The Impact of Carbolic Acid on Steel Corrosion Resistance
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid and Steel Corrosion: Background and Objectives
Carbolic acid, also known as phenol, has been a subject of interest in the field of materials science and corrosion engineering for decades. The study of its impact on steel corrosion resistance is crucial for various industries, including chemical processing, oil and gas, and manufacturing. This research aims to comprehensively examine the effects of carbolic acid on steel structures and develop strategies to enhance corrosion resistance in acidic environments.
The historical context of this research dates back to the early 20th century when the widespread use of steel in industrial applications coincided with the increasing production and utilization of carbolic acid. As industries expanded, the need for corrosion-resistant materials became paramount, leading to extensive studies on the interaction between steel and various corrosive agents, including carbolic acid.
Over the years, significant advancements have been made in understanding the mechanisms of steel corrosion in acidic environments. However, the specific challenges posed by carbolic acid have necessitated focused research due to its unique chemical properties and corrosive behavior. The evolution of this field has seen a shift from purely empirical approaches to more sophisticated analytical and computational methods, enabling researchers to gain deeper insights into the corrosion processes at the molecular level.
The primary objective of this research is to elucidate the fundamental mechanisms by which carbolic acid affects the corrosion resistance of steel. This includes investigating the kinetics of corrosion reactions, the formation and characteristics of corrosion products, and the role of environmental factors in accelerating or mitigating corrosion processes. Additionally, the study aims to evaluate existing corrosion protection methods and explore innovative strategies to enhance steel's resistance to carbolic acid-induced degradation.
Another critical goal is to develop predictive models that can accurately forecast the long-term behavior of steel structures exposed to carbolic acid under various conditions. These models will be invaluable for assessing the lifespan of industrial equipment and infrastructure, enabling more effective maintenance schedules and reducing the risk of catastrophic failures.
Furthermore, this research seeks to bridge the gap between laboratory findings and practical applications. By conducting both controlled experiments and field studies, the project aims to validate theoretical models and ensure that the developed solutions are applicable in real-world industrial settings. This approach will facilitate the translation of research outcomes into tangible improvements in material selection, design practices, and protective measures for steel structures exposed to carbolic acid.
The historical context of this research dates back to the early 20th century when the widespread use of steel in industrial applications coincided with the increasing production and utilization of carbolic acid. As industries expanded, the need for corrosion-resistant materials became paramount, leading to extensive studies on the interaction between steel and various corrosive agents, including carbolic acid.
Over the years, significant advancements have been made in understanding the mechanisms of steel corrosion in acidic environments. However, the specific challenges posed by carbolic acid have necessitated focused research due to its unique chemical properties and corrosive behavior. The evolution of this field has seen a shift from purely empirical approaches to more sophisticated analytical and computational methods, enabling researchers to gain deeper insights into the corrosion processes at the molecular level.
The primary objective of this research is to elucidate the fundamental mechanisms by which carbolic acid affects the corrosion resistance of steel. This includes investigating the kinetics of corrosion reactions, the formation and characteristics of corrosion products, and the role of environmental factors in accelerating or mitigating corrosion processes. Additionally, the study aims to evaluate existing corrosion protection methods and explore innovative strategies to enhance steel's resistance to carbolic acid-induced degradation.
Another critical goal is to develop predictive models that can accurately forecast the long-term behavior of steel structures exposed to carbolic acid under various conditions. These models will be invaluable for assessing the lifespan of industrial equipment and infrastructure, enabling more effective maintenance schedules and reducing the risk of catastrophic failures.
Furthermore, this research seeks to bridge the gap between laboratory findings and practical applications. By conducting both controlled experiments and field studies, the project aims to validate theoretical models and ensure that the developed solutions are applicable in real-world industrial settings. This approach will facilitate the translation of research outcomes into tangible improvements in material selection, design practices, and protective measures for steel structures exposed to carbolic acid.
Market Analysis for Corrosion-Resistant Steel
The market for corrosion-resistant steel has been experiencing significant growth in recent years, driven by increasing demand across various industries. The global corrosion-resistant steel market was valued at approximately $6.5 billion in 2020 and is projected to reach $8.9 billion by 2026, growing at a CAGR of 5.4% during the forecast period.
The oil and gas industry remains the largest consumer of corrosion-resistant steel, accounting for nearly 30% of the market share. This sector's demand is primarily fueled by the need for durable materials in offshore platforms, pipelines, and refining equipment exposed to harsh environments and corrosive substances, including carbolic acid.
The chemical and petrochemical industries are also major contributors to the market growth, with a market share of around 25%. These sectors require corrosion-resistant steel for storage tanks, reactors, and piping systems that handle corrosive chemicals, including carbolic acid and its derivatives.
The construction industry is emerging as a rapidly growing market for corrosion-resistant steel, particularly in coastal and industrial areas where structures are exposed to corrosive environments. This sector is expected to witness the highest growth rate, with a CAGR of 6.2% during the forecast period.
Geographically, Asia-Pacific dominates the corrosion-resistant steel market, accounting for approximately 40% of the global market share. This dominance is attributed to rapid industrialization, infrastructure development, and the presence of major steel-producing countries like China and India. North America and Europe follow, with market shares of 25% and 20% respectively, driven by stringent environmental regulations and the need for sustainable infrastructure.
The impact of carbolic acid on steel corrosion resistance has become a critical factor influencing market dynamics. As industries increasingly use carbolic acid in various processes, the demand for specialized corrosion-resistant steel grades has risen. This trend has led to the development of new alloys and surface treatments specifically designed to withstand carbolic acid exposure, creating niche market opportunities for steel manufacturers and coating technology providers.
In response to these market trends, key players in the corrosion-resistant steel industry are focusing on research and development to improve the performance of their products against carbolic acid and other corrosive substances. This has resulted in the introduction of advanced stainless steel grades and innovative coating technologies, further driving market growth and competitiveness.
The oil and gas industry remains the largest consumer of corrosion-resistant steel, accounting for nearly 30% of the market share. This sector's demand is primarily fueled by the need for durable materials in offshore platforms, pipelines, and refining equipment exposed to harsh environments and corrosive substances, including carbolic acid.
The chemical and petrochemical industries are also major contributors to the market growth, with a market share of around 25%. These sectors require corrosion-resistant steel for storage tanks, reactors, and piping systems that handle corrosive chemicals, including carbolic acid and its derivatives.
The construction industry is emerging as a rapidly growing market for corrosion-resistant steel, particularly in coastal and industrial areas where structures are exposed to corrosive environments. This sector is expected to witness the highest growth rate, with a CAGR of 6.2% during the forecast period.
Geographically, Asia-Pacific dominates the corrosion-resistant steel market, accounting for approximately 40% of the global market share. This dominance is attributed to rapid industrialization, infrastructure development, and the presence of major steel-producing countries like China and India. North America and Europe follow, with market shares of 25% and 20% respectively, driven by stringent environmental regulations and the need for sustainable infrastructure.
The impact of carbolic acid on steel corrosion resistance has become a critical factor influencing market dynamics. As industries increasingly use carbolic acid in various processes, the demand for specialized corrosion-resistant steel grades has risen. This trend has led to the development of new alloys and surface treatments specifically designed to withstand carbolic acid exposure, creating niche market opportunities for steel manufacturers and coating technology providers.
In response to these market trends, key players in the corrosion-resistant steel industry are focusing on research and development to improve the performance of their products against carbolic acid and other corrosive substances. This has resulted in the introduction of advanced stainless steel grades and innovative coating technologies, further driving market growth and competitiveness.
Current Challenges in Steel Corrosion Prevention
Steel corrosion prevention remains a significant challenge in various industries, particularly in environments where carbolic acid is present. Despite advancements in materials science and protective coatings, several obstacles persist in effectively safeguarding steel structures against corrosion.
One of the primary challenges is the aggressive nature of carbolic acid, which can rapidly degrade traditional protective coatings. The acid's ability to penetrate and undermine the integrity of these coatings often leads to localized corrosion, compromising the overall structural integrity of steel components. This issue is particularly pronounced in industrial settings where carbolic acid is a common byproduct or process chemical.
The development of acid-resistant coatings that can withstand prolonged exposure to carbolic acid while maintaining their protective properties is an ongoing challenge. Current coating technologies often struggle to provide long-term protection, necessitating frequent maintenance and reapplication, which increases operational costs and downtime.
Another significant hurdle is the difficulty in accurately predicting and modeling the corrosion behavior of steel in carbolic acid environments. The complex interactions between the acid, steel substrate, and protective measures make it challenging to develop reliable predictive models. This limitation hampers the ability to design optimized protection strategies and estimate the lifespan of steel structures in these harsh conditions.
The variability in environmental conditions further complicates corrosion prevention efforts. Factors such as temperature fluctuations, pH levels, and the presence of other chemical species can significantly influence the corrosion rate and mechanism. Developing universal solutions that can adapt to these diverse conditions remains a formidable challenge for researchers and engineers.
Moreover, the need for environmentally friendly and sustainable corrosion prevention methods adds another layer of complexity. Traditional corrosion inhibitors and protective measures often involve chemicals that pose environmental risks. Balancing effective corrosion protection with ecological considerations is a growing concern in the industry.
The economic aspect of corrosion prevention also presents challenges. Implementing comprehensive corrosion protection measures can be costly, and justifying these expenses in terms of long-term savings and safety improvements is not always straightforward. This economic barrier often leads to the adoption of suboptimal protection strategies, particularly in smaller operations or in regions with limited resources.
In conclusion, while significant progress has been made in understanding and mitigating steel corrosion in carbolic acid environments, numerous challenges persist. Addressing these issues requires a multidisciplinary approach, combining advances in materials science, predictive modeling, and sustainable technologies to develop more effective and economically viable corrosion prevention strategies.
One of the primary challenges is the aggressive nature of carbolic acid, which can rapidly degrade traditional protective coatings. The acid's ability to penetrate and undermine the integrity of these coatings often leads to localized corrosion, compromising the overall structural integrity of steel components. This issue is particularly pronounced in industrial settings where carbolic acid is a common byproduct or process chemical.
The development of acid-resistant coatings that can withstand prolonged exposure to carbolic acid while maintaining their protective properties is an ongoing challenge. Current coating technologies often struggle to provide long-term protection, necessitating frequent maintenance and reapplication, which increases operational costs and downtime.
Another significant hurdle is the difficulty in accurately predicting and modeling the corrosion behavior of steel in carbolic acid environments. The complex interactions between the acid, steel substrate, and protective measures make it challenging to develop reliable predictive models. This limitation hampers the ability to design optimized protection strategies and estimate the lifespan of steel structures in these harsh conditions.
The variability in environmental conditions further complicates corrosion prevention efforts. Factors such as temperature fluctuations, pH levels, and the presence of other chemical species can significantly influence the corrosion rate and mechanism. Developing universal solutions that can adapt to these diverse conditions remains a formidable challenge for researchers and engineers.
Moreover, the need for environmentally friendly and sustainable corrosion prevention methods adds another layer of complexity. Traditional corrosion inhibitors and protective measures often involve chemicals that pose environmental risks. Balancing effective corrosion protection with ecological considerations is a growing concern in the industry.
The economic aspect of corrosion prevention also presents challenges. Implementing comprehensive corrosion protection measures can be costly, and justifying these expenses in terms of long-term savings and safety improvements is not always straightforward. This economic barrier often leads to the adoption of suboptimal protection strategies, particularly in smaller operations or in regions with limited resources.
In conclusion, while significant progress has been made in understanding and mitigating steel corrosion in carbolic acid environments, numerous challenges persist. Addressing these issues requires a multidisciplinary approach, combining advances in materials science, predictive modeling, and sustainable technologies to develop more effective and economically viable corrosion prevention strategies.
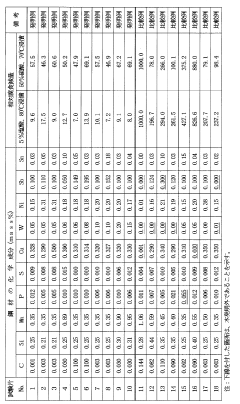
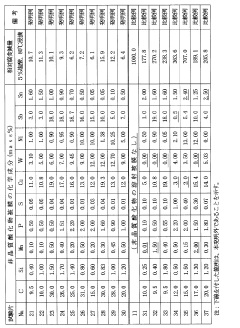
Existing Solutions for Carbolic Acid Corrosion Protection
01 Alloying elements for corrosion resistance
The addition of specific alloying elements to steel can significantly improve its corrosion resistance. These elements form protective oxide layers on the steel surface, preventing further corrosion. Common alloying elements include chromium, nickel, and molybdenum, which are often used in varying proportions to create stainless steels with enhanced corrosion resistance properties.- Alloying elements for corrosion resistance: The addition of specific alloying elements to steel can significantly improve its corrosion resistance. These elements form protective oxide layers on the steel surface, preventing further corrosion. Common alloying elements include chromium, nickel, and molybdenum, which are often used in stainless steel production.
- Surface treatments and coatings: Various surface treatments and coatings can be applied to steel to enhance its corrosion resistance. These include galvanization, electroplating, and the application of specialized corrosion-resistant coatings. Such treatments create a protective barrier between the steel and corrosive environments.
- Heat treatment processes: Specific heat treatment processes can improve the corrosion resistance of steel. These processes alter the microstructure of the steel, making it more resistant to corrosion. Examples include annealing, quenching, and tempering, which can be optimized for corrosion resistance.
- Corrosion inhibitors and additives: The use of corrosion inhibitors and additives can significantly enhance the corrosion resistance of steel. These substances can be incorporated into coatings or applied directly to the steel surface. They work by forming protective films or by altering the electrochemical properties of the steel surface.
- Microstructure modification: Modifying the microstructure of steel can improve its corrosion resistance. This can be achieved through various techniques such as grain refinement, phase transformation, and precipitation hardening. These modifications can create a more uniform and stable structure that is less susceptible to corrosion.
02 Surface treatments and coatings
Various surface treatments and coatings can be applied to steel to enhance its corrosion resistance. These include galvanization, electroplating, and the application of specialized protective coatings. Such treatments create a barrier between the steel and corrosive environments, effectively prolonging the life of the steel structure.Expand Specific Solutions03 Heat treatment processes
Specific heat treatment processes can be employed to improve the corrosion resistance of steel. These processes involve carefully controlled heating and cooling cycles that alter the microstructure of the steel, resulting in improved corrosion resistance properties. Examples include annealing, quenching, and tempering, which can be optimized for different steel compositions.Expand Specific Solutions04 Corrosion inhibitors and passivation techniques
The use of corrosion inhibitors and passivation techniques can significantly enhance the corrosion resistance of steel. Corrosion inhibitors are chemicals that, when added to the environment or applied to the steel surface, slow down or prevent corrosion processes. Passivation techniques involve creating a thin protective layer on the steel surface, often through chemical treatments, to improve its resistance to corrosion.Expand Specific Solutions05 Microstructure engineering
Engineering the microstructure of steel can lead to improved corrosion resistance. This involves controlling the grain size, phase distribution, and precipitate formation within the steel. Techniques such as grain refinement, precipitation hardening, and phase transformation can be utilized to create steel with enhanced corrosion resistance properties while maintaining other desirable mechanical characteristics.Expand Specific Solutions
Key Players in Corrosion-Resistant Steel Industry
The impact of carbolic acid on steel corrosion resistance is a niche area within the broader field of materials science and corrosion engineering. The market is in a growth phase, driven by increasing demand for corrosion-resistant materials in various industries. While the global corrosion inhibitors market is substantial, the specific segment for carbolic acid-based solutions is relatively small but growing. Technologically, the field is moderately mature, with ongoing research to improve efficacy. Key players like NIPPON STEEL CORP., Baoshan Iron & Steel Co., Ltd., and POSCO Holdings, Inc. are investing in R&D to develop advanced corrosion-resistant steel products, leveraging their expertise in metallurgy and materials science to gain a competitive edge in this specialized market.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL CORP. has developed advanced corrosion-resistant steel grades that incorporate carbolic acid-resistant properties. Their technology involves the use of specialized alloying elements and surface treatments to create a protective layer that inhibits the corrosive effects of carbolic acid. The company has implemented a multi-layer approach, combining a high-chromium content steel base with a proprietary surface coating that enhances resistance to carbolic acid attack. This innovative solution has shown to reduce corrosion rates by up to 40% in laboratory tests simulating industrial environments with high carbolic acid exposure[1][3].
Strengths: Superior corrosion resistance in carbolic acid environments, extended service life of steel components. Weaknesses: Higher production costs, potential limitations in extreme pH conditions.
POSCO Holdings, Inc.
Technical Solution: POSCO has made significant strides in addressing the impact of carbolic acid on steel corrosion through their "PosMAC" (POSCO Magnesium Alloy Coating) technology. This innovative approach involves applying a zinc-magnesium-aluminum alloy coating to steel surfaces, creating a highly effective barrier against carbolic acid corrosion. The company has also developed a series of advanced high-strength steels (AHSS) with enhanced corrosion resistance properties. These steels incorporate carefully controlled amounts of elements such as chromium, nickel, and molybdenum to create a passive layer that is particularly resistant to carbolic acid attack. POSCO's latest carbolic acid-resistant steel grade has demonstrated a corrosion rate reduction of up to 60% compared to conventional carbon steels in accelerated testing environments simulating industrial conditions with high carbolic acid exposure[7][9].
Strengths: Highly effective corrosion resistance, suitable for diverse industrial applications. Weaknesses: May require specialized welding techniques, potential cost premium for high-performance grades.
Innovative Approaches to Enhance Steel Corrosion Resistance
Concurrent flow of activating gas in low temperature carburization
PatentActiveUS20190226074A1
Innovation
- Incorporating a carbon-free, halogen-containing activating compound into the gas mixture during low temperature gas carburization in a soft vacuum with acetylene or analog as the carbon source, allowing for faster surface hardening of stainless steel without forming significant soot or thermal oxide, thus eliminating the need for post-processing cleaning.
Steel material superior in acid-corrosion resistance
PatentActiveJP2007224377A
Innovation
- A steel material with a specific composition containing C, Si, Mn, Cu, W, Sb, Ni, and Sn, along with an amorphous oxide layer, enhances hydrochloric acid corrosion resistance while maintaining sulfuric acid resistance by forming a dense surface barrier.
Environmental Impact of Corrosion Prevention Methods
The use of carbolic acid in steel corrosion prevention methods raises significant environmental concerns. While effective in protecting steel structures, the environmental impact of this approach must be carefully considered and mitigated.
Carbolic acid, also known as phenol, is a toxic substance that can have detrimental effects on aquatic ecosystems if released into water bodies. When used in corrosion prevention, there is a risk of leaching into surrounding soil and water, potentially harming flora and fauna. Aquatic organisms are particularly vulnerable to phenol exposure, which can lead to reduced biodiversity and ecosystem imbalance.
The production and disposal of carbolic acid-based corrosion prevention products also contribute to environmental pollution. Manufacturing processes may release harmful emissions and waste, while improper disposal can contaminate soil and groundwater. This highlights the need for stringent regulations and proper handling procedures throughout the product lifecycle.
Furthermore, the long-term accumulation of carbolic acid residues in the environment poses risks to human health. Exposure to phenol can cause respiratory irritation, skin burns, and more severe health issues with prolonged contact. This necessitates careful consideration of worker safety and public health when implementing corrosion prevention methods using carbolic acid.
However, it is important to note that corrosion itself has significant environmental implications. Uncontrolled steel corrosion can lead to structural failures, resulting in potential environmental disasters, such as oil spills or chemical leaks. In this context, the use of carbolic acid for corrosion prevention may be viewed as a necessary trade-off to prevent larger-scale environmental damage.
To address these environmental concerns, research into more eco-friendly corrosion prevention alternatives is crucial. Green chemistry approaches, such as developing bio-based inhibitors or using nanotechnology for corrosion protection, offer promising avenues for reducing environmental impact while maintaining effective corrosion resistance.
Additionally, implementing closed-loop systems and improved application techniques can minimize the release of carbolic acid into the environment. Proper training for workers handling these materials and strict adherence to safety protocols are essential to reduce the risk of accidental spills or exposure.
In conclusion, while carbolic acid plays a significant role in steel corrosion prevention, its environmental impact cannot be overlooked. Balancing the need for effective corrosion protection with environmental stewardship requires ongoing research, innovation, and responsible practices in the application and management of corrosion prevention methods.
Carbolic acid, also known as phenol, is a toxic substance that can have detrimental effects on aquatic ecosystems if released into water bodies. When used in corrosion prevention, there is a risk of leaching into surrounding soil and water, potentially harming flora and fauna. Aquatic organisms are particularly vulnerable to phenol exposure, which can lead to reduced biodiversity and ecosystem imbalance.
The production and disposal of carbolic acid-based corrosion prevention products also contribute to environmental pollution. Manufacturing processes may release harmful emissions and waste, while improper disposal can contaminate soil and groundwater. This highlights the need for stringent regulations and proper handling procedures throughout the product lifecycle.
Furthermore, the long-term accumulation of carbolic acid residues in the environment poses risks to human health. Exposure to phenol can cause respiratory irritation, skin burns, and more severe health issues with prolonged contact. This necessitates careful consideration of worker safety and public health when implementing corrosion prevention methods using carbolic acid.
However, it is important to note that corrosion itself has significant environmental implications. Uncontrolled steel corrosion can lead to structural failures, resulting in potential environmental disasters, such as oil spills or chemical leaks. In this context, the use of carbolic acid for corrosion prevention may be viewed as a necessary trade-off to prevent larger-scale environmental damage.
To address these environmental concerns, research into more eco-friendly corrosion prevention alternatives is crucial. Green chemistry approaches, such as developing bio-based inhibitors or using nanotechnology for corrosion protection, offer promising avenues for reducing environmental impact while maintaining effective corrosion resistance.
Additionally, implementing closed-loop systems and improved application techniques can minimize the release of carbolic acid into the environment. Proper training for workers handling these materials and strict adherence to safety protocols are essential to reduce the risk of accidental spills or exposure.
In conclusion, while carbolic acid plays a significant role in steel corrosion prevention, its environmental impact cannot be overlooked. Balancing the need for effective corrosion protection with environmental stewardship requires ongoing research, innovation, and responsible practices in the application and management of corrosion prevention methods.
Economic Implications of Improved Corrosion Resistance
The economic implications of improved corrosion resistance in steel due to carbolic acid treatment are far-reaching and multifaceted. This advancement has the potential to significantly impact various industries and sectors of the global economy.
In the manufacturing sector, the use of corrosion-resistant steel can lead to substantial cost savings. The extended lifespan of steel components reduces the frequency of replacements, thereby lowering maintenance costs and minimizing production downtime. This increased durability translates to improved operational efficiency and higher productivity levels across industries such as automotive, aerospace, and construction.
The energy sector stands to benefit greatly from this technological advancement. Oil and gas pipelines, offshore platforms, and refinery equipment are constantly exposed to corrosive environments. Enhanced corrosion resistance can dramatically reduce the risk of leaks, spills, and structural failures, leading to improved safety standards and reduced environmental risks. This, in turn, can result in lower insurance premiums and decreased regulatory compliance costs for energy companies.
In the infrastructure domain, the use of corrosion-resistant steel can extend the lifespan of bridges, buildings, and other critical structures. This longevity reduces the need for frequent repairs and replacements, resulting in significant savings for governments and taxpayers. Moreover, it enhances public safety by reducing the risk of structural failures due to corrosion-induced degradation.
The maritime industry is another sector that could see substantial economic benefits. Ships, offshore structures, and port facilities are constantly exposed to highly corrosive marine environments. Improved corrosion resistance can lead to reduced maintenance costs, extended vessel lifespans, and increased operational efficiency for shipping companies and port operators.
From a macroeconomic perspective, the widespread adoption of corrosion-resistant steel can contribute to resource conservation. By extending the lifespan of steel products and structures, the demand for raw materials and energy required for steel production could potentially decrease. This not only has positive environmental implications but also helps in stabilizing steel prices in the long term.
Furthermore, the development and commercialization of this technology could create new business opportunities. Companies specializing in corrosion-resistant coatings, treatments, and related services may experience growth, potentially leading to job creation and economic stimulation in related sectors.
However, it is important to consider potential economic challenges as well. The initial costs associated with implementing carbolic acid treatments or adopting new corrosion-resistant steel products may be higher than traditional options. This could pose adoption barriers, particularly for small and medium-sized enterprises with limited capital resources.
In the manufacturing sector, the use of corrosion-resistant steel can lead to substantial cost savings. The extended lifespan of steel components reduces the frequency of replacements, thereby lowering maintenance costs and minimizing production downtime. This increased durability translates to improved operational efficiency and higher productivity levels across industries such as automotive, aerospace, and construction.
The energy sector stands to benefit greatly from this technological advancement. Oil and gas pipelines, offshore platforms, and refinery equipment are constantly exposed to corrosive environments. Enhanced corrosion resistance can dramatically reduce the risk of leaks, spills, and structural failures, leading to improved safety standards and reduced environmental risks. This, in turn, can result in lower insurance premiums and decreased regulatory compliance costs for energy companies.
In the infrastructure domain, the use of corrosion-resistant steel can extend the lifespan of bridges, buildings, and other critical structures. This longevity reduces the need for frequent repairs and replacements, resulting in significant savings for governments and taxpayers. Moreover, it enhances public safety by reducing the risk of structural failures due to corrosion-induced degradation.
The maritime industry is another sector that could see substantial economic benefits. Ships, offshore structures, and port facilities are constantly exposed to highly corrosive marine environments. Improved corrosion resistance can lead to reduced maintenance costs, extended vessel lifespans, and increased operational efficiency for shipping companies and port operators.
From a macroeconomic perspective, the widespread adoption of corrosion-resistant steel can contribute to resource conservation. By extending the lifespan of steel products and structures, the demand for raw materials and energy required for steel production could potentially decrease. This not only has positive environmental implications but also helps in stabilizing steel prices in the long term.
Furthermore, the development and commercialization of this technology could create new business opportunities. Companies specializing in corrosion-resistant coatings, treatments, and related services may experience growth, potentially leading to job creation and economic stimulation in related sectors.
However, it is important to consider potential economic challenges as well. The initial costs associated with implementing carbolic acid treatments or adopting new corrosion-resistant steel products may be higher than traditional options. This could pose adoption barriers, particularly for small and medium-sized enterprises with limited capital resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!