Dimethyl Ether Effectiveness in Identifying New Energy Potentials
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME Technology Background and Objectives
Dimethyl ether (DME) has emerged as a promising alternative fuel and energy carrier, attracting significant attention in the quest for sustainable energy solutions. The technology surrounding DME has evolved rapidly over the past few decades, driven by the global need for cleaner and more efficient energy sources. Initially developed as a propellant and refrigerant, DME's potential as a fuel was recognized in the 1990s, leading to extensive research and development efforts worldwide.
The primary objective of DME technology in the context of new energy potentials is to establish it as a viable and environmentally friendly alternative to conventional fossil fuels. This goal encompasses several key aspects, including improving production efficiency, enhancing storage and distribution infrastructure, and developing DME-compatible engines and fuel systems. Researchers and industry players are focusing on optimizing DME synthesis processes, particularly from renewable feedstocks, to reduce production costs and increase sustainability.
One of the most significant trends in DME technology is its integration with renewable energy sources. The ability to produce DME from biomass, waste materials, and even captured CO2 aligns with the global push towards a circular economy and carbon neutrality. This synergy between DME and renewable energy sources presents a compelling pathway for decarbonizing various sectors, including transportation, power generation, and industrial processes.
The evolution of DME technology is closely tied to advancements in catalysis and process engineering. Ongoing research aims to develop more efficient catalysts for DME synthesis, improve reactor designs, and optimize overall production systems. These efforts are crucial for scaling up DME production and making it economically competitive with traditional fuels.
In the transportation sector, DME is being explored as a clean-burning diesel substitute, capable of significantly reducing particulate matter and NOx emissions. The automotive industry is actively developing DME-compatible engines and fuel systems, with several successful pilot projects demonstrating the feasibility of DME as a vehicle fuel. This trend is expected to accelerate as environmental regulations become more stringent and the demand for low-emission vehicles increases.
The potential of DME in identifying new energy potentials extends beyond its use as a direct fuel. Its role as an energy carrier and storage medium is gaining attention, particularly in the context of renewable energy integration and grid stabilization. DME can be produced using excess renewable electricity during off-peak hours, effectively storing energy in a chemical form that can be easily transported and utilized when needed.
As the technology continues to mature, the focus is shifting towards large-scale implementation and commercialization. This involves addressing challenges related to infrastructure development, standardization of DME specifications, and policy support to create a favorable market environment for DME adoption. The success of DME in unlocking new energy potentials will depend on collaborative efforts between researchers, industry stakeholders, and policymakers to overcome these challenges and realize the full potential of this versatile energy carrier.
The primary objective of DME technology in the context of new energy potentials is to establish it as a viable and environmentally friendly alternative to conventional fossil fuels. This goal encompasses several key aspects, including improving production efficiency, enhancing storage and distribution infrastructure, and developing DME-compatible engines and fuel systems. Researchers and industry players are focusing on optimizing DME synthesis processes, particularly from renewable feedstocks, to reduce production costs and increase sustainability.
One of the most significant trends in DME technology is its integration with renewable energy sources. The ability to produce DME from biomass, waste materials, and even captured CO2 aligns with the global push towards a circular economy and carbon neutrality. This synergy between DME and renewable energy sources presents a compelling pathway for decarbonizing various sectors, including transportation, power generation, and industrial processes.
The evolution of DME technology is closely tied to advancements in catalysis and process engineering. Ongoing research aims to develop more efficient catalysts for DME synthesis, improve reactor designs, and optimize overall production systems. These efforts are crucial for scaling up DME production and making it economically competitive with traditional fuels.
In the transportation sector, DME is being explored as a clean-burning diesel substitute, capable of significantly reducing particulate matter and NOx emissions. The automotive industry is actively developing DME-compatible engines and fuel systems, with several successful pilot projects demonstrating the feasibility of DME as a vehicle fuel. This trend is expected to accelerate as environmental regulations become more stringent and the demand for low-emission vehicles increases.
The potential of DME in identifying new energy potentials extends beyond its use as a direct fuel. Its role as an energy carrier and storage medium is gaining attention, particularly in the context of renewable energy integration and grid stabilization. DME can be produced using excess renewable electricity during off-peak hours, effectively storing energy in a chemical form that can be easily transported and utilized when needed.
As the technology continues to mature, the focus is shifting towards large-scale implementation and commercialization. This involves addressing challenges related to infrastructure development, standardization of DME specifications, and policy support to create a favorable market environment for DME adoption. The success of DME in unlocking new energy potentials will depend on collaborative efforts between researchers, industry stakeholders, and policymakers to overcome these challenges and realize the full potential of this versatile energy carrier.
Market Demand for DME as New Energy Source
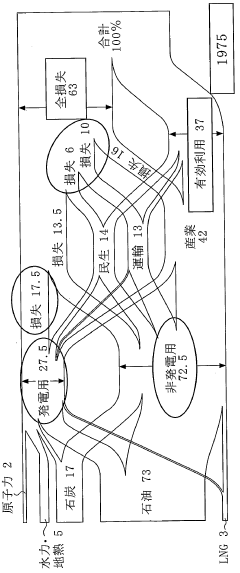
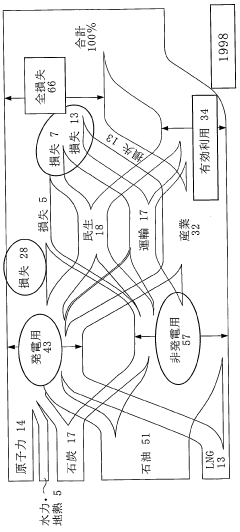
The market demand for Dimethyl Ether (DME) as a new energy source has been steadily growing in recent years, driven by increasing environmental concerns and the search for cleaner alternative fuels. DME is gaining attention due to its potential as a versatile and eco-friendly energy carrier, particularly in sectors such as transportation, power generation, and domestic heating.
In the transportation sector, DME has shown promise as a substitute for diesel fuel in heavy-duty vehicles. Its high cetane number and clean-burning properties make it an attractive option for reducing emissions and improving air quality in urban areas. Several major automotive manufacturers have conducted trials and pilot projects using DME-powered trucks, indicating a growing interest in this technology.
The power generation industry is also exploring DME as a potential fuel for gas turbines and combined cycle power plants. Its low emissions profile and compatibility with existing natural gas infrastructure make it an appealing option for utilities seeking to reduce their carbon footprint while maintaining operational flexibility.
In the domestic heating sector, DME is being considered as a replacement for liquefied petroleum gas (LPG) in regions where natural gas infrastructure is limited. Its similar properties to LPG allow for easy adoption with minimal modifications to existing appliances and distribution systems.
Market analysts project significant growth potential for DME in the coming years. The global DME market is expected to expand at a compound annual growth rate (CAGR) of over 9% from 2021 to 2026. This growth is primarily attributed to increasing demand from emerging economies in Asia-Pacific, particularly China and India, where government initiatives to promote cleaner fuels are driving adoption.
However, the market demand for DME faces several challenges. The lack of widespread infrastructure for production, distribution, and storage remains a significant barrier to large-scale adoption. Additionally, competition from other alternative fuels, such as hydrogen and advanced biofuels, may impact DME's market penetration in certain sectors.
Despite these challenges, the market outlook for DME remains positive. Ongoing research and development efforts are focused on improving production efficiency and reducing costs, which could further enhance its competitiveness as a new energy source. As governments worldwide implement stricter emissions regulations and push for decarbonization, the demand for clean-burning fuels like DME is expected to increase across various industries.
In the transportation sector, DME has shown promise as a substitute for diesel fuel in heavy-duty vehicles. Its high cetane number and clean-burning properties make it an attractive option for reducing emissions and improving air quality in urban areas. Several major automotive manufacturers have conducted trials and pilot projects using DME-powered trucks, indicating a growing interest in this technology.
The power generation industry is also exploring DME as a potential fuel for gas turbines and combined cycle power plants. Its low emissions profile and compatibility with existing natural gas infrastructure make it an appealing option for utilities seeking to reduce their carbon footprint while maintaining operational flexibility.
In the domestic heating sector, DME is being considered as a replacement for liquefied petroleum gas (LPG) in regions where natural gas infrastructure is limited. Its similar properties to LPG allow for easy adoption with minimal modifications to existing appliances and distribution systems.
Market analysts project significant growth potential for DME in the coming years. The global DME market is expected to expand at a compound annual growth rate (CAGR) of over 9% from 2021 to 2026. This growth is primarily attributed to increasing demand from emerging economies in Asia-Pacific, particularly China and India, where government initiatives to promote cleaner fuels are driving adoption.
However, the market demand for DME faces several challenges. The lack of widespread infrastructure for production, distribution, and storage remains a significant barrier to large-scale adoption. Additionally, competition from other alternative fuels, such as hydrogen and advanced biofuels, may impact DME's market penetration in certain sectors.
Despite these challenges, the market outlook for DME remains positive. Ongoing research and development efforts are focused on improving production efficiency and reducing costs, which could further enhance its competitiveness as a new energy source. As governments worldwide implement stricter emissions regulations and push for decarbonization, the demand for clean-burning fuels like DME is expected to increase across various industries.
DME Technical Challenges and Limitations
Despite the promising potential of Dimethyl Ether (DME) as a new energy source, several technical challenges and limitations hinder its widespread adoption and effectiveness in identifying new energy potentials. One of the primary obstacles is the production process of DME, which currently relies heavily on fossil fuels, particularly natural gas or coal. This dependency contradicts the goal of developing sustainable and environmentally friendly energy alternatives, as the production process itself contributes to carbon emissions.
Another significant challenge lies in the storage and transportation of DME. Due to its low boiling point and high vapor pressure, DME requires specialized infrastructure for storage and distribution. This necessitates substantial investments in new or modified equipment, pipelines, and storage facilities, which can be a significant barrier to widespread implementation.
The energy density of DME is lower compared to conventional diesel fuel, which impacts its efficiency in certain applications. This limitation is particularly noticeable in the transportation sector, where vehicles using DME may require larger fuel tanks or more frequent refueling, potentially reducing their practicality for long-distance travel.
DME's corrosive properties pose another technical hurdle. It can degrade certain materials commonly used in fuel systems, such as some plastics and rubber compounds. This necessitates the development and use of compatible materials in engines, fuel lines, and storage tanks, adding complexity and cost to the implementation of DME-based systems.
The combustion characteristics of DME also present challenges in engine design and optimization. While DME has a high cetane number, which is beneficial for diesel engines, its low viscosity and lubricity can lead to increased wear on engine components. This requires the development of specialized lubricants or engine modifications to ensure long-term reliability and performance.
Furthermore, the scalability of DME production remains a significant limitation. Current production methods are not sufficiently efficient or cost-effective to meet large-scale energy demands. Developing more efficient catalysts and production processes is crucial to make DME a viable alternative on a global scale.
Lastly, the lack of standardization in DME production, quality, and usage across different regions and industries poses challenges for widespread adoption. Establishing uniform standards and regulations is essential to ensure consistency in product quality, safety measures, and compatibility across various applications and markets.
Another significant challenge lies in the storage and transportation of DME. Due to its low boiling point and high vapor pressure, DME requires specialized infrastructure for storage and distribution. This necessitates substantial investments in new or modified equipment, pipelines, and storage facilities, which can be a significant barrier to widespread implementation.
The energy density of DME is lower compared to conventional diesel fuel, which impacts its efficiency in certain applications. This limitation is particularly noticeable in the transportation sector, where vehicles using DME may require larger fuel tanks or more frequent refueling, potentially reducing their practicality for long-distance travel.
DME's corrosive properties pose another technical hurdle. It can degrade certain materials commonly used in fuel systems, such as some plastics and rubber compounds. This necessitates the development and use of compatible materials in engines, fuel lines, and storage tanks, adding complexity and cost to the implementation of DME-based systems.
The combustion characteristics of DME also present challenges in engine design and optimization. While DME has a high cetane number, which is beneficial for diesel engines, its low viscosity and lubricity can lead to increased wear on engine components. This requires the development of specialized lubricants or engine modifications to ensure long-term reliability and performance.
Furthermore, the scalability of DME production remains a significant limitation. Current production methods are not sufficiently efficient or cost-effective to meet large-scale energy demands. Developing more efficient catalysts and production processes is crucial to make DME a viable alternative on a global scale.
Lastly, the lack of standardization in DME production, quality, and usage across different regions and industries poses challenges for widespread adoption. Establishing uniform standards and regulations is essential to ensure consistency in product quality, safety measures, and compatibility across various applications and markets.
Current DME Production Methods
01 Dimethyl ether production methods
Various methods for producing dimethyl ether (DME) are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of hydrocarbons. These processes aim to improve efficiency and yield in DME production, which is important for its use as a clean-burning fuel and chemical feedstock.- Synthesis and production of dimethyl ether: Various methods and processes for synthesizing and producing dimethyl ether are described. These include catalytic dehydration of methanol, direct synthesis from syngas, and other novel approaches. The effectiveness of different catalysts, reaction conditions, and process configurations are explored to improve yield and efficiency.
- Dimethyl ether as a fuel or fuel additive: The effectiveness of dimethyl ether as an alternative fuel or fuel additive is investigated. Its properties as a clean-burning fuel with low emissions are highlighted. Various applications in internal combustion engines, gas turbines, and other energy systems are discussed, along with its potential to replace or supplement conventional fuels.
- Catalysts for dimethyl ether-related processes: Development and optimization of catalysts for dimethyl ether synthesis, conversion, and related processes are explored. Various catalyst compositions, preparation methods, and their effectiveness in improving reaction rates, selectivity, and stability are discussed. The impact of catalyst properties on the overall efficiency of dimethyl ether production is analyzed.
- Dimethyl ether in chemical synthesis: The effectiveness of dimethyl ether as a reactant or intermediate in various chemical synthesis processes is investigated. Its use in the production of other chemicals, such as light olefins, aromatics, or oxygenates, is explored. The advantages and challenges of using dimethyl ether in these applications are discussed.
- Environmental and safety aspects of dimethyl ether: The environmental impact and safety considerations of dimethyl ether production and use are examined. Its potential as a more environmentally friendly alternative to conventional fuels and chemicals is discussed. Safety measures, handling procedures, and risk assessments related to dimethyl ether are explored to ensure its effective and responsible use.
02 Catalysts for dimethyl ether synthesis
Different catalysts are employed to enhance the effectiveness of dimethyl ether production. These include zeolites, metal oxides, and composite catalysts. The choice and optimization of catalysts significantly impact the conversion rate, selectivity, and overall efficiency of DME synthesis processes.Expand Specific Solutions03 Applications of dimethyl ether
Dimethyl ether demonstrates effectiveness in various applications, including use as a clean fuel alternative, aerosol propellant, and chemical intermediate. Its properties make it suitable for diesel engines, household products, and as a building block in chemical synthesis, showcasing its versatility and potential environmental benefits.Expand Specific Solutions04 Purification and separation of dimethyl ether
Techniques for purifying and separating dimethyl ether from reaction mixtures are crucial for its effectiveness in various applications. Methods include distillation, adsorption, and membrane separation, which aim to produce high-purity DME and improve overall process efficiency.Expand Specific Solutions05 Environmental and safety aspects of dimethyl ether use
The effectiveness of dimethyl ether is also evaluated in terms of its environmental impact and safety considerations. Studies focus on its low emissions when used as a fuel, biodegradability, and handling precautions. These aspects are crucial for assessing the overall viability and sustainability of DME in various applications.Expand Specific Solutions
Key Players in DME Industry
The development of Dimethyl Ether (DME) as an alternative energy source is in a transitional phase, with growing market potential but still facing technological challenges. The global DME market is expanding, driven by increasing demand for clean energy solutions. Key players in this field include major petrochemical companies like China Petroleum & Chemical Corp., SK Energy, and BASF, as well as research institutions such as the University of Southern California and the Chinese Academy of Science Guangzhou Energy Research Institute. These organizations are actively working on improving DME production efficiency and exploring new applications. The technology's maturity varies, with some companies having commercial-scale production facilities, while others are still in the research and development stage, indicating a diverse competitive landscape.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for dimethyl ether (DME) production from syngas. Their technology utilizes a bifunctional catalyst system that combines methanol synthesis and dehydration in a single step, improving efficiency and reducing costs[1]. Sinopec has also explored the use of DME as a clean-burning alternative fuel for diesel engines, conducting extensive field trials in vehicles and power generation units[2]. The company has invested in large-scale DME production facilities, with a capacity of over 1 million tons per year, demonstrating the commercial viability of DME as an energy source[3]. Additionally, Sinopec has researched the potential of DME as a hydrogen carrier for fuel cell applications, exploring its role in the transition to a low-carbon energy system[4].
Strengths: Extensive experience in large-scale DME production and commercialization; integrated approach from production to end-use applications. Weaknesses: Heavy reliance on fossil fuel feedstocks for DME production; potential competition from other alternative fuels and electrification in the transportation sector.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative catalysts and process technologies for efficient DME production. Their proprietary MegaMax® catalyst system enables high-yield, single-step conversion of syngas to DME, reducing energy consumption and capital costs[5]. BASF has also explored the use of renewable feedstocks, such as biomass and CO2, for DME synthesis, aligning with the growing demand for sustainable energy solutions[6]. The company's research extends to novel applications of DME, including its use as a propellant in aerosol products and as a refrigerant with low global warming potential[7]. BASF has collaborated with engine manufacturers to optimize DME combustion characteristics, enhancing its potential as a clean-burning fuel for heavy-duty vehicles[8].
Strengths: Advanced catalyst technology for efficient DME production; diverse research portfolio covering multiple applications. Weaknesses: Limited direct involvement in large-scale DME production and distribution infrastructure.
DME Synthesis Innovations
Catalytic system and process for direct synthesis of dimethyl ether from synthesis gas
PatentInactiveUS20090326281A1
Innovation
- A mixed-bed catalytic system comprising a catalyst for methanol synthesis and acid form zeolite ferrierite, with a silica/alumina ratio of 10 and specific potassium and sodium content, is physically mixed and activated, providing a high concentration of Brønsted acid sites for efficient dehydration without forming unwanted products.
Energy supply method and system
PatentWO2006004140A1
Innovation
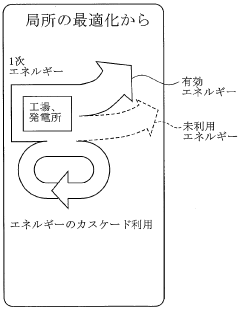
- The introduction of Dimethyl Ether (DME) as a versatile energy circulation medium, which can be derived from biomass, waste, and petroleum residues, and used for power generation, transportation, and heating, allowing for efficient energy storage and distribution without relying on pipelines, and enabling the conversion of waste heat into usable energy.
Environmental Impact of DME
The environmental impact of Dimethyl Ether (DME) as a potential new energy source is a critical aspect to consider in evaluating its effectiveness. DME offers several environmental advantages compared to traditional fossil fuels, particularly in terms of reduced emissions and improved air quality.
One of the primary environmental benefits of DME is its lower carbon footprint. When used as a fuel, DME produces significantly fewer greenhouse gas emissions compared to conventional diesel or gasoline. This reduction in carbon dioxide emissions can contribute to mitigating climate change and meeting global carbon reduction targets. Additionally, DME combustion results in near-zero particulate matter emissions, which is a major advantage in addressing air pollution concerns, especially in urban areas where particulate matter is a significant health hazard.
DME also exhibits lower nitrogen oxide (NOx) emissions compared to conventional fuels. NOx is a major contributor to smog formation and acid rain, and its reduction can lead to improved air quality and reduced environmental impact. The clean-burning properties of DME make it an attractive option for use in various applications, including transportation and power generation, where emission reduction is a priority.
From a lifecycle perspective, DME can be produced from a variety of feedstocks, including renewable sources such as biomass and waste materials. This flexibility in production pathways offers the potential for a more sustainable fuel cycle compared to fossil fuels. When produced from renewable sources, DME can be considered a carbon-neutral fuel, further enhancing its environmental credentials.
However, it is important to note that the environmental impact of DME is closely tied to its production methods. While DME itself is a clean-burning fuel, the production process can have varying environmental implications depending on the feedstock and technology used. For instance, DME production from coal or natural gas may still result in significant carbon emissions during the manufacturing phase.
Water usage and land use changes associated with DME production, particularly when derived from biomass, are additional environmental factors that require careful consideration. Large-scale production of DME from biomass could potentially compete with food crops for agricultural land, raising concerns about food security and biodiversity.
In terms of handling and storage, DME has some environmental advantages. It is non-toxic and biodegradable, reducing the risk of environmental contamination in case of spills or leaks. Furthermore, DME does not persist in the environment, minimizing long-term ecological impacts.
As research and development in DME technology continue, ongoing assessments of its full lifecycle environmental impact are crucial. This includes evaluating the energy efficiency of production processes, optimizing feedstock selection, and developing more sustainable manufacturing methods. Such comprehensive analyses will be essential in determining the true environmental effectiveness of DME as a new energy potential and its role in a sustainable energy future.
One of the primary environmental benefits of DME is its lower carbon footprint. When used as a fuel, DME produces significantly fewer greenhouse gas emissions compared to conventional diesel or gasoline. This reduction in carbon dioxide emissions can contribute to mitigating climate change and meeting global carbon reduction targets. Additionally, DME combustion results in near-zero particulate matter emissions, which is a major advantage in addressing air pollution concerns, especially in urban areas where particulate matter is a significant health hazard.
DME also exhibits lower nitrogen oxide (NOx) emissions compared to conventional fuels. NOx is a major contributor to smog formation and acid rain, and its reduction can lead to improved air quality and reduced environmental impact. The clean-burning properties of DME make it an attractive option for use in various applications, including transportation and power generation, where emission reduction is a priority.
From a lifecycle perspective, DME can be produced from a variety of feedstocks, including renewable sources such as biomass and waste materials. This flexibility in production pathways offers the potential for a more sustainable fuel cycle compared to fossil fuels. When produced from renewable sources, DME can be considered a carbon-neutral fuel, further enhancing its environmental credentials.
However, it is important to note that the environmental impact of DME is closely tied to its production methods. While DME itself is a clean-burning fuel, the production process can have varying environmental implications depending on the feedstock and technology used. For instance, DME production from coal or natural gas may still result in significant carbon emissions during the manufacturing phase.
Water usage and land use changes associated with DME production, particularly when derived from biomass, are additional environmental factors that require careful consideration. Large-scale production of DME from biomass could potentially compete with food crops for agricultural land, raising concerns about food security and biodiversity.
In terms of handling and storage, DME has some environmental advantages. It is non-toxic and biodegradable, reducing the risk of environmental contamination in case of spills or leaks. Furthermore, DME does not persist in the environment, minimizing long-term ecological impacts.
As research and development in DME technology continue, ongoing assessments of its full lifecycle environmental impact are crucial. This includes evaluating the energy efficiency of production processes, optimizing feedstock selection, and developing more sustainable manufacturing methods. Such comprehensive analyses will be essential in determining the true environmental effectiveness of DME as a new energy potential and its role in a sustainable energy future.
DME Safety and Regulations
The safety and regulatory aspects of dimethyl ether (DME) are crucial considerations in its potential use as an alternative energy source. DME is generally considered a safe and environmentally friendly fuel, but proper handling and storage procedures are essential to mitigate potential risks.
From a safety perspective, DME is non-toxic and non-carcinogenic, making it less hazardous than many conventional fuels. It has a relatively high autoignition temperature and a narrow flammability range, which reduces the risk of accidental ignition. However, as DME is a colorless and odorless gas at room temperature and pressure, leak detection systems are necessary to ensure safe operation.
In terms of storage and transportation, DME can be easily liquefied under moderate pressure, similar to liquefied petroleum gas (LPG). This characteristic allows for the use of existing LPG infrastructure with minimal modifications, reducing implementation costs. Nevertheless, proper sealing and materials selection are crucial to prevent leaks, as DME can cause swelling and degradation of certain elastomers and plastics.
Regulatory frameworks for DME vary across different regions and countries. In the United States, the Environmental Protection Agency (EPA) has approved DME as a renewable fuel under the Renewable Fuel Standard program. This recognition has paved the way for increased adoption of DME in the transportation sector. The European Union has also shown interest in DME as a potential alternative fuel, with ongoing efforts to develop specific regulations and standards.
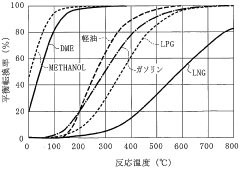
International standards organizations, such as ISO and ASTM, have been working on developing comprehensive standards for DME production, handling, and use. These standards aim to ensure consistency in quality and safety across the global DME industry. For instance, ASTM D7901 provides specifications for DME fuel quality, which is essential for its use in various applications.
Occupational safety regulations for DME handling and use are generally aligned with those for other flammable gases. Workers involved in DME production, storage, and distribution must receive proper training on safety procedures, emergency response, and the use of personal protective equipment. Regular inspections and maintenance of DME facilities and equipment are also mandated to ensure compliance with safety standards.
As the interest in DME as a potential energy source grows, regulatory bodies are likely to develop more specific guidelines and regulations. This evolving regulatory landscape will play a crucial role in shaping the future of DME adoption and its integration into existing energy infrastructures.
From a safety perspective, DME is non-toxic and non-carcinogenic, making it less hazardous than many conventional fuels. It has a relatively high autoignition temperature and a narrow flammability range, which reduces the risk of accidental ignition. However, as DME is a colorless and odorless gas at room temperature and pressure, leak detection systems are necessary to ensure safe operation.
In terms of storage and transportation, DME can be easily liquefied under moderate pressure, similar to liquefied petroleum gas (LPG). This characteristic allows for the use of existing LPG infrastructure with minimal modifications, reducing implementation costs. Nevertheless, proper sealing and materials selection are crucial to prevent leaks, as DME can cause swelling and degradation of certain elastomers and plastics.
Regulatory frameworks for DME vary across different regions and countries. In the United States, the Environmental Protection Agency (EPA) has approved DME as a renewable fuel under the Renewable Fuel Standard program. This recognition has paved the way for increased adoption of DME in the transportation sector. The European Union has also shown interest in DME as a potential alternative fuel, with ongoing efforts to develop specific regulations and standards.
International standards organizations, such as ISO and ASTM, have been working on developing comprehensive standards for DME production, handling, and use. These standards aim to ensure consistency in quality and safety across the global DME industry. For instance, ASTM D7901 provides specifications for DME fuel quality, which is essential for its use in various applications.
Occupational safety regulations for DME handling and use are generally aligned with those for other flammable gases. Workers involved in DME production, storage, and distribution must receive proper training on safety procedures, emergency response, and the use of personal protective equipment. Regular inspections and maintenance of DME facilities and equipment are also mandated to ensure compliance with safety standards.
As the interest in DME as a potential energy source grows, regulatory bodies are likely to develop more specific guidelines and regulations. This evolving regulatory landscape will play a crucial role in shaping the future of DME adoption and its integration into existing energy infrastructures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!