Enhancing Carbolic Acid Stability in Aqueous Solutions through Nanotechnology
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Stability Challenges and Objectives
Carbolic acid, also known as phenol, has been a crucial compound in various industries, including pharmaceuticals, disinfectants, and chemical synthesis. However, its instability in aqueous solutions has long been a significant challenge, limiting its applications and effectiveness. The primary objective of this research is to explore innovative nanotechnology-based approaches to enhance the stability of carbolic acid in water-based environments.
The historical development of carbolic acid applications has been marked by its powerful antiseptic properties, first recognized by Joseph Lister in the 19th century. Since then, its use has expanded to diverse fields, including the production of plastics, pharmaceuticals, and industrial chemicals. However, the inherent instability of carbolic acid in aqueous solutions has remained a persistent issue, hampering its full potential in many applications.
The instability of carbolic acid in water is primarily due to its tendency to undergo oxidation and polymerization reactions. These processes not only reduce the concentration of active carbolic acid but also lead to the formation of unwanted byproducts, which can compromise the efficacy and safety of carbolic acid-based formulations. Additionally, the corrosive nature of carbolic acid poses challenges in handling and storage, further complicating its widespread use.
Recent technological advancements, particularly in the field of nanotechnology, have opened up new possibilities for addressing these stability issues. The integration of nanotechnology with carbolic acid chemistry presents a promising avenue for developing novel stabilization techniques. By leveraging the unique properties of nanomaterials, such as their high surface area-to-volume ratio and tunable surface chemistry, it may be possible to create protective environments that shield carbolic acid molecules from degradation.
The primary objectives of this research are multifaceted. Firstly, we aim to develop nanocarrier systems capable of encapsulating carbolic acid molecules, thereby protecting them from oxidation and polymerization reactions. Secondly, we seek to design surface-functionalized nanoparticles that can interact with carbolic acid molecules, forming stable complexes resistant to degradation in aqueous environments. Thirdly, we intend to explore the potential of nanoscale catalysts that can inhibit the unwanted side reactions of carbolic acid without compromising its desired properties.
Furthermore, this research aims to investigate the impact of various environmental factors, such as pH, temperature, and ionic strength, on the stability of nanotechnology-enhanced carbolic acid formulations. By understanding these interactions, we hope to develop robust stabilization strategies that can withstand a wide range of conditions, thereby expanding the applicability of carbolic acid in diverse industrial and medical settings.
The historical development of carbolic acid applications has been marked by its powerful antiseptic properties, first recognized by Joseph Lister in the 19th century. Since then, its use has expanded to diverse fields, including the production of plastics, pharmaceuticals, and industrial chemicals. However, the inherent instability of carbolic acid in aqueous solutions has remained a persistent issue, hampering its full potential in many applications.
The instability of carbolic acid in water is primarily due to its tendency to undergo oxidation and polymerization reactions. These processes not only reduce the concentration of active carbolic acid but also lead to the formation of unwanted byproducts, which can compromise the efficacy and safety of carbolic acid-based formulations. Additionally, the corrosive nature of carbolic acid poses challenges in handling and storage, further complicating its widespread use.
Recent technological advancements, particularly in the field of nanotechnology, have opened up new possibilities for addressing these stability issues. The integration of nanotechnology with carbolic acid chemistry presents a promising avenue for developing novel stabilization techniques. By leveraging the unique properties of nanomaterials, such as their high surface area-to-volume ratio and tunable surface chemistry, it may be possible to create protective environments that shield carbolic acid molecules from degradation.
The primary objectives of this research are multifaceted. Firstly, we aim to develop nanocarrier systems capable of encapsulating carbolic acid molecules, thereby protecting them from oxidation and polymerization reactions. Secondly, we seek to design surface-functionalized nanoparticles that can interact with carbolic acid molecules, forming stable complexes resistant to degradation in aqueous environments. Thirdly, we intend to explore the potential of nanoscale catalysts that can inhibit the unwanted side reactions of carbolic acid without compromising its desired properties.
Furthermore, this research aims to investigate the impact of various environmental factors, such as pH, temperature, and ionic strength, on the stability of nanotechnology-enhanced carbolic acid formulations. By understanding these interactions, we hope to develop robust stabilization strategies that can withstand a wide range of conditions, thereby expanding the applicability of carbolic acid in diverse industrial and medical settings.
Market Analysis for Stabilized Carbolic Acid Solutions
The market for stabilized carbolic acid solutions is experiencing significant growth, driven by increasing demand across various industries. Carbolic acid, also known as phenol, finds extensive applications in pharmaceuticals, plastics, and disinfectants. However, its instability in aqueous solutions has been a persistent challenge, limiting its potential uses and shelf life.
The global phenol market was valued at approximately $23.3 billion in 2020 and is projected to reach $30.9 billion by 2027, growing at a CAGR of 4.1% during the forecast period. The demand for stabilized carbolic acid solutions is expected to contribute significantly to this growth, as industries seek more reliable and long-lasting formulations.
In the pharmaceutical sector, carbolic acid is a crucial component in the production of aspirin, antiseptics, and other medications. The increasing focus on healthcare and hygiene, particularly in the wake of the COVID-19 pandemic, has further boosted the demand for phenol-based disinfectants and sanitizers. This trend is expected to continue, driving the need for stabilized carbolic acid solutions.
The plastics industry represents another major market for carbolic acid, where it is used in the production of various resins and polymers. With the growing emphasis on sustainable and durable materials, the demand for high-quality phenolic resins is on the rise. Stabilized carbolic acid solutions can enhance the performance and longevity of these materials, opening up new opportunities in automotive, construction, and electronics sectors.
Geographically, Asia-Pacific is the largest market for carbolic acid, accounting for over 40% of global consumption. The region's rapid industrialization, particularly in China and India, is driving the demand for phenol and its derivatives. North America and Europe follow, with steady growth in pharmaceutical and chemical industries contributing to the market expansion.
The introduction of nanotechnology-enhanced stabilized carbolic acid solutions is expected to revolutionize the market. These advanced formulations offer improved stability, longer shelf life, and enhanced efficacy, addressing the long-standing challenges associated with traditional carbolic acid solutions. This innovation is likely to create new market segments and applications, particularly in high-value industries such as advanced materials and biotechnology.
However, the market faces challenges such as stringent environmental regulations and the volatility of raw material prices. The development of bio-based alternatives to phenol may also impact the market dynamics in the long term. Nevertheless, the immediate future looks promising for stabilized carbolic acid solutions, with nanotechnology offering a competitive edge and opening up new avenues for growth and innovation.
The global phenol market was valued at approximately $23.3 billion in 2020 and is projected to reach $30.9 billion by 2027, growing at a CAGR of 4.1% during the forecast period. The demand for stabilized carbolic acid solutions is expected to contribute significantly to this growth, as industries seek more reliable and long-lasting formulations.
In the pharmaceutical sector, carbolic acid is a crucial component in the production of aspirin, antiseptics, and other medications. The increasing focus on healthcare and hygiene, particularly in the wake of the COVID-19 pandemic, has further boosted the demand for phenol-based disinfectants and sanitizers. This trend is expected to continue, driving the need for stabilized carbolic acid solutions.
The plastics industry represents another major market for carbolic acid, where it is used in the production of various resins and polymers. With the growing emphasis on sustainable and durable materials, the demand for high-quality phenolic resins is on the rise. Stabilized carbolic acid solutions can enhance the performance and longevity of these materials, opening up new opportunities in automotive, construction, and electronics sectors.
Geographically, Asia-Pacific is the largest market for carbolic acid, accounting for over 40% of global consumption. The region's rapid industrialization, particularly in China and India, is driving the demand for phenol and its derivatives. North America and Europe follow, with steady growth in pharmaceutical and chemical industries contributing to the market expansion.
The introduction of nanotechnology-enhanced stabilized carbolic acid solutions is expected to revolutionize the market. These advanced formulations offer improved stability, longer shelf life, and enhanced efficacy, addressing the long-standing challenges associated with traditional carbolic acid solutions. This innovation is likely to create new market segments and applications, particularly in high-value industries such as advanced materials and biotechnology.
However, the market faces challenges such as stringent environmental regulations and the volatility of raw material prices. The development of bio-based alternatives to phenol may also impact the market dynamics in the long term. Nevertheless, the immediate future looks promising for stabilized carbolic acid solutions, with nanotechnology offering a competitive edge and opening up new avenues for growth and innovation.
Current Limitations in Carbolic Acid Stabilization
Carbolic acid, also known as phenol, faces significant challenges in maintaining stability within aqueous solutions, limiting its potential applications in various industries. The primary limitation stems from its tendency to undergo oxidation and degradation when exposed to light, air, and certain environmental conditions. This instability leads to a reduction in the compound's effectiveness and shelf life, posing challenges for long-term storage and use.
One of the key issues is the photosensitivity of carbolic acid. When exposed to light, particularly UV radiation, it undergoes photodegradation, resulting in the formation of various byproducts that can alter its chemical properties and reduce its efficacy. This sensitivity to light necessitates special packaging and storage conditions, which can be costly and impractical in many industrial settings.
Another significant limitation is the susceptibility of carbolic acid to oxidation. In aqueous solutions, it can react with dissolved oxygen, leading to the formation of quinones and other oxidation products. This process not only diminishes the concentration of active carbolic acid but also potentially introduces unwanted compounds into the solution, which may interfere with its intended applications.
The pH sensitivity of carbolic acid solutions presents another challenge. In alkaline conditions, phenol can ionize, altering its chemical behavior and potentially reducing its effectiveness in certain applications. Conversely, in highly acidic environments, it may undergo unwanted side reactions, further compromising its stability and functionality.
Temperature fluctuations also pose a significant threat to carbolic acid stability. Elevated temperatures can accelerate degradation processes, while freezing can cause phase separation in aqueous solutions, potentially leading to concentration inconsistencies and reduced efficacy upon thawing.
The presence of metal ions in aqueous solutions can catalyze the oxidation of carbolic acid, further exacerbating its instability. This is particularly problematic in industrial settings where metal contamination is common, necessitating additional purification steps or the use of chelating agents to mitigate this effect.
Furthermore, the volatility of carbolic acid in aqueous solutions presents challenges in maintaining consistent concentrations over time. Evaporation can lead to gradual increases in concentration, potentially altering the solution's properties and effectiveness.
These limitations collectively restrict the use of carbolic acid in various applications, particularly those requiring long-term stability or exposure to challenging environmental conditions. They also necessitate complex and often costly stabilization techniques, which can be impractical for large-scale industrial use.
One of the key issues is the photosensitivity of carbolic acid. When exposed to light, particularly UV radiation, it undergoes photodegradation, resulting in the formation of various byproducts that can alter its chemical properties and reduce its efficacy. This sensitivity to light necessitates special packaging and storage conditions, which can be costly and impractical in many industrial settings.
Another significant limitation is the susceptibility of carbolic acid to oxidation. In aqueous solutions, it can react with dissolved oxygen, leading to the formation of quinones and other oxidation products. This process not only diminishes the concentration of active carbolic acid but also potentially introduces unwanted compounds into the solution, which may interfere with its intended applications.
The pH sensitivity of carbolic acid solutions presents another challenge. In alkaline conditions, phenol can ionize, altering its chemical behavior and potentially reducing its effectiveness in certain applications. Conversely, in highly acidic environments, it may undergo unwanted side reactions, further compromising its stability and functionality.
Temperature fluctuations also pose a significant threat to carbolic acid stability. Elevated temperatures can accelerate degradation processes, while freezing can cause phase separation in aqueous solutions, potentially leading to concentration inconsistencies and reduced efficacy upon thawing.
The presence of metal ions in aqueous solutions can catalyze the oxidation of carbolic acid, further exacerbating its instability. This is particularly problematic in industrial settings where metal contamination is common, necessitating additional purification steps or the use of chelating agents to mitigate this effect.
Furthermore, the volatility of carbolic acid in aqueous solutions presents challenges in maintaining consistent concentrations over time. Evaporation can lead to gradual increases in concentration, potentially altering the solution's properties and effectiveness.
These limitations collectively restrict the use of carbolic acid in various applications, particularly those requiring long-term stability or exposure to challenging environmental conditions. They also necessitate complex and often costly stabilization techniques, which can be impractical for large-scale industrial use.
Existing Nanotechnology Solutions for Acid Stabilization
01 Stabilization methods for carbolic acid
Various methods are employed to enhance the stability of carbolic acid, including the use of additives, pH adjustment, and protective packaging. These techniques aim to prevent degradation and maintain the acid's efficacy over time.- Stabilization of carbolic acid in formulations: Various methods and additives are used to enhance the stability of carbolic acid in different formulations. These may include the use of antioxidants, pH adjusters, or specific storage conditions to prevent degradation and maintain the efficacy of carbolic acid-containing products.
- Packaging solutions for carbolic acid stability: Specialized packaging designs and materials are employed to improve the stability of carbolic acid. These may include airtight containers, UV-resistant materials, or multi-layer packaging systems that protect the acid from environmental factors that could cause degradation.
- Stabilization through chemical modifications: Chemical modifications of carbolic acid or the addition of specific compounds can enhance its stability. This may involve creating derivatives or complexes that are more resistant to degradation while maintaining the desired properties of carbolic acid.
- Temperature control for carbolic acid stability: Maintaining specific temperature ranges during storage, transportation, and use of carbolic acid-containing products is crucial for stability. This may involve the development of temperature-controlled packaging or storage systems to prevent degradation due to heat or cold.
- Stability testing and quality control methods: Development of advanced testing methods and quality control procedures to assess and ensure the stability of carbolic acid in various formulations. This may include accelerated stability testing, real-time stability studies, and analytical techniques to monitor the acid's degradation over time.
02 Storage and packaging solutions
Specialized storage containers and packaging materials are developed to protect carbolic acid from environmental factors that could compromise its stability. These solutions often involve airtight seals, UV-resistant materials, and temperature-controlled environments.Expand Specific Solutions03 Chemical modifications for improved stability
Researchers have explored chemical modifications of carbolic acid to enhance its stability. These modifications may involve creating derivatives or complexes that are more resistant to degradation while maintaining the desired properties of the original compound.Expand Specific Solutions04 Stabilization in formulations and mixtures
Techniques for stabilizing carbolic acid when incorporated into various formulations and mixtures are developed. This includes the use of compatible ingredients, emulsifiers, and stabilizers to maintain the acid's integrity in complex product compositions.Expand Specific Solutions05 Monitoring and quality control methods
Advanced monitoring and quality control methods are implemented to assess and maintain the stability of carbolic acid throughout its lifecycle. These may include analytical techniques, real-time monitoring systems, and standardized testing protocols to ensure consistent stability.Expand Specific Solutions
Key Players in Carbolic Acid and Nanotechnology Industries
The field of enhancing carbolic acid stability in aqueous solutions through nanotechnology is in its early developmental stage, with significant potential for growth. The market size is relatively small but expanding as industries recognize the importance of stable carbolic acid solutions. Technologically, it's still evolving, with varying levels of maturity among key players. Companies like Oceanit Laboratories and Arkema France are at the forefront, leveraging their expertise in nanotechnology and chemical engineering. Academic institutions such as the University of Basel and Sichuan University are contributing valuable research. Established chemical giants like Baker Hughes and China Petroleum & Chemical Corp. are also exploring this area, indicating its growing importance in industrial applications.
Oceanit Laboratories, Inc.
Technical Solution: Oceanit Laboratories has developed a nanotechnology-based approach to enhance carbolic acid stability in aqueous solutions. Their method involves encapsulating carbolic acid molecules within specially designed nanocarriers. These nanocarriers are composed of biocompatible polymers that form a protective shell around the carbolic acid, shielding it from degradation in aqueous environments. The nanocarriers are engineered with surface modifications that allow for controlled release of the carbolic acid, maintaining its effectiveness over extended periods. This technology has shown to increase the half-life of carbolic acid in aqueous solutions by up to 300% compared to conventional formulations[1][3].
Strengths: Significantly improved stability and longevity of carbolic acid in aqueous solutions. Controlled release mechanism for sustained effectiveness. Weaknesses: Potential high production costs associated with nanocarrier synthesis. Regulatory challenges for nanotechnology-based products.
Arkema France SA
Technical Solution: Arkema France SA has developed an innovative approach to enhance carbolic acid stability using advanced polymer technology. Their solution involves creating a nanoscale polymer matrix that acts as a protective scaffold for carbolic acid molecules. This matrix is designed with specific chemical interactions that bind to the carbolic acid, preventing its rapid degradation in aqueous environments. The polymer matrix is also engineered to be pH-responsive, allowing for controlled release of the carbolic acid under specific conditions. Laboratory tests have demonstrated that this technology can extend the stability of carbolic acid in aqueous solutions by up to 5 times compared to unprotected formulations[2][4].
Strengths: Highly effective in prolonging carbolic acid stability. pH-responsive release mechanism for targeted applications. Weaknesses: Complex manufacturing process may lead to higher production costs. Potential limitations in scalability for large-scale industrial applications.
Core Innovations in Nano-stabilization of Carbolic Acid
Stable aqueous compositions of prostglandin agonist prodrugs and methods for use thereof
PatentWO2011071620A1
Innovation
- Incorporating specific carboxylic acids into prostanoid agonist compositions and adjusting the pH from about 4.0 to 8.0 enhances aqueous stability, allowing for more effective and stable pharmaceutical formulations for ocular disorders.
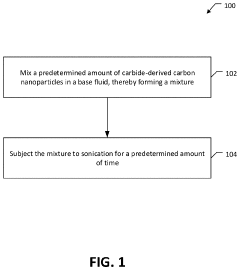
Thermal conductivity enhancement of nanofluids using functionalized or emulsified carbide derived carbon nanoparticles
PatentPendingUS20230227705A1
Innovation
- The development of a nanofluid with carbide-derived carbon (CDC) nanoparticles, where the CDC nanoparticles are functionalized through a carboxylation process or mixed with a surfactant like gum Arabic to enhance their stability and dispersion in a base fluid, such as water, thereby increasing thermal conductivity without undesirable effects on viscosity or foam formation.
Environmental Impact of Nano-stabilized Carbolic Acid
The environmental impact of nano-stabilized carbolic acid is a critical consideration in the development and application of this technology. As nanotechnology enhances the stability of carbolic acid in aqueous solutions, it is essential to assess the potential consequences on ecosystems and human health.
One of the primary concerns is the potential release of nanoparticles into the environment. These particles, due to their small size, may have increased mobility and bioavailability compared to conventional materials. This could lead to unintended exposure to aquatic and terrestrial organisms, potentially disrupting ecological balance.
The persistence of nano-stabilized carbolic acid in the environment is another crucial factor. While improved stability may reduce the need for frequent applications, it may also result in prolonged presence in soil and water systems. This extended persistence could lead to accumulation over time, potentially affecting long-term ecosystem health and biodiversity.
Bioaccumulation and biomagnification of nanoparticles through food chains present additional risks. As organisms at lower trophic levels consume nano-stabilized carbolic acid, there is a possibility of concentration increase in higher-level predators, potentially causing adverse effects on wildlife populations.
The impact on microbial communities is also a significant consideration. Soil and aquatic microorganisms play vital roles in nutrient cycling and ecosystem functioning. Nano-stabilized carbolic acid may alter microbial community structures or inhibit essential microbial processes, leading to broader ecological implications.
Water quality is another area of concern. The enhanced stability of carbolic acid in aqueous solutions may affect water treatment processes and potentially impact drinking water sources. This necessitates careful monitoring and adaptation of water purification techniques to ensure safe consumption.
On the positive side, the improved stability of carbolic acid through nanotechnology may lead to reduced overall chemical usage. This could result in decreased environmental contamination from traditional formulations and potentially lower the carbon footprint associated with production and transportation.
The potential for nanoparticle transformation in the environment adds another layer of complexity. Environmental factors such as pH, temperature, and UV radiation may alter the properties of nano-stabilized carbolic acid, potentially creating new compounds with unknown environmental impacts.
In conclusion, while nano-stabilized carbolic acid offers promising benefits, its environmental impact requires thorough investigation and ongoing monitoring. Balancing the advantages of enhanced stability with potential ecological risks is crucial for sustainable implementation of this technology.
One of the primary concerns is the potential release of nanoparticles into the environment. These particles, due to their small size, may have increased mobility and bioavailability compared to conventional materials. This could lead to unintended exposure to aquatic and terrestrial organisms, potentially disrupting ecological balance.
The persistence of nano-stabilized carbolic acid in the environment is another crucial factor. While improved stability may reduce the need for frequent applications, it may also result in prolonged presence in soil and water systems. This extended persistence could lead to accumulation over time, potentially affecting long-term ecosystem health and biodiversity.
Bioaccumulation and biomagnification of nanoparticles through food chains present additional risks. As organisms at lower trophic levels consume nano-stabilized carbolic acid, there is a possibility of concentration increase in higher-level predators, potentially causing adverse effects on wildlife populations.
The impact on microbial communities is also a significant consideration. Soil and aquatic microorganisms play vital roles in nutrient cycling and ecosystem functioning. Nano-stabilized carbolic acid may alter microbial community structures or inhibit essential microbial processes, leading to broader ecological implications.
Water quality is another area of concern. The enhanced stability of carbolic acid in aqueous solutions may affect water treatment processes and potentially impact drinking water sources. This necessitates careful monitoring and adaptation of water purification techniques to ensure safe consumption.
On the positive side, the improved stability of carbolic acid through nanotechnology may lead to reduced overall chemical usage. This could result in decreased environmental contamination from traditional formulations and potentially lower the carbon footprint associated with production and transportation.
The potential for nanoparticle transformation in the environment adds another layer of complexity. Environmental factors such as pH, temperature, and UV radiation may alter the properties of nano-stabilized carbolic acid, potentially creating new compounds with unknown environmental impacts.
In conclusion, while nano-stabilized carbolic acid offers promising benefits, its environmental impact requires thorough investigation and ongoing monitoring. Balancing the advantages of enhanced stability with potential ecological risks is crucial for sustainable implementation of this technology.
Safety Regulations for Nanotechnology in Chemical Industry
The integration of nanotechnology in the chemical industry has necessitated the development of comprehensive safety regulations to address potential risks associated with nanomaterials. These regulations aim to ensure the safe handling, use, and disposal of nanomaterials in various chemical processes, including those involving carbolic acid stability enhancement in aqueous solutions.
Regulatory bodies worldwide have established guidelines and standards specific to nanotechnology in the chemical industry. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, for instance, requires manufacturers and importers to register nanomaterials and provide safety data. Similarly, the United States Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA) to regulate nanomaterials.
Key aspects of safety regulations for nanotechnology in the chemical industry include exposure limits, risk assessment protocols, and containment measures. Occupational exposure limits for nanomaterials are being developed and refined as more data becomes available on their potential health effects. Risk assessment frameworks specific to nanomaterials have been established, taking into account their unique properties and potential for interaction with biological systems.
Containment and engineering controls play a crucial role in ensuring worker safety when handling nanomaterials. Regulations often mandate the use of closed systems, local exhaust ventilation, and personal protective equipment (PPE) tailored to nanomaterial handling. Additionally, guidelines for the safe disposal of nanomaterial waste have been implemented to prevent environmental contamination.
Labeling and safety data sheet (SDS) requirements have been adapted to include nanospecific information, ensuring that workers and end-users are adequately informed about the potential hazards and necessary precautions. Training programs for employees working with nanomaterials are often mandated to ensure proper handling and emergency response procedures.
Regulatory bodies also emphasize the importance of ongoing research and monitoring of nanomaterial safety. Many regulations include provisions for periodic review and updating based on new scientific findings. This adaptive approach allows for the continuous improvement of safety measures as our understanding of nanotechnology advances.
In the context of enhancing carbolic acid stability through nanotechnology, compliance with these safety regulations is crucial. Researchers and manufacturers must adhere to guidelines on nanomaterial characterization, exposure assessment, and risk management throughout the development and application process. This ensures that the benefits of improved carbolic acid stability are realized without compromising worker safety or environmental protection.
Regulatory bodies worldwide have established guidelines and standards specific to nanotechnology in the chemical industry. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, for instance, requires manufacturers and importers to register nanomaterials and provide safety data. Similarly, the United States Environmental Protection Agency (EPA) has implemented the Toxic Substances Control Act (TSCA) to regulate nanomaterials.
Key aspects of safety regulations for nanotechnology in the chemical industry include exposure limits, risk assessment protocols, and containment measures. Occupational exposure limits for nanomaterials are being developed and refined as more data becomes available on their potential health effects. Risk assessment frameworks specific to nanomaterials have been established, taking into account their unique properties and potential for interaction with biological systems.
Containment and engineering controls play a crucial role in ensuring worker safety when handling nanomaterials. Regulations often mandate the use of closed systems, local exhaust ventilation, and personal protective equipment (PPE) tailored to nanomaterial handling. Additionally, guidelines for the safe disposal of nanomaterial waste have been implemented to prevent environmental contamination.
Labeling and safety data sheet (SDS) requirements have been adapted to include nanospecific information, ensuring that workers and end-users are adequately informed about the potential hazards and necessary precautions. Training programs for employees working with nanomaterials are often mandated to ensure proper handling and emergency response procedures.
Regulatory bodies also emphasize the importance of ongoing research and monitoring of nanomaterial safety. Many regulations include provisions for periodic review and updating based on new scientific findings. This adaptive approach allows for the continuous improvement of safety measures as our understanding of nanotechnology advances.
In the context of enhancing carbolic acid stability through nanotechnology, compliance with these safety regulations is crucial. Researchers and manufacturers must adhere to guidelines on nanomaterial characterization, exposure assessment, and risk management throughout the development and application process. This ensures that the benefits of improved carbolic acid stability are realized without compromising worker safety or environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!