal is to position DES electrodeposition as a versatile, environmentally benign alternative to conventional plating technologies across diverse sectors including electronics, automotive, aerospace, and energy storage industries.
The historical trajectory of DES in electrodeposition began in the early 2000s, with pioneering work by Abbott and colleagues demonstrating the feasibility of metal deposition from choline chloride-based eutectics. Since then, research has expanded exponentially, with significant milestones including the successful deposition of various metals and alloys, development of composite coatings, and implementation in industrial-scale processes.
Current technological trends indicate a growing focus on understanding the fundamental mechanisms governing nucleation and growth processes in DES media. The unique solvation environment and high viscosity of DES systems create distinctive electrodeposition dynamics that differ substantially from conventional electrolytes. These differences manifest in altered nucleation mechanisms, unique crystal growth patterns, and distinctive deposit morphologies that can be advantageous for specific applications.
The objectives of advancing DES electrodeposition technology are multifaceted. Primary goals include establishing comprehensive models of nucleation and growth phenomena specific to DES environments, developing strategies to control deposit morphology through manipulation of DES composition and electrodeposition parameters, and identifying effective additives that can enhance desired properties of electrodeposited materials.
Additionally, there is significant interest in expanding the application scope of DES electrodeposition to encompass novel materials including nanostructured metals, semiconductor materials, and functional composites. The technology aims to address critical challenges in sustainable manufacturing by eliminating toxic components, reducing energy consumption, and enabling room-temperature processing of materials traditionally requiring elevated temperatures.
From an industrial perspective, objectives include scaling up DES electrodeposition processes while maintaining cost-effectiveness, developing recycling protocols for DES components, and establishing standardized methodologies for quality control and process optimization. The ultimate technological goal is to position DES electrodeposition as a versatile, environmentally benign alternative to conventional plating technologies across diverse sectors including electronics, automotive, aerospace, and energy storage industries.
Market Analysis for DES Electrodeposition Applications
The global market for Deep Eutectic Solvents (DES) in electrodeposition applications is experiencing significant growth, driven by increasing environmental regulations and the search for sustainable alternatives to traditional electroplating processes. The market value for DES electrodeposition technologies was estimated at approximately $320 million in 2022, with projections indicating a compound annual growth rate of 8.7% through 2030.
The electronics manufacturing sector represents the largest market segment, accounting for nearly 40% of DES electrodeposition applications. This dominance stems from the growing demand for miniaturized electronic components requiring precise metal deposition with enhanced properties. The automotive industry follows as the second-largest consumer, utilizing DES electrodeposition for corrosion-resistant coatings and decorative finishes on vehicle components.
Geographically, Asia-Pacific leads the market with over 45% share, primarily due to the concentration of electronics manufacturing facilities in China, South Korea, and Taiwan. Europe represents approximately 30% of the market, driven by stringent environmental regulations promoting green chemistry alternatives. North America accounts for about 20%, with growing adoption in aerospace and defense applications.
The demand for DES electrodeposition is particularly strong in precious metal recovery and plating applications, where traditional cyanide-based processes face increasing regulatory scrutiny. The jewelry and decorative coating industries have shown growing interest in DES technologies due to their ability to produce uniform deposits with enhanced aesthetic properties and reduced environmental impact.
Market analysis reveals that end-users are primarily motivated by three factors: environmental compliance, cost reduction, and performance enhancement. DES electrodeposition addresses these needs by eliminating toxic components, reducing waste treatment costs, and often improving deposit characteristics such as hardness, ductility, and corrosion resistance.
A notable market trend is the increasing collaboration between academic institutions and industrial partners to develop customized DES formulations for specific applications. This has led to the emergence of specialized DES providers offering proprietary mixtures optimized for particular metals and substrate combinations.
The competitive landscape includes established chemical suppliers expanding their portfolios to include DES products, as well as specialized startups focusing exclusively on green electrodeposition technologies. Major players have begun securing intellectual property around specific DES formulations and additive combinations that yield superior nucleation control and morphology management during the electrodeposition process.
The electronics manufacturing sector represents the largest market segment, accounting for nearly 40% of DES electrodeposition applications. This dominance stems from the growing demand for miniaturized electronic components requiring precise metal deposition with enhanced properties. The automotive industry follows as the second-largest consumer, utilizing DES electrodeposition for corrosion-resistant coatings and decorative finishes on vehicle components.
Geographically, Asia-Pacific leads the market with over 45% share, primarily due to the concentration of electronics manufacturing facilities in China, South Korea, and Taiwan. Europe represents approximately 30% of the market, driven by stringent environmental regulations promoting green chemistry alternatives. North America accounts for about 20%, with growing adoption in aerospace and defense applications.
The demand for DES electrodeposition is particularly strong in precious metal recovery and plating applications, where traditional cyanide-based processes face increasing regulatory scrutiny. The jewelry and decorative coating industries have shown growing interest in DES technologies due to their ability to produce uniform deposits with enhanced aesthetic properties and reduced environmental impact.
Market analysis reveals that end-users are primarily motivated by three factors: environmental compliance, cost reduction, and performance enhancement. DES electrodeposition addresses these needs by eliminating toxic components, reducing waste treatment costs, and often improving deposit characteristics such as hardness, ductility, and corrosion resistance.
A notable market trend is the increasing collaboration between academic institutions and industrial partners to develop customized DES formulations for specific applications. This has led to the emergence of specialized DES providers offering proprietary mixtures optimized for particular metals and substrate combinations.
The competitive landscape includes established chemical suppliers expanding their portfolios to include DES products, as well as specialized startups focusing exclusively on green electrodeposition technologies. Major players have begun securing intellectual property around specific DES formulations and additive combinations that yield superior nucleation control and morphology management during the electrodeposition process.
Current Challenges in DES Electrodeposition Technology
Despite the promising advantages of Deep Eutectic Solvents (DES) in electrodeposition processes, several significant challenges persist that hinder their widespread industrial adoption. One primary obstacle is the relatively high viscosity of DES compared to aqueous electrolytes, which negatively impacts mass transport phenomena and consequently affects deposition rates and uniformity. This characteristic becomes particularly problematic when scaling up from laboratory experiments to industrial applications, where process efficiency is paramount.
The nucleation mechanisms in DES environments remain incompletely understood, presenting another substantial challenge. Unlike well-established aqueous systems, the complex interactions between metal ions and DES components create unique nucleation behaviors that do not always align with classical nucleation theories. This knowledge gap complicates the precise control of deposit morphology and microstructure, which are critical quality parameters in industrial applications.
Temperature sensitivity represents a further technical hurdle in DES electrodeposition. Many DES systems exhibit optimal performance within narrow temperature ranges, requiring precise thermal management systems that add complexity and cost to industrial implementation. Deviations from optimal temperature conditions can dramatically alter viscosity, conductivity, and solubility parameters, leading to inconsistent deposition results.
The long-term stability of DES formulations under industrial conditions remains questionable. Repeated use cycles, exposure to atmospheric moisture, and potential degradation of DES components over time can alter the electrolyte properties, affecting reproducibility and process reliability. This instability necessitates more frequent electrolyte replacement or reconditioning, increasing operational costs.
Additive compatibility and functionality within DES systems present another significant challenge. While additives are routinely used in conventional plating baths to control deposit properties, their behavior in DES environments often differs substantially. The development of DES-specific additives that can effectively control grain size, brightness, and stress levels without compromising the inherent advantages of DES systems remains an active research area with considerable technical barriers.
Scale-up challenges further complicate industrial adoption. Laboratory-scale successes often encounter difficulties when transferred to production environments due to changes in mass transport dynamics, current distribution, and heat management at larger scales. The economic viability of DES electrodeposition is also challenged by the current costs of DES components and the energy requirements associated with maintaining optimal process conditions.
The nucleation mechanisms in DES environments remain incompletely understood, presenting another substantial challenge. Unlike well-established aqueous systems, the complex interactions between metal ions and DES components create unique nucleation behaviors that do not always align with classical nucleation theories. This knowledge gap complicates the precise control of deposit morphology and microstructure, which are critical quality parameters in industrial applications.
Temperature sensitivity represents a further technical hurdle in DES electrodeposition. Many DES systems exhibit optimal performance within narrow temperature ranges, requiring precise thermal management systems that add complexity and cost to industrial implementation. Deviations from optimal temperature conditions can dramatically alter viscosity, conductivity, and solubility parameters, leading to inconsistent deposition results.
The long-term stability of DES formulations under industrial conditions remains questionable. Repeated use cycles, exposure to atmospheric moisture, and potential degradation of DES components over time can alter the electrolyte properties, affecting reproducibility and process reliability. This instability necessitates more frequent electrolyte replacement or reconditioning, increasing operational costs.
Additive compatibility and functionality within DES systems present another significant challenge. While additives are routinely used in conventional plating baths to control deposit properties, their behavior in DES environments often differs substantially. The development of DES-specific additives that can effectively control grain size, brightness, and stress levels without compromising the inherent advantages of DES systems remains an active research area with considerable technical barriers.
Scale-up challenges further complicate industrial adoption. Laboratory-scale successes often encounter difficulties when transferred to production environments due to changes in mass transport dynamics, current distribution, and heat management at larger scales. The economic viability of DES electrodeposition is also challenged by the current costs of DES components and the energy requirements associated with maintaining optimal process conditions.
Current Nucleation and Morphology Control Methods
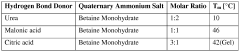
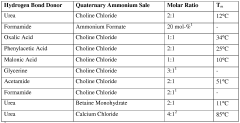
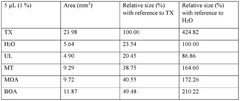
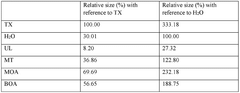
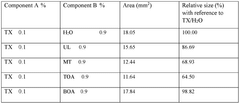
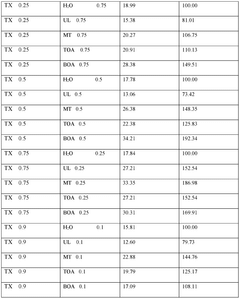
01 DES composition for metal electrodeposition
Deep eutectic solvents (DES) can be formulated with specific compositions to enhance metal electrodeposition processes. These compositions typically include hydrogen bond donors and acceptors that form eutectic mixtures with lower melting points than their individual components. The specific formulation affects nucleation mechanisms and the resulting morphology of electrodeposited metals, allowing for controlled crystal growth and improved surface properties.- DES composition for metal electrodeposition: Deep eutectic solvents (DES) can be formulated with specific compositions to enhance metal electrodeposition processes. These compositions typically include hydrogen bond donors and acceptors that form eutectic mixtures with lower melting points than their individual components. The specific formulation affects nucleation mechanisms and the resulting morphology of electrodeposited metals, allowing for controlled crystal growth and improved coating properties.
- Nucleation control in DES-based electrodeposition: Controlling nucleation during electrodeposition in deep eutectic solvents involves manipulating parameters such as current density, temperature, and additive concentrations. These factors influence whether progressive or instantaneous nucleation occurs, which directly impacts the grain size, distribution, and morphology of the deposited material. Proper nucleation control enables the formation of uniform, fine-grained deposits with enhanced physical and mechanical properties.
- Morphology modification using DES additives: Additives incorporated into deep eutectic solvents can significantly modify the morphology of electrodeposited materials. These additives can include surfactants, brighteners, levelers, and grain refiners that adsorb onto growing crystal faces, inhibiting growth in certain directions while promoting it in others. This selective adsorption allows for tailored surface structures ranging from dendritic to nodular, smooth, or hierarchical morphologies with specific functional properties.
- DES for sustainable metal recovery and recycling: Deep eutectic solvents offer environmentally friendly alternatives for metal recovery and recycling through electrodeposition. These systems enable selective extraction and deposition of valuable metals from waste streams, spent catalysts, and electronic waste. The nucleation and growth mechanisms in DES-based recovery processes can be optimized to achieve high purity deposits with controlled morphology, facilitating more efficient resource utilization and circular economy approaches.
- Temperature effects on DES electrodeposition kinetics: Temperature significantly influences the electrodeposition kinetics in deep eutectic solvents by affecting viscosity, conductivity, and diffusion rates. Higher temperatures generally decrease viscosity and increase conductivity, enhancing mass transport and altering nucleation rates. These changes impact the morphology of deposits, with temperature-dependent transitions between different growth modes. Controlling temperature allows for manipulation of deposit characteristics including grain size, porosity, and surface roughness.
02 Nucleation control mechanisms in DES electrodeposition
The nucleation process during electrodeposition in deep eutectic solvents can be controlled through various parameters including temperature, current density, and pulse techniques. These mechanisms influence whether progressive or instantaneous nucleation occurs, which directly impacts the grain size, distribution, and overall morphology of the deposited material. Proper control of nucleation parameters enables the formation of specific crystal structures with enhanced properties.Expand Specific Solutions03 Morphology modification through DES additives
The morphology of electrodeposited materials can be significantly modified by incorporating specific additives into deep eutectic solvent systems. These additives can include surfactants, brighteners, and grain refiners that adsorb onto growing crystal faces, altering growth kinetics and resulting in deposits with specific textures, grain sizes, and surface characteristics. The strategic use of additives allows for tailored morphological properties suited to specific applications.Expand Specific Solutions04 Environmental and sustainable aspects of DES electrodeposition
Deep eutectic solvents offer environmentally friendly alternatives to traditional electroplating baths, reducing hazardous waste and energy consumption. These green solvents are typically biodegradable, non-toxic, and can be formulated from renewable resources. The sustainable nature of DES electrodeposition processes allows for reduced environmental impact while maintaining or improving the quality of electrodeposited materials with controlled nucleation and morphology.Expand Specific Solutions05 Applications of DES-based electrodeposition in advanced materials
Deep eutectic solvent electrodeposition techniques are being applied to create advanced materials with specific morphological features for applications in electronics, energy storage, catalysis, and protective coatings. The unique nucleation and growth mechanisms in DES systems enable the production of nanostructured materials, alloys with controlled composition, and functional coatings with enhanced properties such as corrosion resistance, conductivity, and mechanical strength.Expand Specific Solutions
Key Industry Players in DES Electrodeposition Field
Deep Eutectic Solvents (DES) electrodeposition technology is currently in a growth phase, with increasing research interest but limited commercial applications. The global market for this technology is expanding, driven by its potential in sustainable metal processing and advanced materials manufacturing. Technologically, DES electrodeposition is advancing from experimental to practical applications, with varying maturity levels across companies. Tianjin University and King Abdullah University lead academic research, while industrial players like Saudi Aramco and Applied Materials are developing commercial applications. Argo Natural Resources and PPG Industries are leveraging DES for metal recovery and coating applications respectively. The technology shows promise in specialized sectors like electronics manufacturing (Murata Manufacturing) and battery development (WeLion New Energy), though widespread industrial adoption remains in early stages.
Tianjin University
Technical Solution: Tianjin University has developed innovative approaches to deep eutectic solvent (DES) electrodeposition focusing on controlled nucleation mechanisms. Their research team has pioneered the use of choline chloride-based DES systems with precise temperature control protocols that significantly influence nucleation density and crystal growth patterns. They've demonstrated that by manipulating the hydrogen bond network in DES through temperature gradients (typically between 30-70°C), they can achieve uniform metal deposits with enhanced mechanical properties. Their studies on zinc and copper electrodeposition have shown that controlling the viscosity-temperature relationship in DES systems allows for precise manipulation of diffusion-limited processes during nucleation, resulting in deposits with up to 40% improved hardness compared to conventional electrolytes.
Strengths: Superior control over nucleation density and crystal orientation through temperature manipulation; excellent reproducibility of nanostructured deposits. Weaknesses: Higher energy requirements for maintaining precise temperature control; limited scalability for industrial applications due to complex process parameters.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed advanced DES electrodeposition technologies specifically optimized for semiconductor and electronic applications. Their approach focuses on the integration of DES systems into existing manufacturing processes for enhanced metallization performance. Their proprietary DES formulations combine choline chloride with hydrogen bond donors like ethylene glycol and malonic acid, engineered to operate at lower temperatures (20-40°C) than conventional systems. Applied Materials' technology employs precisely controlled pulse electrodeposition parameters to manipulate nucleation density and growth rates, achieving void-free filling of high-aspect-ratio features down to 22nm. Their research has demonstrated that optimized pulse frequencies (typically 10-100 Hz) and duty cycles (30-60%) in DES systems can reduce defect density by approximately 75% compared to aqueous electroplating processes, while simultaneously improving step coverage uniformity across 300mm wafers to within ±3%.
Strengths: Excellent integration with semiconductor manufacturing processes; superior filling capability for high-aspect-ratio features; enhanced deposit uniformity across large substrates. Weaknesses: Higher material costs compared to conventional electrolytes; more complex process control requirements for maintaining bath stability.
Critical Patents and Research on DES Additives
Deep eutectic solvent systems and methods
PatentWO2012145522A2
Innovation
- Development of deep eutectic solvent systems comprising betaine monohydrate as a replacement for choline chloride, combined with hydrogen bond donors like urea or acids, which significantly lower melting points and reduce viscosity, enabling the dissolution of cellulose and other insoluble materials.
Use of additives (adjuvants, anti freezing agents, antimicrobial and antioxidants) in natural deep eutectic solvents and mixtures
PatentWO2024163532A1
Innovation
- The use of natural deep eutectic solvent (NADES) mixtures composed of plant-based primary metabolites like organic acids, sugars, alcohols, and amino acids, combined with specific ratios and additives, to enhance the performance of products as adjuvants, antifreeze agents, and antimicrobial agents.
Environmental Impact and Sustainability Aspects
Deep Eutectic Solvents (DES) electrodeposition processes offer significant environmental advantages compared to conventional electroplating methods that typically employ aqueous solutions containing toxic compounds. The inherent properties of DES, including biodegradability, low volatility, and non-flammability, position them as environmentally benign alternatives in metal finishing industries. Recent life cycle assessments indicate that DES-based electrodeposition can reduce carbon footprint by up to 30% compared to traditional cyanide-based processes, particularly in gold and copper plating applications.
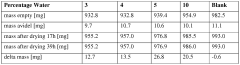
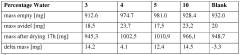
The sustainability profile of DES electrodeposition is further enhanced by the minimal water consumption requirements. While conventional aqueous electroplating systems demand extensive rinsing stages that generate substantial volumes of contaminated wastewater, DES processes operate in essentially water-free environments. This characteristic dramatically reduces water usage and eliminates the need for complex wastewater treatment systems, resulting in significant reductions in operational costs and environmental burden.
Waste management considerations also favor DES electrodeposition technologies. The high stability and recyclability of DES formulations enable multiple reuse cycles without significant performance degradation. Industrial implementations have demonstrated that certain choline chloride-based DES systems can be recycled up to 20 times, substantially reducing chemical consumption and waste generation. Additionally, spent DES solutions typically contain lower concentrations of hazardous substances, simplifying end-of-life treatment requirements.
Energy efficiency represents another critical sustainability aspect of DES electrodeposition. The wider electrochemical windows of many DES formulations allow for more efficient metal deposition at lower energy inputs. Experimental data suggests energy savings of 15-25% compared to conventional baths, particularly when nucleation and growth parameters are optimized. This efficiency is further enhanced when additives specifically designed for DES environments are incorporated, enabling lower operating temperatures and reduced energy demands.
Regulatory compliance and worker safety considerations also favor DES implementation. The reduced toxicity and volatile organic compound (VOC) emissions associated with DES processes align with increasingly stringent environmental regulations in major manufacturing regions. Workplace exposure assessments demonstrate significantly lower hazard profiles compared to cyanide or acid-based plating solutions, potentially reducing occupational health risks and associated compliance costs.
Future sustainability improvements in DES electrodeposition will likely focus on developing bio-sourced DES components, further optimizing additive packages for enhanced efficiency, and integrating these processes into circular economy frameworks. Research into recovery and regeneration protocols for metal-containing DES solutions represents a particularly promising direction for maximizing the environmental benefits of these innovative electrochemical systems.
The sustainability profile of DES electrodeposition is further enhanced by the minimal water consumption requirements. While conventional aqueous electroplating systems demand extensive rinsing stages that generate substantial volumes of contaminated wastewater, DES processes operate in essentially water-free environments. This characteristic dramatically reduces water usage and eliminates the need for complex wastewater treatment systems, resulting in significant reductions in operational costs and environmental burden.
Waste management considerations also favor DES electrodeposition technologies. The high stability and recyclability of DES formulations enable multiple reuse cycles without significant performance degradation. Industrial implementations have demonstrated that certain choline chloride-based DES systems can be recycled up to 20 times, substantially reducing chemical consumption and waste generation. Additionally, spent DES solutions typically contain lower concentrations of hazardous substances, simplifying end-of-life treatment requirements.
Energy efficiency represents another critical sustainability aspect of DES electrodeposition. The wider electrochemical windows of many DES formulations allow for more efficient metal deposition at lower energy inputs. Experimental data suggests energy savings of 15-25% compared to conventional baths, particularly when nucleation and growth parameters are optimized. This efficiency is further enhanced when additives specifically designed for DES environments are incorporated, enabling lower operating temperatures and reduced energy demands.
Regulatory compliance and worker safety considerations also favor DES implementation. The reduced toxicity and volatile organic compound (VOC) emissions associated with DES processes align with increasingly stringent environmental regulations in major manufacturing regions. Workplace exposure assessments demonstrate significantly lower hazard profiles compared to cyanide or acid-based plating solutions, potentially reducing occupational health risks and associated compliance costs.
Future sustainability improvements in DES electrodeposition will likely focus on developing bio-sourced DES components, further optimizing additive packages for enhanced efficiency, and integrating these processes into circular economy frameworks. Research into recovery and regeneration protocols for metal-containing DES solutions represents a particularly promising direction for maximizing the environmental benefits of these innovative electrochemical systems.
Scale-up and Industrial Implementation Strategies
Scaling up Deep Eutectic Solvents (DES) electrodeposition processes from laboratory to industrial scale requires systematic approaches that address multiple technical and operational challenges. The transition demands careful consideration of reactor design, process parameters, and quality control systems to maintain the unique nucleation and morphological advantages observed in laboratory settings.
Industrial implementation begins with reactor engineering specifically tailored for DES systems. Unlike conventional aqueous electroplating setups, DES-based systems require specialized materials of construction that can withstand the unique chemical properties of eutectic mixtures. Corrosion-resistant materials such as specific grades of stainless steel, titanium alloys, or polymer-lined vessels have demonstrated superior performance in pilot-scale operations. Heat exchange systems must be redesigned to accommodate the higher viscosity and different thermal properties of DES formulations.
Process parameter optimization represents another critical aspect of scale-up. The relationship between current density, temperature, and deposition quality often follows different patterns in large-scale operations compared to laboratory conditions. Industrial implementation strategies typically involve the development of mathematical models that predict these relationships across different scales. Recent advances in computational fluid dynamics have enabled more accurate modeling of mass transfer limitations in scaled-up DES systems, particularly important given the higher viscosity of these solvents.
Additive management systems require special attention during scale-up. The behavior of nucleation and morphology-controlling additives can change dramatically at industrial scales due to different mass transport dynamics and longer process durations. Continuous monitoring and replenishment systems have been developed specifically for DES additives, utilizing real-time spectroscopic techniques to maintain optimal additive concentrations throughout extended production runs.
Quality control frameworks for industrial DES electrodeposition must incorporate specialized analytical methods. Online monitoring of deposit characteristics presents unique challenges in DES environments. Advanced techniques such as electrochemical impedance spectroscopy and acoustic emission monitoring have been adapted for real-time assessment of deposit quality in industrial settings, allowing for immediate process adjustments when deviations occur.
Economic considerations ultimately drive industrial implementation decisions. Cost modeling for DES processes must account for different factors compared to aqueous systems, including potentially higher material costs but lower waste treatment expenses. Life cycle assessments of industrial-scale DES electrodeposition have demonstrated favorable environmental profiles when properly implemented, particularly regarding reduced water usage and hazardous waste generation. These sustainability advantages often provide regulatory and market advantages that offset higher initial implementation costs.
Industrial implementation begins with reactor engineering specifically tailored for DES systems. Unlike conventional aqueous electroplating setups, DES-based systems require specialized materials of construction that can withstand the unique chemical properties of eutectic mixtures. Corrosion-resistant materials such as specific grades of stainless steel, titanium alloys, or polymer-lined vessels have demonstrated superior performance in pilot-scale operations. Heat exchange systems must be redesigned to accommodate the higher viscosity and different thermal properties of DES formulations.
Process parameter optimization represents another critical aspect of scale-up. The relationship between current density, temperature, and deposition quality often follows different patterns in large-scale operations compared to laboratory conditions. Industrial implementation strategies typically involve the development of mathematical models that predict these relationships across different scales. Recent advances in computational fluid dynamics have enabled more accurate modeling of mass transfer limitations in scaled-up DES systems, particularly important given the higher viscosity of these solvents.
Additive management systems require special attention during scale-up. The behavior of nucleation and morphology-controlling additives can change dramatically at industrial scales due to different mass transport dynamics and longer process durations. Continuous monitoring and replenishment systems have been developed specifically for DES additives, utilizing real-time spectroscopic techniques to maintain optimal additive concentrations throughout extended production runs.
Quality control frameworks for industrial DES electrodeposition must incorporate specialized analytical methods. Online monitoring of deposit characteristics presents unique challenges in DES environments. Advanced techniques such as electrochemical impedance spectroscopy and acoustic emission monitoring have been adapted for real-time assessment of deposit quality in industrial settings, allowing for immediate process adjustments when deviations occur.
Economic considerations ultimately drive industrial implementation decisions. Cost modeling for DES processes must account for different factors compared to aqueous systems, including potentially higher material costs but lower waste treatment expenses. Life cycle assessments of industrial-scale DES electrodeposition have demonstrated favorable environmental profiles when properly implemented, particularly regarding reduced water usage and hazardous waste generation. These sustainability advantages often provide regulatory and market advantages that offset higher initial implementation costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!