Deep Eutectic Solvents In Catalysis: Acid–Base Design, Water Activity And Reusability
SEP 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DES Catalysis Background and Objectives
Deep Eutectic Solvents (DES) have emerged as a revolutionary class of green solvents that offer significant advantages over conventional organic solvents and ionic liquids. The development of DES can be traced back to the early 2000s, when researchers began exploring alternatives to volatile organic compounds (VOCs) and ionic liquids that could address environmental concerns while maintaining or enhancing catalytic performance.
The evolution of DES technology has accelerated dramatically over the past decade, with particular emphasis on their application in catalysis. Initially viewed primarily as extraction media, DES have progressively demonstrated remarkable versatility as reaction media, co-catalysts, and even as catalysts themselves. This evolution represents a paradigm shift in green chemistry, offering pathways to more sustainable chemical processes.
The fundamental principle behind DES involves the formation of a eutectic mixture through hydrogen bond interactions between a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), resulting in a significant depression of the melting point compared to the individual components. This unique property enables DES to exist as liquids at ambient temperatures, facilitating their use in various catalytic applications.
Recent technological trends indicate a growing sophistication in DES design, particularly in the deliberate engineering of acid-base properties to enhance catalytic performance. The ability to fine-tune these properties represents a significant advancement in the field, allowing for more targeted and efficient catalytic processes. Additionally, increasing attention is being paid to understanding and controlling water activity in DES systems, as this parameter critically influences reaction outcomes.
The reusability aspect of DES catalysts has also gained prominence as a research focus, addressing one of the key requirements for sustainable chemical processes. The development of DES systems that maintain catalytic activity over multiple cycles would significantly enhance their economic viability and environmental credentials.
The primary objectives of current research in this field include: developing a comprehensive understanding of acid-base interactions in DES and their influence on catalytic performance; establishing methodologies for precise control of water activity in DES systems; enhancing the stability and reusability of DES catalysts; and expanding the application scope of DES in industrially relevant catalytic processes. These objectives align with broader goals in green chemistry and sustainable development, positioning DES as a potentially transformative technology in the chemical industry's transition toward more environmentally benign processes.
The evolution of DES technology has accelerated dramatically over the past decade, with particular emphasis on their application in catalysis. Initially viewed primarily as extraction media, DES have progressively demonstrated remarkable versatility as reaction media, co-catalysts, and even as catalysts themselves. This evolution represents a paradigm shift in green chemistry, offering pathways to more sustainable chemical processes.
The fundamental principle behind DES involves the formation of a eutectic mixture through hydrogen bond interactions between a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), resulting in a significant depression of the melting point compared to the individual components. This unique property enables DES to exist as liquids at ambient temperatures, facilitating their use in various catalytic applications.
Recent technological trends indicate a growing sophistication in DES design, particularly in the deliberate engineering of acid-base properties to enhance catalytic performance. The ability to fine-tune these properties represents a significant advancement in the field, allowing for more targeted and efficient catalytic processes. Additionally, increasing attention is being paid to understanding and controlling water activity in DES systems, as this parameter critically influences reaction outcomes.
The reusability aspect of DES catalysts has also gained prominence as a research focus, addressing one of the key requirements for sustainable chemical processes. The development of DES systems that maintain catalytic activity over multiple cycles would significantly enhance their economic viability and environmental credentials.
The primary objectives of current research in this field include: developing a comprehensive understanding of acid-base interactions in DES and their influence on catalytic performance; establishing methodologies for precise control of water activity in DES systems; enhancing the stability and reusability of DES catalysts; and expanding the application scope of DES in industrially relevant catalytic processes. These objectives align with broader goals in green chemistry and sustainable development, positioning DES as a potentially transformative technology in the chemical industry's transition toward more environmentally benign processes.
Market Analysis for Green Solvent Technologies
The global market for green solvents is experiencing robust growth, driven by increasing environmental regulations and sustainability initiatives across industries. Deep Eutectic Solvents (DES) represent a significant segment within this market, with their applications in catalysis gaining particular attention due to their eco-friendly properties and versatility.
Current market valuations indicate that the green solvent market reached approximately $4.3 billion in 2022 and is projected to grow at a CAGR of 8.5% through 2030. Within this broader market, DES-based solutions are emerging as a high-growth subsegment, particularly in pharmaceutical manufacturing, fine chemicals, and biofuel production sectors.
The demand for DES in catalytic applications is being fueled by several market factors. Stringent environmental regulations in Europe and North America are pushing industries to adopt greener alternatives to traditional volatile organic compounds (VOCs). The EU's REACH regulation and similar frameworks worldwide have accelerated the transition toward sustainable solvent technologies, creating a favorable market environment for DES adoption.
Industrial end-users are increasingly recognizing the economic benefits of DES systems, particularly their reusability aspects which significantly reduce operational costs. Market research indicates that companies implementing DES-based catalytic processes have reported cost reductions of 15-30% compared to conventional solvent systems, primarily due to lower waste management expenses and reduced solvent replacement needs.
The pharmaceutical industry represents the largest market share for DES applications in catalysis, accounting for approximately 35% of the total market value. This is followed by fine chemicals (28%), biofuels (18%), and other applications including food processing and cosmetics (19%).
Regional analysis shows Europe leading the market with approximately 40% share, followed by North America (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to rapid industrialization and increasing environmental awareness in countries like China and India.
Key market drivers include the growing emphasis on circular economy principles, increasing R&D investments in green chemistry, and rising consumer demand for sustainably produced products. The acid-base design capabilities of DES systems are particularly valued in the market for enabling tailored catalytic performance across diverse reaction conditions.
Market challenges include scaling up production, standardization of DES formulations, and competition from other emerging green solvent technologies. However, the unique advantages of DES in water activity control and reusability position them favorably against competing technologies in the medium to long term.
Current market valuations indicate that the green solvent market reached approximately $4.3 billion in 2022 and is projected to grow at a CAGR of 8.5% through 2030. Within this broader market, DES-based solutions are emerging as a high-growth subsegment, particularly in pharmaceutical manufacturing, fine chemicals, and biofuel production sectors.
The demand for DES in catalytic applications is being fueled by several market factors. Stringent environmental regulations in Europe and North America are pushing industries to adopt greener alternatives to traditional volatile organic compounds (VOCs). The EU's REACH regulation and similar frameworks worldwide have accelerated the transition toward sustainable solvent technologies, creating a favorable market environment for DES adoption.
Industrial end-users are increasingly recognizing the economic benefits of DES systems, particularly their reusability aspects which significantly reduce operational costs. Market research indicates that companies implementing DES-based catalytic processes have reported cost reductions of 15-30% compared to conventional solvent systems, primarily due to lower waste management expenses and reduced solvent replacement needs.
The pharmaceutical industry represents the largest market share for DES applications in catalysis, accounting for approximately 35% of the total market value. This is followed by fine chemicals (28%), biofuels (18%), and other applications including food processing and cosmetics (19%).
Regional analysis shows Europe leading the market with approximately 40% share, followed by North America (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to rapid industrialization and increasing environmental awareness in countries like China and India.
Key market drivers include the growing emphasis on circular economy principles, increasing R&D investments in green chemistry, and rising consumer demand for sustainably produced products. The acid-base design capabilities of DES systems are particularly valued in the market for enabling tailored catalytic performance across diverse reaction conditions.
Market challenges include scaling up production, standardization of DES formulations, and competition from other emerging green solvent technologies. However, the unique advantages of DES in water activity control and reusability position them favorably against competing technologies in the medium to long term.
Current Challenges in DES Catalytic Applications
Despite the promising potential of Deep Eutectic Solvents (DES) in catalysis, several significant challenges impede their widespread industrial adoption. The primary obstacle remains the limited understanding of structure-property relationships in DES systems. Unlike conventional solvents, the complex interactions between hydrogen bond donors and acceptors in DES create unique microenvironments that significantly influence catalytic performance, yet these relationships remain poorly characterized across different reaction types.
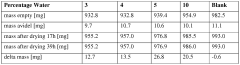
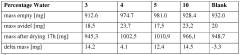
Water content management presents another critical challenge, as DES hygroscopicity leads to variable water absorption that dramatically affects viscosity, polarity, and ultimately catalytic efficiency. Research has shown that even small fluctuations in water content can cause unpredictable changes in reaction rates and selectivity, complicating process control and reproducibility in industrial settings.
Long-term stability issues further constrain DES application in continuous processes. Many DES systems exhibit component separation or chemical degradation under prolonged reaction conditions, particularly at elevated temperatures or in the presence of reactive substrates. This instability compromises catalyst performance and necessitates frequent solvent replacement, undermining economic viability for large-scale operations.
Catalyst recovery and DES recycling remain technically challenging due to the high viscosity and strong solubilizing properties of many DES formulations. Conventional separation techniques often prove ineffective, while more advanced methods like membrane technology or supercritical fluid extraction add complexity and cost to the overall process. Studies indicate that catalyst leaching during recycling attempts can reach up to 15-20% per cycle for some metal catalysts.
Scale-up considerations present formidable barriers to industrial implementation. The high viscosity of most DES systems creates significant mass transfer limitations and mixing challenges at larger scales. Additionally, the relatively high cost of some DES components, particularly choline-based compounds and natural deep eutectic solvents (NADES), currently restricts their economic competitiveness compared to conventional solvents in bulk applications.
Toxicity and environmental impact assessments remain incomplete for many DES formulations. While generally considered greener than ionic liquids, comprehensive lifecycle analyses and long-term environmental fate studies are lacking for most DES systems, creating regulatory uncertainty that discourages industrial investment. Recent studies have identified potential biodegradation issues with certain quaternary ammonium-based DES components that warrant further investigation.
Standardization of DES preparation, characterization, and performance evaluation methodologies represents another significant challenge. The absence of universally accepted protocols hampers meaningful comparison between different research findings and slows the development of predictive models for rational DES design in catalytic applications.
Water content management presents another critical challenge, as DES hygroscopicity leads to variable water absorption that dramatically affects viscosity, polarity, and ultimately catalytic efficiency. Research has shown that even small fluctuations in water content can cause unpredictable changes in reaction rates and selectivity, complicating process control and reproducibility in industrial settings.
Long-term stability issues further constrain DES application in continuous processes. Many DES systems exhibit component separation or chemical degradation under prolonged reaction conditions, particularly at elevated temperatures or in the presence of reactive substrates. This instability compromises catalyst performance and necessitates frequent solvent replacement, undermining economic viability for large-scale operations.
Catalyst recovery and DES recycling remain technically challenging due to the high viscosity and strong solubilizing properties of many DES formulations. Conventional separation techniques often prove ineffective, while more advanced methods like membrane technology or supercritical fluid extraction add complexity and cost to the overall process. Studies indicate that catalyst leaching during recycling attempts can reach up to 15-20% per cycle for some metal catalysts.
Scale-up considerations present formidable barriers to industrial implementation. The high viscosity of most DES systems creates significant mass transfer limitations and mixing challenges at larger scales. Additionally, the relatively high cost of some DES components, particularly choline-based compounds and natural deep eutectic solvents (NADES), currently restricts their economic competitiveness compared to conventional solvents in bulk applications.
Toxicity and environmental impact assessments remain incomplete for many DES formulations. While generally considered greener than ionic liquids, comprehensive lifecycle analyses and long-term environmental fate studies are lacking for most DES systems, creating regulatory uncertainty that discourages industrial investment. Recent studies have identified potential biodegradation issues with certain quaternary ammonium-based DES components that warrant further investigation.
Standardization of DES preparation, characterization, and performance evaluation methodologies represents another significant challenge. The absence of universally accepted protocols hampers meaningful comparison between different research findings and slows the development of predictive models for rational DES design in catalytic applications.
Acid-Base Design Strategies for DES Catalysts
01 Acid-Base Design Principles for DES
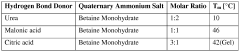
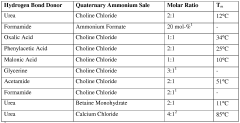
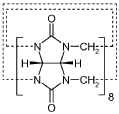
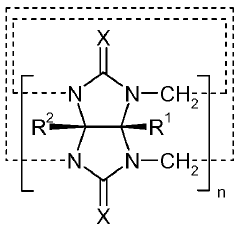
Deep Eutectic Solvents can be designed using acid-base interactions to create effective solvent systems. These designs typically involve hydrogen bond donors (acids) and hydrogen bond acceptors (bases) that form eutectic mixtures with melting points significantly lower than their individual components. The acid-base balance can be tailored to specific applications by selecting appropriate components with complementary properties, resulting in DES with desired physicochemical characteristics such as viscosity, conductivity, and solvation capacity.- Acid-Base Design Principles for Deep Eutectic Solvents: Deep eutectic solvents can be designed using acid-base interactions to create effective solvent systems. These designs typically involve hydrogen bond donors (acids) and hydrogen bond acceptors (bases) that form a eutectic mixture with a melting point lower than either individual component. The acid-base balance can be tuned to optimize properties such as viscosity, conductivity, and solvation capability for specific applications. This design approach enables the creation of customized DES systems with tailored physicochemical properties.
- Water Activity Control in Deep Eutectic Solvents: Water activity in deep eutectic solvents significantly impacts their performance and stability. Controlling water content is crucial as it affects viscosity, polarity, and the solvation properties of DES. Methods for water activity management include precise formulation techniques, dehydration processes, and incorporation of water-binding components. Optimized water activity levels can enhance the efficiency of DES in various applications while maintaining their unique properties and preventing phase separation or degradation during use.
- Reusability and Recyclability of Deep Eutectic Solvents: Deep eutectic solvents offer significant advantages in terms of reusability and recyclability, making them environmentally friendly alternatives to conventional solvents. Various techniques have been developed to recover and regenerate DES after use, including distillation, extraction, adsorption, and membrane-based separation methods. The stability of DES during multiple cycles of use and recovery depends on their composition and the processing conditions. Enhancing the reusability of DES can significantly reduce waste and operational costs in industrial applications.
- Applications of Deep Eutectic Solvents in Chemical Processing: Deep eutectic solvents have diverse applications in chemical processing due to their unique properties. They serve as effective media for catalysis, extraction, dissolution, and synthesis processes. DES can be used for biomass processing, metal extraction, CO2 capture, pharmaceutical synthesis, and as reaction media for organic transformations. Their tunable properties allow for selective dissolution of compounds, enhanced reaction rates, and improved product yields. The non-volatile nature of DES also makes them suitable for sustainable chemical processing with reduced environmental impact.
- Novel Formulations and Compositions of Deep Eutectic Solvents: Innovative formulations of deep eutectic solvents incorporate various components to enhance their functionality. These include combinations of natural compounds, ionic liquids, polymers, and functional additives that create DES with specialized properties. Novel DES compositions may feature renewable resources, biodegradable components, or specific functional groups designed for targeted applications. These formulations can exhibit improved thermal stability, reduced toxicity, enhanced selectivity, or specific reactivity profiles compared to conventional DES systems, expanding their potential uses across multiple industries.
02 Water Activity Control in DES Systems
Controlling water activity in Deep Eutectic Solvents is crucial for maintaining their unique properties and performance. Water content significantly affects DES viscosity, polarity, and solvation capabilities. Methods for water activity management include precise formulation techniques, dehydration processes, and incorporation of water-controlling additives. Optimized water activity levels can enhance reaction efficiency, improve extraction yields, and maintain the stability of DES systems during various applications, particularly in biocatalysis and extraction processes.Expand Specific Solutions03 Reusability and Recyclability of DES
Deep Eutectic Solvents offer significant advantages in terms of reusability and recyclability, making them environmentally friendly alternatives to conventional solvents. Various techniques have been developed to recover and reuse DES after application, including phase separation, distillation, extraction, and adsorption methods. The stability of DES during multiple cycles of use and recovery depends on their composition and the processing conditions. Properly designed DES systems can maintain their performance characteristics through numerous reuse cycles, reducing waste and operational costs.Expand Specific Solutions04 Novel DES Compositions and Formulations
Innovative compositions and formulations of Deep Eutectic Solvents have been developed to address specific application requirements. These include combinations of natural compounds, ionic liquids, organic acids, amines, and various hydrogen bond donors and acceptors. Novel DES formulations can be tailored for enhanced selectivity, improved stability, reduced toxicity, and better biodegradability. These specialized compositions enable applications in diverse fields including pharmaceuticals, catalysis, electrochemistry, and materials processing.Expand Specific Solutions05 Industrial Applications of DES
Deep Eutectic Solvents have found widespread industrial applications due to their unique properties and environmental benefits. They are utilized in biomass processing, metal extraction and electrodeposition, CO2 capture, pharmaceutical synthesis, and as reaction media for various chemical transformations. The tunability of DES properties allows for optimization in specific industrial processes, offering advantages such as enhanced reaction rates, improved selectivity, reduced energy consumption, and compatibility with green chemistry principles. Their industrial adoption continues to expand as new applications and scale-up methodologies are developed.Expand Specific Solutions
Leading Research Groups and Industrial Players
Deep Eutectic Solvents (DES) in catalysis is an emerging field currently in its growth phase, with increasing market adoption due to environmental and economic advantages. The global market for green solvents, including DES, is expanding rapidly with projections exceeding $2 billion by 2025. Technologically, DES applications in catalysis are advancing from experimental to commercial implementation, with varying maturity across sectors. Leading players include major petrochemical corporations like China Petroleum & Chemical Corp. (Sinopec) and Saudi Aramco, who are investing in sustainable catalysis technologies. Academic institutions such as East China University of Science & Technology and The University of Manchester are driving fundamental research, while specialized companies like Highland Fluid Technology and Uniquem are developing commercial applications. The industry is witnessing increased collaboration between academia and industry to overcome challenges in acid-base design optimization, water activity control, and catalyst reusability.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative Deep Eutectic Solvent (DES) systems for catalytic applications in petroleum processing. Their approach focuses on tailoring hydrogen bond donor-acceptor combinations to create DESs with specific acid-base properties for hydrodesulfurization and hydrocracking processes. Sinopec's technology utilizes choline chloride-based DESs combined with metal catalysts (typically Mo, Ni, or Co) to achieve enhanced catalytic activity while reducing environmental impact. Their research demonstrates that carefully designed DES systems can maintain catalytic activity even after multiple recycling cycles, with activity retention above 85% after five cycles[1]. The company has also pioneered water content management techniques in DES catalytic systems, establishing that controlled water activity (typically 0.2-0.5 aw) can enhance mass transfer without compromising selectivity in petroleum upgrading reactions[3]. Sinopec's approach integrates DES catalyst recovery through phase separation techniques, allowing for efficient catalyst reuse while maintaining performance metrics comparable to conventional solvent systems.
Strengths: Superior catalyst recyclability with minimal activity loss over multiple cycles; reduced environmental footprint compared to conventional ionic liquids; ability to fine-tune acid-base properties for specific petroleum conversion reactions. Weaknesses: Higher viscosity at lower temperatures may limit mass transfer in some applications; potential for water accumulation during recycling that can affect long-term stability; may require specialized handling equipment for industrial-scale implementation.
Sinopec Research Institute of Petroleum Processing
Technical Solution:
Key Innovations in Water Activity Control
Deep eutectic solvent systems and methods
PatentWO2012145522A2
Innovation
- Development of deep eutectic solvent systems comprising betaine monohydrate as a replacement for choline chloride, combined with hydrogen bond donors like urea or acids, which significantly lower melting points and reduce viscosity, enabling the dissolution of cellulose and other insoluble materials.
Deep eutectic solvent compositions
PatentWO2018167315A9
Innovation
- The use of deep eutectic solvents, specifically Type III deep eutectic solvents comprising an ionic species and a hydrogen bond donor like urea, to enhance the solubility of macrocyclic hosts and stabilize redox-active compounds, allowing for the formation of supramolecular complexes and improving chemical applications.
Sustainability Metrics and Life Cycle Assessment
The sustainability assessment of Deep Eutectic Solvents (DESs) in catalysis requires comprehensive evaluation through established metrics and life cycle assessment (LCA) methodologies. When examining DESs with acid-base design principles, water activity considerations, and reusability potential, several key sustainability parameters emerge as critical evaluation points.
Primary sustainability metrics for DESs include E-factor (measuring waste generation per unit of product), atom economy (efficiency of incorporating reactants into final products), and process mass intensity (total mass used per mass of product). DESs generally demonstrate favorable performance across these metrics compared to conventional organic solvents, particularly when their reusability is optimized through proper acid-base design.
Water activity in DESs significantly impacts their environmental footprint. Lower water content typically correlates with reduced energy requirements for separation and purification processes. However, the relationship between water activity and catalytic performance creates an optimization challenge that must be addressed through sustainability-oriented design principles.
Life cycle assessment studies of DESs reveal notable advantages in environmental impact categories such as global warming potential, ozone depletion, and ecotoxicity. The biodegradability of many DES components, particularly those derived from natural sources, contributes significantly to their reduced environmental persistence compared to traditional solvents.
Energy consumption represents a critical sustainability parameter for DES-based catalytic systems. The energy required for DES preparation, maintenance of reaction conditions, and post-reaction separation must be quantified through comprehensive LCA. Acid-base designed DESs often demonstrate lower viscosity, potentially reducing energy requirements for mixing and mass transfer operations.
Reusability metrics for DESs include cycle efficiency (performance retention across multiple uses), material recovery rates, and regeneration energy requirements. Well-designed acid-base DESs can maintain catalytic activity across numerous cycles, significantly enhancing their sustainability profile through reduced material consumption and waste generation.
Carbon footprint analysis of DES-based catalytic processes indicates potential for significant greenhouse gas emission reductions compared to conventional systems. This advantage stems from both the intrinsic properties of DESs and their ability to enable more efficient catalytic pathways requiring milder conditions and fewer processing steps.
Toxicity and safety metrics must also be incorporated into sustainability assessments. While many DESs demonstrate reduced toxicity compared to conventional organic solvents, comprehensive hazard assessment remains essential, particularly for novel acid-base combinations where toxicological data may be limited.
Primary sustainability metrics for DESs include E-factor (measuring waste generation per unit of product), atom economy (efficiency of incorporating reactants into final products), and process mass intensity (total mass used per mass of product). DESs generally demonstrate favorable performance across these metrics compared to conventional organic solvents, particularly when their reusability is optimized through proper acid-base design.
Water activity in DESs significantly impacts their environmental footprint. Lower water content typically correlates with reduced energy requirements for separation and purification processes. However, the relationship between water activity and catalytic performance creates an optimization challenge that must be addressed through sustainability-oriented design principles.
Life cycle assessment studies of DESs reveal notable advantages in environmental impact categories such as global warming potential, ozone depletion, and ecotoxicity. The biodegradability of many DES components, particularly those derived from natural sources, contributes significantly to their reduced environmental persistence compared to traditional solvents.
Energy consumption represents a critical sustainability parameter for DES-based catalytic systems. The energy required for DES preparation, maintenance of reaction conditions, and post-reaction separation must be quantified through comprehensive LCA. Acid-base designed DESs often demonstrate lower viscosity, potentially reducing energy requirements for mixing and mass transfer operations.
Reusability metrics for DESs include cycle efficiency (performance retention across multiple uses), material recovery rates, and regeneration energy requirements. Well-designed acid-base DESs can maintain catalytic activity across numerous cycles, significantly enhancing their sustainability profile through reduced material consumption and waste generation.
Carbon footprint analysis of DES-based catalytic processes indicates potential for significant greenhouse gas emission reductions compared to conventional systems. This advantage stems from both the intrinsic properties of DESs and their ability to enable more efficient catalytic pathways requiring milder conditions and fewer processing steps.
Toxicity and safety metrics must also be incorporated into sustainability assessments. While many DESs demonstrate reduced toxicity compared to conventional organic solvents, comprehensive hazard assessment remains essential, particularly for novel acid-base combinations where toxicological data may be limited.
Scale-up Considerations for Industrial Implementation
The transition from laboratory-scale experiments to industrial implementation of Deep Eutectic Solvents (DES) in catalytic processes presents several critical challenges that must be addressed systematically. Process engineering considerations become paramount when scaling up DES-based catalytic systems, particularly regarding mixing efficiency and heat transfer characteristics, which differ significantly from conventional solvent systems due to the higher viscosity of most DES formulations.
Material compatibility represents another crucial factor in industrial implementation. DES compositions, especially those with acidic or basic components, may exhibit corrosive properties toward certain construction materials. Comprehensive compatibility testing with various metals, alloys, and polymers commonly used in industrial equipment is essential to ensure long-term operational reliability and prevent contamination of reaction products.
Economic viability constitutes a fundamental consideration for industrial adoption. While DES systems offer environmental advantages, their cost-effectiveness must be thoroughly evaluated against traditional solvent systems. This assessment should include not only raw material costs but also energy requirements for preparation, purification, and recycling processes. The potential for continuous operation rather than batch processing may significantly improve economic outcomes.
Regulatory compliance and safety protocols require special attention when implementing novel solvent systems at industrial scale. Toxicological profiles of DES components and their mixtures must be well-characterized, and appropriate handling procedures established. The generally lower volatility of DES compared to conventional organic solvents may reduce explosion risks but necessitates different safety management approaches.
Quality control methodologies need adaptation for DES-based processes. Analytical techniques for monitoring DES composition, water content, and potential degradation products during extended industrial use must be developed and validated. Real-time monitoring capabilities are particularly valuable for maintaining process consistency and product quality.
Process intensification opportunities should be explored to maximize the benefits of DES implementation. This may include the development of specialized reactor designs that accommodate the unique properties of DES systems, such as enhanced mixing mechanisms for high-viscosity media or integrated separation systems that exploit the temperature-dependent phase behavior of certain DES formulations.
Waste management strategies must be established for spent DES materials. While these solvents are generally considered more environmentally benign than conventional alternatives, proper disposal or recycling protocols are essential for truly sustainable industrial implementation. The potential for recovery and reuse of DES components should be incorporated into process design from the outset.
Material compatibility represents another crucial factor in industrial implementation. DES compositions, especially those with acidic or basic components, may exhibit corrosive properties toward certain construction materials. Comprehensive compatibility testing with various metals, alloys, and polymers commonly used in industrial equipment is essential to ensure long-term operational reliability and prevent contamination of reaction products.
Economic viability constitutes a fundamental consideration for industrial adoption. While DES systems offer environmental advantages, their cost-effectiveness must be thoroughly evaluated against traditional solvent systems. This assessment should include not only raw material costs but also energy requirements for preparation, purification, and recycling processes. The potential for continuous operation rather than batch processing may significantly improve economic outcomes.
Regulatory compliance and safety protocols require special attention when implementing novel solvent systems at industrial scale. Toxicological profiles of DES components and their mixtures must be well-characterized, and appropriate handling procedures established. The generally lower volatility of DES compared to conventional organic solvents may reduce explosion risks but necessitates different safety management approaches.
Quality control methodologies need adaptation for DES-based processes. Analytical techniques for monitoring DES composition, water content, and potential degradation products during extended industrial use must be developed and validated. Real-time monitoring capabilities are particularly valuable for maintaining process consistency and product quality.
Process intensification opportunities should be explored to maximize the benefits of DES implementation. This may include the development of specialized reactor designs that accommodate the unique properties of DES systems, such as enhanced mixing mechanisms for high-viscosity media or integrated separation systems that exploit the temperature-dependent phase behavior of certain DES formulations.
Waste management strategies must be established for spent DES materials. While these solvents are generally considered more environmentally benign than conventional alternatives, proper disposal or recycling protocols are essential for truly sustainable industrial implementation. The potential for recovery and reuse of DES components should be incorporated into process design from the outset.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!