Enhancing Carbolic Acid Solubility for Industrial Applications
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Solubility Enhancement Objectives
Carbolic acid, also known as phenol, is a crucial compound in various industrial applications, including pharmaceuticals, plastics, and disinfectants. However, its limited solubility in water poses significant challenges for many processes. The primary objective of enhancing carbolic acid solubility is to improve its versatility and efficiency in industrial applications while maintaining its essential properties.
One key goal is to develop novel formulations that increase the aqueous solubility of carbolic acid without compromising its effectiveness. This involves exploring various solubilization techniques, such as the use of co-solvents, surfactants, or complexing agents. By achieving higher solubility, we aim to enhance the compound's bioavailability in pharmaceutical applications and improve its performance in water-based industrial processes.
Another important objective is to optimize the pH-dependent solubility of carbolic acid. As the compound's solubility is influenced by pH, developing methods to control and manipulate the pH environment can lead to improved solubility profiles. This approach could potentially expand the range of conditions under which carbolic acid can be effectively utilized in industrial settings.
Enhancing the thermal stability of carbolic acid solutions is also a critical goal. Many industrial processes involve elevated temperatures, and improving the solubility and stability of carbolic acid under these conditions can significantly broaden its applicability. This objective includes researching heat-resistant additives or developing new molecular structures that maintain solubility at higher temperatures.
Furthermore, we aim to explore green chemistry approaches to enhance carbolic acid solubility. This involves investigating environmentally friendly solvents or solubilization methods that reduce the reliance on harmful organic solvents. By aligning with sustainable practices, we can address both the technical challenges of solubility enhancement and the growing demand for eco-friendly industrial processes.
Lastly, a crucial objective is to develop scalable and cost-effective methods for enhancing carbolic acid solubility. While laboratory-scale solutions may demonstrate promising results, their feasibility for large-scale industrial applications must be carefully evaluated. This includes considering factors such as raw material costs, process complexity, and equipment requirements to ensure that the developed solutions are economically viable for widespread adoption in various industries.
One key goal is to develop novel formulations that increase the aqueous solubility of carbolic acid without compromising its effectiveness. This involves exploring various solubilization techniques, such as the use of co-solvents, surfactants, or complexing agents. By achieving higher solubility, we aim to enhance the compound's bioavailability in pharmaceutical applications and improve its performance in water-based industrial processes.
Another important objective is to optimize the pH-dependent solubility of carbolic acid. As the compound's solubility is influenced by pH, developing methods to control and manipulate the pH environment can lead to improved solubility profiles. This approach could potentially expand the range of conditions under which carbolic acid can be effectively utilized in industrial settings.
Enhancing the thermal stability of carbolic acid solutions is also a critical goal. Many industrial processes involve elevated temperatures, and improving the solubility and stability of carbolic acid under these conditions can significantly broaden its applicability. This objective includes researching heat-resistant additives or developing new molecular structures that maintain solubility at higher temperatures.
Furthermore, we aim to explore green chemistry approaches to enhance carbolic acid solubility. This involves investigating environmentally friendly solvents or solubilization methods that reduce the reliance on harmful organic solvents. By aligning with sustainable practices, we can address both the technical challenges of solubility enhancement and the growing demand for eco-friendly industrial processes.
Lastly, a crucial objective is to develop scalable and cost-effective methods for enhancing carbolic acid solubility. While laboratory-scale solutions may demonstrate promising results, their feasibility for large-scale industrial applications must be carefully evaluated. This includes considering factors such as raw material costs, process complexity, and equipment requirements to ensure that the developed solutions are economically viable for widespread adoption in various industries.
Industrial Demand for Improved Carbolic Acid Solutions
The industrial demand for improved carbolic acid solutions has been steadily increasing due to the wide-ranging applications of this versatile compound across various sectors. Carbolic acid, also known as phenol, plays a crucial role in the production of numerous products, including plastics, pharmaceuticals, and disinfectants. However, its limited solubility in water has been a significant challenge for many industrial processes, driving the need for enhanced solubility solutions.
In the chemical industry, carbolic acid is a key raw material for the synthesis of various polymers, such as phenolic resins and polycarbonates. These materials are extensively used in automotive, construction, and electronics industries. The demand for higher-quality and more efficient production processes has led to a growing interest in improving carbolic acid solubility, as it directly impacts the reaction kinetics and product quality.
The pharmaceutical sector has also shown a strong demand for improved carbolic acid solutions. Phenol derivatives are essential components in many drugs and medicinal products. Enhanced solubility of carbolic acid can lead to more efficient drug formulations, improved bioavailability, and reduced production costs. This is particularly important in the development of novel drug delivery systems and the optimization of existing pharmaceutical processes.
In the field of disinfectants and antiseptics, carbolic acid has long been recognized for its potent antimicrobial properties. The recent global health crises have further emphasized the importance of effective disinfection solutions. Improved solubility of carbolic acid can lead to the development of more powerful and fast-acting disinfectants, meeting the growing demand for superior hygiene products in healthcare facilities, public spaces, and households.
The water treatment industry is another sector driving the demand for enhanced carbolic acid solubility. Phenolic compounds are common water pollutants, and improved solubility can aid in their more efficient removal from wastewater streams. This is crucial for meeting increasingly stringent environmental regulations and ensuring the safety of water resources.
Furthermore, the agrochemical industry has shown interest in improved carbolic acid solutions for the development of more effective pesticides and herbicides. Enhanced solubility can lead to better absorption and distribution of active ingredients in plants, potentially reducing the required application rates and minimizing environmental impact.
As industries continue to evolve and face new challenges, the demand for innovative solutions to enhance carbolic acid solubility is expected to grow. This presents significant opportunities for research and development in areas such as novel solvent systems, surfactant technologies, and advanced formulation techniques. The successful development of improved carbolic acid solutions has the potential to drive efficiency, sustainability, and innovation across multiple industrial sectors.
In the chemical industry, carbolic acid is a key raw material for the synthesis of various polymers, such as phenolic resins and polycarbonates. These materials are extensively used in automotive, construction, and electronics industries. The demand for higher-quality and more efficient production processes has led to a growing interest in improving carbolic acid solubility, as it directly impacts the reaction kinetics and product quality.
The pharmaceutical sector has also shown a strong demand for improved carbolic acid solutions. Phenol derivatives are essential components in many drugs and medicinal products. Enhanced solubility of carbolic acid can lead to more efficient drug formulations, improved bioavailability, and reduced production costs. This is particularly important in the development of novel drug delivery systems and the optimization of existing pharmaceutical processes.
In the field of disinfectants and antiseptics, carbolic acid has long been recognized for its potent antimicrobial properties. The recent global health crises have further emphasized the importance of effective disinfection solutions. Improved solubility of carbolic acid can lead to the development of more powerful and fast-acting disinfectants, meeting the growing demand for superior hygiene products in healthcare facilities, public spaces, and households.
The water treatment industry is another sector driving the demand for enhanced carbolic acid solubility. Phenolic compounds are common water pollutants, and improved solubility can aid in their more efficient removal from wastewater streams. This is crucial for meeting increasingly stringent environmental regulations and ensuring the safety of water resources.
Furthermore, the agrochemical industry has shown interest in improved carbolic acid solutions for the development of more effective pesticides and herbicides. Enhanced solubility can lead to better absorption and distribution of active ingredients in plants, potentially reducing the required application rates and minimizing environmental impact.
As industries continue to evolve and face new challenges, the demand for innovative solutions to enhance carbolic acid solubility is expected to grow. This presents significant opportunities for research and development in areas such as novel solvent systems, surfactant technologies, and advanced formulation techniques. The successful development of improved carbolic acid solutions has the potential to drive efficiency, sustainability, and innovation across multiple industrial sectors.
Current Limitations in Carbolic Acid Solubility
Carbolic acid, also known as phenol, faces significant solubility limitations that hinder its widespread industrial applications. The primary challenge lies in its limited solubility in water, which is approximately 8.3 g/100 mL at room temperature. This low solubility restricts the concentration of phenol that can be effectively utilized in aqueous solutions, limiting its efficiency in various industrial processes.
The hydrophobic nature of the phenyl ring in carbolic acid contributes to its poor water solubility. While the hydroxyl group provides some hydrophilicity, it is insufficient to overcome the overall hydrophobic character of the molecule. This results in a tendency for phenol to form separate phases in water at higher concentrations, leading to handling and processing difficulties in industrial settings.
Temperature dependency further complicates the solubility issue. Although phenol's solubility in water increases with temperature, this relationship is not linear and can lead to precipitation problems during cooling processes. This temperature sensitivity necessitates careful control of process conditions, adding complexity and cost to industrial applications.
The limited solubility also impacts the extraction and purification of phenol from industrial mixtures. Traditional liquid-liquid extraction methods may not be sufficiently effective due to the partitioning behavior of phenol between aqueous and organic phases. This can result in lower yields and increased processing steps, reducing overall efficiency and increasing production costs.
Furthermore, the low solubility of carbolic acid affects its reactivity in aqueous media. Many industrial applications require phenol to be in solution for optimal reaction kinetics and yield. The solubility limitation can lead to slower reaction rates, incomplete conversions, and the need for excess reagents or catalysts, all of which negatively impact process economics and sustainability.
Safety concerns also arise from the solubility limitations. Concentrated phenol solutions can be highly corrosive and toxic, necessitating specialized handling and storage procedures. The potential for phase separation and crystallization during storage or transport poses additional safety risks and complicates logistics in industrial settings.
These solubility constraints have prompted ongoing research into alternative formulations and processing methods. Efforts to enhance carbolic acid solubility include the development of co-solvent systems, surfactant-based solutions, and chemical modifications of phenol to produce more soluble derivatives. However, these approaches often introduce additional complexities and costs, highlighting the need for more innovative solutions to overcome the current limitations in carbolic acid solubility for industrial applications.
The hydrophobic nature of the phenyl ring in carbolic acid contributes to its poor water solubility. While the hydroxyl group provides some hydrophilicity, it is insufficient to overcome the overall hydrophobic character of the molecule. This results in a tendency for phenol to form separate phases in water at higher concentrations, leading to handling and processing difficulties in industrial settings.
Temperature dependency further complicates the solubility issue. Although phenol's solubility in water increases with temperature, this relationship is not linear and can lead to precipitation problems during cooling processes. This temperature sensitivity necessitates careful control of process conditions, adding complexity and cost to industrial applications.
The limited solubility also impacts the extraction and purification of phenol from industrial mixtures. Traditional liquid-liquid extraction methods may not be sufficiently effective due to the partitioning behavior of phenol between aqueous and organic phases. This can result in lower yields and increased processing steps, reducing overall efficiency and increasing production costs.
Furthermore, the low solubility of carbolic acid affects its reactivity in aqueous media. Many industrial applications require phenol to be in solution for optimal reaction kinetics and yield. The solubility limitation can lead to slower reaction rates, incomplete conversions, and the need for excess reagents or catalysts, all of which negatively impact process economics and sustainability.
Safety concerns also arise from the solubility limitations. Concentrated phenol solutions can be highly corrosive and toxic, necessitating specialized handling and storage procedures. The potential for phase separation and crystallization during storage or transport poses additional safety risks and complicates logistics in industrial settings.
These solubility constraints have prompted ongoing research into alternative formulations and processing methods. Efforts to enhance carbolic acid solubility include the development of co-solvent systems, surfactant-based solutions, and chemical modifications of phenol to produce more soluble derivatives. However, these approaches often introduce additional complexities and costs, highlighting the need for more innovative solutions to overcome the current limitations in carbolic acid solubility for industrial applications.
Existing Methods for Enhancing Carbolic Acid Solubility
01 Solubility enhancement techniques
Various methods are employed to enhance the solubility of carbolic acid, including the use of co-solvents, surfactants, and pH adjustment. These techniques aim to improve the dissolution of carbolic acid in different media, making it more suitable for various applications in pharmaceuticals, chemical processes, and industrial formulations.- Solubility in water and organic solvents: Carbolic acid, also known as phenol, exhibits varying solubility in different solvents. It is moderately soluble in water and highly soluble in many organic solvents such as alcohols, ethers, and chloroform. The solubility of carbolic acid is influenced by temperature and pH, with higher temperatures generally increasing its solubility.
- pH-dependent solubility: The solubility of carbolic acid is significantly affected by pH. In alkaline solutions, it forms phenolate salts, which are more water-soluble than the free acid. Conversely, in acidic conditions, the solubility decreases as the molecule remains in its undissociated form.
- Solubility enhancement techniques: Various methods can be employed to enhance the solubility of carbolic acid, including the use of co-solvents, surfactants, and complexing agents. These techniques are particularly useful in pharmaceutical and industrial applications where increased solubility is desired.
- Temperature effects on solubility: The solubility of carbolic acid generally increases with temperature. This property is important in various industrial processes, such as extraction and purification. Understanding the temperature-solubility relationship is crucial for optimizing these processes and ensuring efficient handling of carbolic acid solutions.
- Solubility in specific applications: The solubility characteristics of carbolic acid are exploited in various applications, including disinfectants, pharmaceuticals, and chemical synthesis. In these contexts, understanding and controlling the solubility is essential for product efficacy and process efficiency. Specialized formulations may be developed to achieve desired solubility profiles for specific uses.
02 Carbolic acid in aqueous solutions
The solubility of carbolic acid in water is an important consideration for many applications. Research focuses on understanding and improving its aqueous solubility through various means, such as temperature control, addition of electrolytes, and use of solubilizing agents. This knowledge is crucial for developing effective disinfectants, antiseptics, and other water-based formulations containing carbolic acid.Expand Specific Solutions03 Carbolic acid in organic solvents
The solubility of carbolic acid in organic solvents is explored for applications in organic synthesis, extraction processes, and analytical chemistry. Different organic solvents exhibit varying degrees of carbolic acid solubility, which is important for selecting appropriate solvents in industrial and laboratory settings.Expand Specific Solutions04 Temperature effects on carbolic acid solubility
The influence of temperature on carbolic acid solubility is studied to optimize dissolution processes and storage conditions. Understanding how temperature affects solubility is crucial for designing efficient extraction methods, crystallization processes, and maintaining stability in various formulations containing carbolic acid.Expand Specific Solutions05 Carbolic acid solubility in complex mixtures
Research investigates the solubility behavior of carbolic acid in complex mixtures and multi-component systems. This knowledge is essential for developing effective formulations in industries such as pharmaceuticals, cosmetics, and chemical manufacturing, where carbolic acid may interact with various other compounds.Expand Specific Solutions
Key Industry Players in Carbolic Acid Production and Application
The market for enhancing carbolic acid solubility in industrial applications is in a growth phase, driven by increasing demand across various sectors. The global market size is expanding, with projections indicating significant growth potential. Technologically, the field is advancing rapidly, with companies like PetroChina, Chevron Oronite, and Nippon Shokubai leading innovation efforts. These firms, along with others such as Baker Hughes and Dow Global Technologies, are investing heavily in R&D to develop more efficient and cost-effective solutions. The competitive landscape is characterized by a mix of established chemical giants and specialized firms, each bringing unique expertise to address the challenges of carbolic acid solubility enhancement.
Nippon Shokubai Co., Ltd.
Technical Solution: Nippon Shokubai has developed a proprietary technology for enhancing carbolic acid solubility using advanced polymer-based solutions. Their approach involves the synthesis of specially designed water-soluble polymers that can form stable complexes with carbolic acid molecules[2]. These polymers are engineered to have specific functional groups that interact favorably with both the hydrophilic and hydrophobic parts of carbolic acid, effectively increasing its solubility in aqueous media. The company has also explored the use of supercritical fluid technology, particularly supercritical CO2, as a green solvent system for carbolic acid, which can significantly enhance solubility under certain pressure and temperature conditions[4]. Furthermore, Nippon Shokubai has developed a novel microencapsulation technique that allows for controlled release of carbolic acid in various industrial applications, improving its handling and efficacy[6].
Strengths: Innovative polymer-based solutions, expertise in green chemistry approaches, and advanced encapsulation technologies. Weaknesses: Potential scalability issues for some technologies, and possible higher production costs compared to traditional methods.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed cutting-edge technologies for enhancing carbolic acid solubility, particularly focused on oilfield applications. Their approach includes the use of advanced chelating agents that form stable complexes with carbolic acid, significantly increasing its solubility in aqueous solutions[8]. These chelating agents are designed to work effectively in high-temperature and high-pressure environments commonly found in oil and gas operations. Baker Hughes has also pioneered the use of nano-engineered materials, such as functionalized carbon nanotubes, to create highly efficient adsorbents for carbolic acid[10]. This technology allows for improved handling and transportation of carbolic acid in industrial settings. Furthermore, the company has developed smart fluid systems that can dynamically adjust their composition to optimize carbolic acid solubility based on real-time downhole conditions, using responsive polymers and in-situ sensors[12].
Strengths: Specialized solutions for extreme conditions, advanced materials science expertise, and smart fluid technologies. Weaknesses: High development costs for some technologies, and potential environmental concerns with nanomaterials in some applications.
Innovative Approaches to Carbolic Acid Solubilization
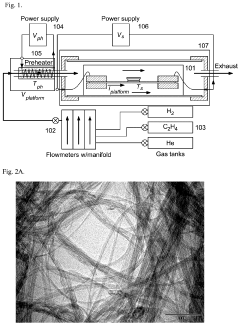
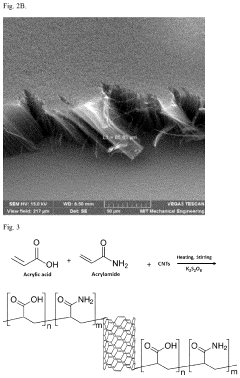
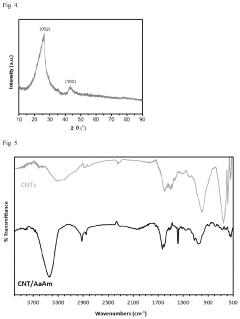
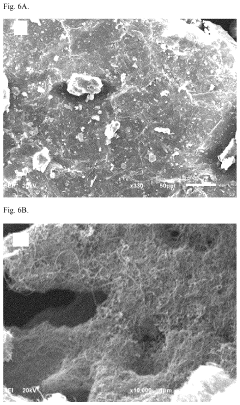
Water purification method with nanocomposite sorbent
PatentActiveUS11976152B2
Innovation
- A nanocomposite sorbent comprising carbon nanotubes grafted with an acrylic acid/acrylamide copolymer composite is developed, which reduces aggregation and enhances adsorption capacity for phenol and other organic pollutants, allowing for convenient reuse without toxic byproducts.
Patent
Innovation
- Novel surfactant formulation to enhance carbolic acid solubility in aqueous solutions for industrial use.
- Development of a pH-responsive solubility enhancement system for carbolic acid.
- Integration of co-solvents with surfactants to create a synergistic solubility enhancement effect.
Environmental Impact of Carbolic Acid Use and Disposal
The environmental impact of carbolic acid, also known as phenol, use and disposal is a critical consideration in industrial applications. Carbolic acid is widely used in various industries, including pharmaceuticals, plastics, and disinfectants. However, its potential environmental hazards necessitate careful management throughout its lifecycle.
When released into the environment, carbolic acid can have detrimental effects on aquatic ecosystems. It is highly toxic to fish and other aquatic organisms, even at low concentrations. Studies have shown that exposure to carbolic acid can lead to reduced growth rates, reproductive impairment, and increased mortality in aquatic species. The compound's persistence in water bodies further exacerbates its ecological impact, as it can accumulate in sediments and affect benthic organisms.
Soil contamination is another significant concern associated with carbolic acid use and disposal. When improperly handled or disposed of, it can leach into soil, potentially affecting soil microorganisms and plant growth. This contamination can persist for extended periods, leading to long-term ecological damage and potential risks to human health through food chain contamination.
Air pollution is also a consideration, particularly in industrial settings where carbolic acid is used or produced. Volatile organic compounds (VOCs) released during its production or use can contribute to smog formation and have adverse effects on air quality. Workers and nearby communities may be at risk of respiratory issues and other health problems if proper emission control measures are not implemented.
The disposal of carbolic acid and its byproducts presents additional environmental challenges. Improper disposal methods, such as direct discharge into water bodies or landfills, can lead to widespread contamination. Incineration, while effective in destroying the compound, may produce harmful emissions if not properly controlled. Therefore, specialized waste treatment facilities and strict disposal protocols are essential to minimize environmental risks.
To mitigate these environmental impacts, industries are increasingly adopting more sustainable practices. These include implementing closed-loop systems to minimize waste generation, developing more efficient production processes to reduce carbolic acid usage, and exploring alternative, less harmful substances where possible. Additionally, advanced wastewater treatment technologies, such as activated carbon adsorption and advanced oxidation processes, are being employed to remove carbolic acid from industrial effluents before discharge.
Regulatory bodies worldwide have established guidelines and standards for the use, handling, and disposal of carbolic acid. These regulations aim to protect both human health and the environment by setting limits on emissions, mandating proper storage and handling procedures, and requiring comprehensive environmental impact assessments for facilities using or producing carbolic acid.
When released into the environment, carbolic acid can have detrimental effects on aquatic ecosystems. It is highly toxic to fish and other aquatic organisms, even at low concentrations. Studies have shown that exposure to carbolic acid can lead to reduced growth rates, reproductive impairment, and increased mortality in aquatic species. The compound's persistence in water bodies further exacerbates its ecological impact, as it can accumulate in sediments and affect benthic organisms.
Soil contamination is another significant concern associated with carbolic acid use and disposal. When improperly handled or disposed of, it can leach into soil, potentially affecting soil microorganisms and plant growth. This contamination can persist for extended periods, leading to long-term ecological damage and potential risks to human health through food chain contamination.
Air pollution is also a consideration, particularly in industrial settings where carbolic acid is used or produced. Volatile organic compounds (VOCs) released during its production or use can contribute to smog formation and have adverse effects on air quality. Workers and nearby communities may be at risk of respiratory issues and other health problems if proper emission control measures are not implemented.
The disposal of carbolic acid and its byproducts presents additional environmental challenges. Improper disposal methods, such as direct discharge into water bodies or landfills, can lead to widespread contamination. Incineration, while effective in destroying the compound, may produce harmful emissions if not properly controlled. Therefore, specialized waste treatment facilities and strict disposal protocols are essential to minimize environmental risks.
To mitigate these environmental impacts, industries are increasingly adopting more sustainable practices. These include implementing closed-loop systems to minimize waste generation, developing more efficient production processes to reduce carbolic acid usage, and exploring alternative, less harmful substances where possible. Additionally, advanced wastewater treatment technologies, such as activated carbon adsorption and advanced oxidation processes, are being employed to remove carbolic acid from industrial effluents before discharge.
Regulatory bodies worldwide have established guidelines and standards for the use, handling, and disposal of carbolic acid. These regulations aim to protect both human health and the environment by setting limits on emissions, mandating proper storage and handling procedures, and requiring comprehensive environmental impact assessments for facilities using or producing carbolic acid.
Safety Regulations for Handling Solubilized Carbolic Acid
The handling of solubilized carbolic acid in industrial applications requires strict adherence to safety regulations to protect workers, the environment, and the integrity of production processes. These regulations typically encompass several key areas of concern, including personal protective equipment (PPE), storage and transportation, emergency response procedures, and environmental safeguards.
Personal protective equipment is a critical component of safety regulations for handling solubilized carbolic acid. Workers must wear appropriate chemical-resistant gloves, protective eyewear, and respiratory protection when working with or near the substance. Full-body protective suits may be required in certain high-risk scenarios or when dealing with large quantities of the solubilized acid.
Storage and transportation regulations for solubilized carbolic acid are designed to prevent accidental releases and ensure proper containment. Facilities must use corrosion-resistant containers and storage tanks, equipped with secondary containment systems to capture potential leaks or spills. Transportation of the substance must comply with hazardous materials regulations, including proper labeling, documentation, and use of approved containers.
Emergency response procedures form a crucial part of safety regulations. Facilities handling solubilized carbolic acid must have detailed emergency plans in place, including spill response protocols, evacuation procedures, and decontamination measures. Regular drills and training sessions should be conducted to ensure all personnel are familiar with these procedures and can respond effectively in case of an incident.
Environmental safeguards are essential to prevent contamination of soil, water, and air. Regulations typically require proper waste disposal methods, including neutralization of acid waste before disposal and the use of approved treatment facilities. Monitoring systems may be mandated to detect and prevent unauthorized releases into the environment.
Workplace exposure limits are strictly regulated to protect workers from the harmful effects of carbolic acid. These limits typically specify the maximum allowable concentration of carbolic acid vapors in the air and the duration of exposure. Regular air quality monitoring and medical surveillance of workers may be required to ensure compliance with these limits.
Training and education requirements are often included in safety regulations. Workers must receive comprehensive training on the hazards of carbolic acid, proper handling techniques, use of PPE, and emergency procedures. This training should be regularly updated and documented to maintain compliance with regulatory standards.
Facility design and engineering controls are also addressed in safety regulations. This may include requirements for proper ventilation systems, enclosed processing areas, and automated handling systems to minimize direct contact with the solubilized acid. Regular inspections and maintenance of these systems are typically mandated to ensure their continued effectiveness.
Personal protective equipment is a critical component of safety regulations for handling solubilized carbolic acid. Workers must wear appropriate chemical-resistant gloves, protective eyewear, and respiratory protection when working with or near the substance. Full-body protective suits may be required in certain high-risk scenarios or when dealing with large quantities of the solubilized acid.
Storage and transportation regulations for solubilized carbolic acid are designed to prevent accidental releases and ensure proper containment. Facilities must use corrosion-resistant containers and storage tanks, equipped with secondary containment systems to capture potential leaks or spills. Transportation of the substance must comply with hazardous materials regulations, including proper labeling, documentation, and use of approved containers.
Emergency response procedures form a crucial part of safety regulations. Facilities handling solubilized carbolic acid must have detailed emergency plans in place, including spill response protocols, evacuation procedures, and decontamination measures. Regular drills and training sessions should be conducted to ensure all personnel are familiar with these procedures and can respond effectively in case of an incident.
Environmental safeguards are essential to prevent contamination of soil, water, and air. Regulations typically require proper waste disposal methods, including neutralization of acid waste before disposal and the use of approved treatment facilities. Monitoring systems may be mandated to detect and prevent unauthorized releases into the environment.
Workplace exposure limits are strictly regulated to protect workers from the harmful effects of carbolic acid. These limits typically specify the maximum allowable concentration of carbolic acid vapors in the air and the duration of exposure. Regular air quality monitoring and medical surveillance of workers may be required to ensure compliance with these limits.
Training and education requirements are often included in safety regulations. Workers must receive comprehensive training on the hazards of carbolic acid, proper handling techniques, use of PPE, and emergency procedures. This training should be regularly updated and documented to maintain compliance with regulatory standards.
Facility design and engineering controls are also addressed in safety regulations. This may include requirements for proper ventilation systems, enclosed processing areas, and automated handling systems to minimize direct contact with the solubilized acid. Regular inspections and maintenance of these systems are typically mandated to ensure their continued effectiveness.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!