Environmental Impact of Carbolic Acid Degradation Byproducts
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Degradation Background and Objectives
Carbolic acid, also known as phenol, has been a significant compound in industrial and medical applications for over a century. Its degradation process and the resulting byproducts have become a growing concern in environmental science and public health. The evolution of carbolic acid usage traces back to the 19th century when it was first isolated from coal tar and subsequently used as an antiseptic in medical practices.
The technological progression in carbolic acid production and application has led to its widespread use in various industries, including pharmaceuticals, plastics, and disinfectants. However, this extensive utilization has also resulted in increased environmental exposure and potential ecological risks. The degradation of carbolic acid in natural environments occurs through various pathways, including photolysis, biodegradation, and chemical oxidation, each producing a unique set of byproducts with varying environmental impacts.
Recent years have witnessed a surge in research focusing on the environmental fate of carbolic acid and its degradation products. This heightened interest stems from the growing awareness of the potential long-term effects these compounds may have on ecosystems and human health. The primary objective of current research is to comprehensively understand the degradation mechanisms, identify the full spectrum of byproducts, and assess their environmental persistence and toxicity.
One of the key challenges in this field is the complexity of the degradation process, which can vary significantly depending on environmental conditions such as pH, temperature, and the presence of other chemical species. This variability leads to a diverse array of byproducts, some of which may be more harmful than the parent compound. Consequently, there is a pressing need for advanced analytical techniques to accurately detect and quantify these byproducts in environmental matrices.
The technological goals in this area are multifaceted. They include developing more efficient and environmentally friendly methods for carbolic acid degradation, creating predictive models for byproduct formation under various environmental conditions, and designing remediation strategies for contaminated sites. Additionally, there is a growing emphasis on green chemistry approaches to minimize the production of harmful byproducts during industrial processes involving carbolic acid.
As environmental regulations become more stringent globally, understanding the full lifecycle of carbolic acid and its degradation products has become crucial for industries and policymakers alike. This has spurred collaborative efforts between academic institutions, regulatory bodies, and industrial partners to address the environmental challenges associated with carbolic acid usage and disposal.
The technological progression in carbolic acid production and application has led to its widespread use in various industries, including pharmaceuticals, plastics, and disinfectants. However, this extensive utilization has also resulted in increased environmental exposure and potential ecological risks. The degradation of carbolic acid in natural environments occurs through various pathways, including photolysis, biodegradation, and chemical oxidation, each producing a unique set of byproducts with varying environmental impacts.
Recent years have witnessed a surge in research focusing on the environmental fate of carbolic acid and its degradation products. This heightened interest stems from the growing awareness of the potential long-term effects these compounds may have on ecosystems and human health. The primary objective of current research is to comprehensively understand the degradation mechanisms, identify the full spectrum of byproducts, and assess their environmental persistence and toxicity.
One of the key challenges in this field is the complexity of the degradation process, which can vary significantly depending on environmental conditions such as pH, temperature, and the presence of other chemical species. This variability leads to a diverse array of byproducts, some of which may be more harmful than the parent compound. Consequently, there is a pressing need for advanced analytical techniques to accurately detect and quantify these byproducts in environmental matrices.
The technological goals in this area are multifaceted. They include developing more efficient and environmentally friendly methods for carbolic acid degradation, creating predictive models for byproduct formation under various environmental conditions, and designing remediation strategies for contaminated sites. Additionally, there is a growing emphasis on green chemistry approaches to minimize the production of harmful byproducts during industrial processes involving carbolic acid.
As environmental regulations become more stringent globally, understanding the full lifecycle of carbolic acid and its degradation products has become crucial for industries and policymakers alike. This has spurred collaborative efforts between academic institutions, regulatory bodies, and industrial partners to address the environmental challenges associated with carbolic acid usage and disposal.
Environmental Concerns and Regulatory Landscape
The environmental impact of carbolic acid degradation byproducts has become a significant concern in recent years, prompting increased regulatory scrutiny and public awareness. Carbolic acid, also known as phenol, is widely used in various industrial processes, including the production of plastics, pharmaceuticals, and disinfectants. However, its degradation can lead to the formation of potentially harmful byproducts, which pose risks to ecosystems and human health.
One of the primary environmental concerns associated with carbolic acid degradation byproducts is their persistence in soil and water systems. These compounds can accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological impacts. Studies have shown that some degradation products, such as chlorophenols and nitrophenols, exhibit toxic effects on aquatic life, including fish, algae, and invertebrates, even at low concentrations.
The potential for groundwater contamination is another critical issue. Carbolic acid and its byproducts can leach into groundwater sources, potentially affecting drinking water quality and posing risks to human health. This has led to increased monitoring and treatment requirements for industrial facilities and waste management sites handling phenol-containing materials.
Air pollution is also a concern, as volatile phenolic compounds can be released during industrial processes or waste treatment. These emissions can contribute to the formation of photochemical smog and may have adverse effects on air quality in surrounding communities.
In response to these environmental concerns, regulatory bodies worldwide have implemented stringent guidelines and standards for the handling, use, and disposal of carbolic acid and its byproducts. The United States Environmental Protection Agency (EPA) has established maximum contaminant levels for phenols in drinking water and effluent discharge limits for industrial facilities. Similarly, the European Union has set environmental quality standards for phenolic compounds under the Water Framework Directive.
Many countries have also introduced regulations requiring industries to implement best available techniques (BAT) for reducing phenol emissions and managing waste streams containing carbolic acid and its degradation products. These measures often include advanced treatment technologies such as activated carbon adsorption, advanced oxidation processes, and biological treatment systems.
The regulatory landscape continues to evolve as new scientific evidence emerges regarding the environmental fate and effects of carbolic acid degradation byproducts. There is a growing emphasis on life cycle assessments and the development of more sustainable alternatives to phenol-based products. Industries are increasingly required to conduct environmental impact assessments and implement monitoring programs to ensure compliance with evolving regulations.
As public awareness of environmental issues grows, there is also increasing pressure on companies to adopt more transparent practices and voluntarily reduce their environmental footprint beyond regulatory requirements. This has led to the development of industry-led initiatives and voluntary standards aimed at minimizing the environmental impact of carbolic acid and its byproducts throughout the product lifecycle.
One of the primary environmental concerns associated with carbolic acid degradation byproducts is their persistence in soil and water systems. These compounds can accumulate in sediments and bioaccumulate in aquatic organisms, leading to long-term ecological impacts. Studies have shown that some degradation products, such as chlorophenols and nitrophenols, exhibit toxic effects on aquatic life, including fish, algae, and invertebrates, even at low concentrations.
The potential for groundwater contamination is another critical issue. Carbolic acid and its byproducts can leach into groundwater sources, potentially affecting drinking water quality and posing risks to human health. This has led to increased monitoring and treatment requirements for industrial facilities and waste management sites handling phenol-containing materials.
Air pollution is also a concern, as volatile phenolic compounds can be released during industrial processes or waste treatment. These emissions can contribute to the formation of photochemical smog and may have adverse effects on air quality in surrounding communities.
In response to these environmental concerns, regulatory bodies worldwide have implemented stringent guidelines and standards for the handling, use, and disposal of carbolic acid and its byproducts. The United States Environmental Protection Agency (EPA) has established maximum contaminant levels for phenols in drinking water and effluent discharge limits for industrial facilities. Similarly, the European Union has set environmental quality standards for phenolic compounds under the Water Framework Directive.
Many countries have also introduced regulations requiring industries to implement best available techniques (BAT) for reducing phenol emissions and managing waste streams containing carbolic acid and its degradation products. These measures often include advanced treatment technologies such as activated carbon adsorption, advanced oxidation processes, and biological treatment systems.
The regulatory landscape continues to evolve as new scientific evidence emerges regarding the environmental fate and effects of carbolic acid degradation byproducts. There is a growing emphasis on life cycle assessments and the development of more sustainable alternatives to phenol-based products. Industries are increasingly required to conduct environmental impact assessments and implement monitoring programs to ensure compliance with evolving regulations.
As public awareness of environmental issues grows, there is also increasing pressure on companies to adopt more transparent practices and voluntarily reduce their environmental footprint beyond regulatory requirements. This has led to the development of industry-led initiatives and voluntary standards aimed at minimizing the environmental impact of carbolic acid and its byproducts throughout the product lifecycle.
Current Challenges in Carbolic Acid Waste Management
The management of carbolic acid waste presents significant challenges in today's environmental landscape. One of the primary concerns is the toxicity of carbolic acid and its degradation byproducts. These compounds can persist in the environment, posing long-term risks to ecosystems and human health. Traditional waste treatment methods often struggle to effectively neutralize or remove these substances, leading to potential contamination of soil and water resources.
Another major challenge lies in the complexity of carbolic acid degradation processes. The breakdown of carbolic acid can result in a variety of byproducts, each with its own environmental impact and treatment requirements. This diversity complicates waste management strategies, as a one-size-fits-all approach is rarely effective. Furthermore, the interaction between these byproducts and other environmental factors can lead to unforeseen consequences, making it difficult to predict and mitigate potential risks.
The scale of carbolic acid waste generation also presents logistical and economic challenges. Industries such as petrochemicals, pharmaceuticals, and plastics produce significant quantities of carbolic acid waste. Handling and treating these large volumes require substantial infrastructure and resources, often straining existing waste management systems. The cost of implementing advanced treatment technologies can be prohibitive for smaller operations, leading to disparities in waste management practices across different sectors and regions.
Regulatory compliance adds another layer of complexity to carbolic acid waste management. Environmental regulations governing the disposal and treatment of hazardous waste are becoming increasingly stringent worldwide. Companies must navigate a complex web of local, national, and international regulations, which can vary significantly between jurisdictions. Staying compliant while maintaining operational efficiency is a delicate balance that many organizations struggle to achieve.
The lack of standardized methods for detecting and quantifying carbolic acid and its byproducts in environmental samples further complicates management efforts. Current analytical techniques may not be sensitive or specific enough to accurately measure low concentrations of these compounds, particularly in complex environmental matrices. This limitation hampers the assessment of treatment efficacy and environmental impact, making it challenging to develop evidence-based management strategies.
Lastly, the public perception and awareness of carbolic acid waste issues play a crucial role in shaping management practices. There is often a lack of understanding among the general public about the risks associated with carbolic acid and its byproducts. This knowledge gap can lead to resistance against necessary waste management initiatives and facilities, complicating efforts to implement comprehensive solutions. Balancing public concerns with scientific evidence and regulatory requirements remains an ongoing challenge in the field of carbolic acid waste management.
Another major challenge lies in the complexity of carbolic acid degradation processes. The breakdown of carbolic acid can result in a variety of byproducts, each with its own environmental impact and treatment requirements. This diversity complicates waste management strategies, as a one-size-fits-all approach is rarely effective. Furthermore, the interaction between these byproducts and other environmental factors can lead to unforeseen consequences, making it difficult to predict and mitigate potential risks.
The scale of carbolic acid waste generation also presents logistical and economic challenges. Industries such as petrochemicals, pharmaceuticals, and plastics produce significant quantities of carbolic acid waste. Handling and treating these large volumes require substantial infrastructure and resources, often straining existing waste management systems. The cost of implementing advanced treatment technologies can be prohibitive for smaller operations, leading to disparities in waste management practices across different sectors and regions.
Regulatory compliance adds another layer of complexity to carbolic acid waste management. Environmental regulations governing the disposal and treatment of hazardous waste are becoming increasingly stringent worldwide. Companies must navigate a complex web of local, national, and international regulations, which can vary significantly between jurisdictions. Staying compliant while maintaining operational efficiency is a delicate balance that many organizations struggle to achieve.
The lack of standardized methods for detecting and quantifying carbolic acid and its byproducts in environmental samples further complicates management efforts. Current analytical techniques may not be sensitive or specific enough to accurately measure low concentrations of these compounds, particularly in complex environmental matrices. This limitation hampers the assessment of treatment efficacy and environmental impact, making it challenging to develop evidence-based management strategies.
Lastly, the public perception and awareness of carbolic acid waste issues play a crucial role in shaping management practices. There is often a lack of understanding among the general public about the risks associated with carbolic acid and its byproducts. This knowledge gap can lead to resistance against necessary waste management initiatives and facilities, complicating efforts to implement comprehensive solutions. Balancing public concerns with scientific evidence and regulatory requirements remains an ongoing challenge in the field of carbolic acid waste management.
Existing Carbolic Acid Degradation Technologies
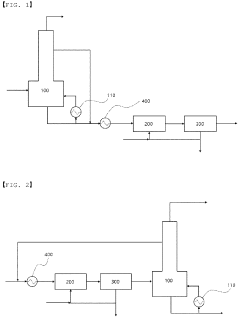
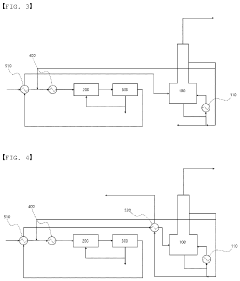
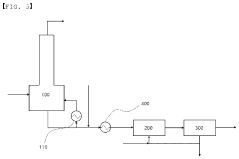
01 Environmental impact assessment of carbolic acid degradation
Studies focus on evaluating the environmental consequences of carbolic acid breakdown products. This includes analyzing their effects on ecosystems, water quality, and soil composition. Research aims to understand the long-term impacts and potential risks associated with these byproducts in various environmental settings.- Environmental impact of carbolic acid degradation: Carbolic acid (phenol) degradation can produce various byproducts that may have significant environmental impacts. These byproducts can affect soil, water, and air quality, potentially harming ecosystems and human health. The environmental fate of these compounds depends on factors such as pH, temperature, and the presence of microorganisms.
- Treatment methods for carbolic acid contamination: Various treatment methods have been developed to address carbolic acid contamination and its degradation byproducts. These include advanced oxidation processes, biological treatment systems, and adsorption techniques. The effectiveness of these methods depends on the specific byproducts present and the environmental conditions.
- Monitoring and analysis of carbolic acid degradation byproducts: Accurate monitoring and analysis of carbolic acid degradation byproducts are crucial for assessing their environmental impact. Advanced analytical techniques, including chromatography and mass spectrometry, are employed to identify and quantify these compounds in various environmental matrices. Real-time monitoring systems have also been developed to provide early warning of potential contamination.
- Ecological effects of carbolic acid degradation byproducts: The byproducts of carbolic acid degradation can have diverse ecological effects, including toxicity to aquatic organisms, bioaccumulation in food chains, and alterations to microbial communities in soil and water. Long-term exposure to these compounds may lead to changes in ecosystem structure and function, affecting biodiversity and ecological balance.
- Regulatory frameworks and risk assessment: Regulatory frameworks have been established to address the environmental impact of carbolic acid degradation byproducts. These include setting environmental quality standards, implementing monitoring programs, and conducting comprehensive risk assessments. The development of these frameworks involves considering the persistence, bioaccumulation potential, and toxicity of the byproducts.
02 Treatment and remediation techniques for carbolic acid byproducts
Development of methods to treat and remediate areas contaminated with carbolic acid degradation byproducts. This involves innovative technologies for soil and water purification, as well as strategies to neutralize or safely dispose of these compounds to minimize environmental harm.Expand Specific Solutions03 Monitoring and detection of carbolic acid degradation products
Advancements in techniques for detecting and monitoring carbolic acid byproducts in the environment. This includes the development of sensitive analytical methods, real-time monitoring systems, and environmental sensors to track the presence and concentration of these compounds in various ecosystems.Expand Specific Solutions04 Biodegradation and natural attenuation of carbolic acid byproducts
Research on the natural breakdown processes of carbolic acid degradation products in the environment. This includes studying microbial communities capable of metabolizing these compounds, as well as investigating the factors that influence the rate and efficiency of natural attenuation in different environmental conditions.Expand Specific Solutions05 Regulatory frameworks and environmental policies
Development and implementation of regulatory guidelines and environmental policies related to carbolic acid degradation byproducts. This includes establishing safety standards, disposal regulations, and monitoring requirements to mitigate the environmental impact of these compounds and ensure compliance with environmental protection laws.Expand Specific Solutions
Key Industry Players and Waste Treatment Facilities
The environmental impact of carbolic acid degradation byproducts presents a complex competitive landscape in the chemical and environmental sectors. The industry is in a growth phase, driven by increasing environmental regulations and corporate sustainability initiatives. The global market for environmental remediation technologies is expanding, with projections reaching billions of dollars annually. Technological maturity varies, with established players like Eastman Chemical Co. and SABIC Global Technologies BV leveraging their extensive R&D capabilities to develop advanced solutions. Emerging companies such as LanzaTech NZ, Inc. and Capro-X, Inc. are introducing innovative biotech approaches, while academic institutions like Monash University and Clemson University contribute cutting-edge research. This diverse ecosystem fosters both collaboration and competition in addressing the challenges of carbolic acid degradation.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has developed an innovative approach to address the environmental impact of carbolic acid degradation byproducts. Their technology focuses on a two-stage treatment process that combines advanced oxidation and biological degradation[1]. In the first stage, they use a catalytic oxidation system that breaks down complex carbolic acid molecules into simpler, more biodegradable compounds. This process employs a proprietary catalyst that operates at lower temperatures, reducing energy consumption[3]. The second stage involves a specialized bioreactor system with carefully selected microbial consortia that further degrade the oxidation products. This biological treatment step not only reduces the overall toxicity of the effluent but also produces valuable byproducts that can be used in other industrial processes[5]. Eastman's system is designed to be modular and scalable, allowing for easy integration into existing industrial setups.
Strengths: High efficiency in breaking down complex molecules, reduced energy consumption, and production of valuable byproducts. Weaknesses: May require significant initial investment and ongoing maintenance of the bioreactor system.
LanzaTech NZ, Inc.
Technical Solution: LanzaTech has pioneered a unique biological approach to mitigate the environmental impact of carbolic acid degradation byproducts. Their technology harnesses the power of gas fermentation, using specially engineered microorganisms to convert carbolic acid and its byproducts into valuable chemicals and fuels[2]. The process begins with a proprietary gas pre-treatment step that optimizes the composition of the waste gas stream containing carbolic acid derivatives. This treated gas is then fed into bioreactors where LanzaTech's patented microbes consume the carbon-rich compounds, effectively removing them from the waste stream[4]. The microorganisms convert these compounds into ethanol, which can be used as a fuel or chemical precursor. Additionally, the process can be tuned to produce other high-value chemicals, depending on market demands[6]. This biological approach not only addresses the environmental concerns but also creates a circular economy model where waste is transformed into valuable products.
Strengths: Converts waste into valuable products, highly adaptable to different waste streams, and creates a circular economy solution. Weaknesses: May be sensitive to variations in waste composition and requires careful control of fermentation conditions.
Innovative Approaches to Byproduct Mitigation
Method of decomposing phenolic by-products
PatentActiveUS11993564B2
Innovation
- A method involving thermal decomposition of phenolic by-products, separating effective components and high-boiling point materials, mixing these with a side discharge stream and process water, and then separating the mixture into an oil and aqueous phase to remove salts and prevent fouling, ensuring minimal process water presence to avoid reboiler fouling.
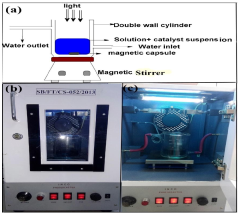
Fluorine doped graphene based slurry type photocatalytic system for water purification
PatentActiveIN201811017744A
Innovation
- A fluorine-doped graphene-based photocatalytic system is developed using an ultrasonication-assisted hydrothermal method to prevent agglomeration, enhancing dispersion and stability, and incorporating Samarium vanadate nanoparticles for efficient degradation of phenol and 2,4-dinitrophenol under visible light, with demonstrated antibacterial activity.
Ecological Risk Assessment of Degradation Byproducts
The ecological risk assessment of carbolic acid degradation byproducts is a critical component in evaluating the environmental impact of these compounds. Carbolic acid, also known as phenol, is widely used in various industrial processes, and its degradation can lead to the formation of potentially harmful byproducts. These byproducts may pose significant risks to ecosystems if released into the environment without proper treatment or management.
To conduct a comprehensive ecological risk assessment, it is essential to identify and characterize the specific degradation byproducts of carbolic acid. Common byproducts include catechol, hydroquinone, and various chlorinated phenols. Each of these compounds may have unique toxicological profiles and environmental fate characteristics, which must be carefully evaluated to determine their potential impacts on different ecological receptors.
The assessment process typically involves several key steps, beginning with hazard identification. This stage focuses on determining the inherent toxicity of the degradation byproducts to various organisms, including aquatic life, terrestrial plants, and soil microorganisms. Toxicity data from standardized tests and published literature are crucial in establishing dose-response relationships and identifying potential ecological endpoints of concern.
Exposure assessment is another critical component of the ecological risk assessment. This step involves evaluating the potential pathways through which the degradation byproducts may enter and move through the environment. Factors such as persistence, bioaccumulation potential, and mobility in different environmental media (e.g., soil, water, air) are considered to estimate the likelihood and magnitude of exposure to ecological receptors.
The integration of hazard and exposure information allows for the characterization of ecological risks associated with carbolic acid degradation byproducts. This process often involves the use of risk quotients, which compare estimated environmental concentrations to toxicity thresholds for various species. Probabilistic approaches may also be employed to account for uncertainties and variability in both exposure and effects data.
It is important to note that the ecological risk assessment should consider potential synergistic or antagonistic effects that may occur when multiple degradation byproducts are present simultaneously. Additionally, the assessment should account for the potential long-term impacts on ecosystem structure and function, including effects on biodiversity, nutrient cycling, and food web dynamics.
The results of the ecological risk assessment can inform decision-making processes related to the management and regulation of carbolic acid and its degradation byproducts. This may include the development of environmental quality standards, the implementation of treatment technologies, or the modification of industrial processes to minimize the formation of harmful byproducts. Ultimately, a thorough ecological risk assessment contributes to the protection of environmental health and the sustainable use of carbolic acid in industrial applications.
To conduct a comprehensive ecological risk assessment, it is essential to identify and characterize the specific degradation byproducts of carbolic acid. Common byproducts include catechol, hydroquinone, and various chlorinated phenols. Each of these compounds may have unique toxicological profiles and environmental fate characteristics, which must be carefully evaluated to determine their potential impacts on different ecological receptors.
The assessment process typically involves several key steps, beginning with hazard identification. This stage focuses on determining the inherent toxicity of the degradation byproducts to various organisms, including aquatic life, terrestrial plants, and soil microorganisms. Toxicity data from standardized tests and published literature are crucial in establishing dose-response relationships and identifying potential ecological endpoints of concern.
Exposure assessment is another critical component of the ecological risk assessment. This step involves evaluating the potential pathways through which the degradation byproducts may enter and move through the environment. Factors such as persistence, bioaccumulation potential, and mobility in different environmental media (e.g., soil, water, air) are considered to estimate the likelihood and magnitude of exposure to ecological receptors.
The integration of hazard and exposure information allows for the characterization of ecological risks associated with carbolic acid degradation byproducts. This process often involves the use of risk quotients, which compare estimated environmental concentrations to toxicity thresholds for various species. Probabilistic approaches may also be employed to account for uncertainties and variability in both exposure and effects data.
It is important to note that the ecological risk assessment should consider potential synergistic or antagonistic effects that may occur when multiple degradation byproducts are present simultaneously. Additionally, the assessment should account for the potential long-term impacts on ecosystem structure and function, including effects on biodiversity, nutrient cycling, and food web dynamics.
The results of the ecological risk assessment can inform decision-making processes related to the management and regulation of carbolic acid and its degradation byproducts. This may include the development of environmental quality standards, the implementation of treatment technologies, or the modification of industrial processes to minimize the formation of harmful byproducts. Ultimately, a thorough ecological risk assessment contributes to the protection of environmental health and the sustainable use of carbolic acid in industrial applications.
Sustainable Practices in Chemical Waste Disposal
Sustainable practices in chemical waste disposal have become increasingly crucial in the face of growing environmental concerns, particularly regarding the byproducts of carbolic acid degradation. The chemical industry has recognized the need for more responsible and eco-friendly approaches to manage waste streams and minimize environmental impact. This shift towards sustainability is driven by a combination of regulatory pressures, public awareness, and corporate social responsibility initiatives.
One of the primary sustainable practices in this field involves the implementation of advanced treatment technologies. These technologies aim to neutralize or transform harmful byproducts into less toxic or even benign substances. For instance, advanced oxidation processes (AOPs) have shown promise in breaking down complex organic compounds, including those resulting from carbolic acid degradation. These processes typically involve the generation of highly reactive hydroxyl radicals, which can effectively oxidize a wide range of organic pollutants.
Another key aspect of sustainable waste disposal is the adoption of circular economy principles. This approach focuses on recovering and reusing valuable resources from waste streams, thereby reducing the overall environmental footprint of chemical processes. In the context of carbolic acid byproducts, this might involve the extraction of useful compounds or the conversion of waste materials into energy through processes such as anaerobic digestion or pyrolysis.
The integration of green chemistry principles into waste management strategies is also gaining traction. This involves designing chemical processes and products that minimize the generation of hazardous substances from the outset. By considering the entire lifecycle of chemical products, including their eventual disposal, manufacturers can significantly reduce the environmental impact of their operations. This proactive approach often leads to the development of alternative synthesis routes or the use of less harmful precursors that result in more manageable waste streams.
Monitoring and analytics play a crucial role in sustainable waste disposal practices. Advanced sensors and real-time monitoring systems allow for the early detection of potential environmental hazards, enabling rapid response and mitigation measures. Additionally, big data analytics and artificial intelligence are being leveraged to optimize waste treatment processes, predict potential issues, and identify opportunities for improving overall sustainability.
Collaboration between industry, academia, and regulatory bodies is essential for developing and implementing sustainable practices in chemical waste disposal. Research partnerships are driving innovation in treatment technologies, while industry consortia are working to establish best practices and share knowledge across the sector. Furthermore, engagement with local communities and environmental organizations helps ensure that waste disposal practices align with broader societal goals and expectations.
One of the primary sustainable practices in this field involves the implementation of advanced treatment technologies. These technologies aim to neutralize or transform harmful byproducts into less toxic or even benign substances. For instance, advanced oxidation processes (AOPs) have shown promise in breaking down complex organic compounds, including those resulting from carbolic acid degradation. These processes typically involve the generation of highly reactive hydroxyl radicals, which can effectively oxidize a wide range of organic pollutants.
Another key aspect of sustainable waste disposal is the adoption of circular economy principles. This approach focuses on recovering and reusing valuable resources from waste streams, thereby reducing the overall environmental footprint of chemical processes. In the context of carbolic acid byproducts, this might involve the extraction of useful compounds or the conversion of waste materials into energy through processes such as anaerobic digestion or pyrolysis.
The integration of green chemistry principles into waste management strategies is also gaining traction. This involves designing chemical processes and products that minimize the generation of hazardous substances from the outset. By considering the entire lifecycle of chemical products, including their eventual disposal, manufacturers can significantly reduce the environmental impact of their operations. This proactive approach often leads to the development of alternative synthesis routes or the use of less harmful precursors that result in more manageable waste streams.
Monitoring and analytics play a crucial role in sustainable waste disposal practices. Advanced sensors and real-time monitoring systems allow for the early detection of potential environmental hazards, enabling rapid response and mitigation measures. Additionally, big data analytics and artificial intelligence are being leveraged to optimize waste treatment processes, predict potential issues, and identify opportunities for improving overall sustainability.
Collaboration between industry, academia, and regulatory bodies is essential for developing and implementing sustainable practices in chemical waste disposal. Research partnerships are driving innovation in treatment technologies, while industry consortia are working to establish best practices and share knowledge across the sector. Furthermore, engagement with local communities and environmental organizations helps ensure that waste disposal practices align with broader societal goals and expectations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!