Evaluate Graphene Oxide for Enhanced Electrocatalysis
Graphene Oxide Electrocatalysis Background
Graphene oxide (GO) has emerged as a promising material in the field of electrocatalysis, attracting significant attention from researchers and industry professionals alike. This two-dimensional carbon-based nanomaterial, derived from the oxidation of graphite, possesses unique structural and chemical properties that make it particularly suitable for catalytic applications.
The journey of graphene oxide in electrocatalysis began with the discovery of graphene in 2004 by Andre Geim and Konstantin Novoselov. As researchers explored the potential of graphene, they soon realized that its oxidized form, graphene oxide, offered distinct advantages in certain applications, including electrocatalysis.
Graphene oxide's structure consists of a single layer of carbon atoms arranged in a hexagonal lattice, with various oxygen-containing functional groups attached to its basal plane and edges. These oxygen functionalities, such as hydroxyl, epoxy, and carboxyl groups, play a crucial role in its electrocatalytic properties by providing active sites for reactions and enhancing its interaction with other materials.
The evolution of graphene oxide in electrocatalysis has been marked by continuous improvements in synthesis methods, functionalization techniques, and composite formation. Early research focused on understanding the fundamental properties of GO and its reduced form (rGO), while later studies explored its application in various electrochemical reactions, such as oxygen reduction, hydrogen evolution, and carbon dioxide reduction.
One of the key trends in graphene oxide electrocatalysis has been the development of hybrid materials and nanocomposites. By combining GO with metal nanoparticles, metal oxides, or other carbon-based materials, researchers have been able to create synergistic effects that enhance catalytic activity, selectivity, and stability.
The field has also witnessed significant advancements in the precise control of GO's oxygen content and defect structure. These parameters have been found to greatly influence its electrocatalytic performance, leading to the development of tailored GO materials for specific reactions.
Recent years have seen an increased focus on the scalable production of high-quality graphene oxide and its derivatives for electrocatalysis. This trend is driven by the growing demand for efficient and cost-effective catalysts in various industrial applications, including fuel cells, electrolyzers, and sensors.
As research in this area continues to progress, the ultimate goal is to develop graphene oxide-based electrocatalysts that can match or surpass the performance of traditional noble metal catalysts while offering advantages in terms of cost, abundance, and sustainability. This objective aligns with broader efforts to transition towards more sustainable energy technologies and reduce reliance on scarce and expensive materials.
Market Demand Analysis
The market demand for graphene oxide in electrocatalysis applications has been steadily growing, driven by the increasing need for efficient and sustainable energy conversion and storage technologies. As global efforts to transition towards clean energy intensify, the demand for advanced materials that can enhance electrocatalytic processes has surged. Graphene oxide, with its unique properties and potential to improve catalytic performance, has emerged as a promising candidate in this field.
The electrocatalysis market, encompassing fuel cells, water splitting, and CO2 reduction, is projected to experience significant growth in the coming years. This expansion is primarily fueled by the rising adoption of renewable energy sources and the push for green hydrogen production. Graphene oxide's ability to enhance the efficiency and durability of electrocatalysts makes it particularly attractive for these applications.
In the fuel cell sector, graphene oxide-based materials have shown potential to improve the performance of both anodes and cathodes. The automotive industry, in particular, has expressed keen interest in utilizing graphene oxide to enhance the efficiency and longevity of fuel cell systems in electric vehicles. This interest is expected to drive substantial market growth as major automakers invest in fuel cell technology.
The water splitting market, crucial for green hydrogen production, is another area where graphene oxide is gaining traction. As countries worldwide set ambitious targets for hydrogen production and utilization, the demand for efficient electrocatalysts for water electrolysis is soaring. Graphene oxide's ability to improve the activity and stability of catalysts for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) positions it as a key material in this rapidly expanding market.
In the field of CO2 reduction, graphene oxide-based catalysts have shown promise in converting carbon dioxide into valuable chemicals and fuels. With increasing focus on carbon capture and utilization technologies, the demand for efficient CO2 reduction catalysts is expected to grow significantly. Graphene oxide's potential to enhance the selectivity and efficiency of these processes makes it an attractive option for researchers and industries alike.
The market for graphene oxide in electrocatalysis is also being driven by its potential to reduce the use of precious metals in catalysts. As the cost and scarcity of materials like platinum and iridium continue to be a concern, graphene oxide offers a pathway to develop more cost-effective and sustainable catalytic systems. This aspect is particularly appealing to industries looking to scale up their electrocatalytic processes while managing material costs.
Current Challenges
Despite the promising potential of graphene oxide (GO) in electrocatalysis, several significant challenges currently hinder its widespread application and commercialization. One of the primary obstacles is the inconsistent quality and reproducibility of GO synthesis. The oxidation process of graphite to produce GO can result in varying degrees of oxidation and defect densities, leading to inconsistent catalytic performance across different batches.
Another major challenge lies in the scalability of GO production. While laboratory-scale synthesis methods have been well-established, scaling up these processes for industrial applications remains problematic. Issues such as maintaining uniform oxidation levels, controlling sheet size distribution, and ensuring cost-effectiveness at larger scales pose significant hurdles for mass production.
The stability of GO in electrocatalytic applications is also a concern. Under certain electrochemical conditions, GO can undergo reduction or further oxidation, altering its structure and potentially compromising its catalytic activity over time. This instability can lead to decreased performance and shorter lifespans for GO-based electrocatalysts, limiting their practical utility in long-term applications.
Furthermore, the electronic properties of GO present a challenge for certain electrocatalytic reactions. While the presence of oxygen-containing functional groups on GO's surface can be beneficial for some catalytic processes, these same groups can also reduce the material's electrical conductivity. This trade-off between catalytic activity and conductivity necessitates careful tuning of GO's oxidation state to optimize performance for specific applications.
The environmental impact and potential toxicity of GO production and usage also raise concerns. The synthesis of GO often involves strong oxidizing agents and acids, which can pose environmental risks if not properly managed. Additionally, the potential long-term effects of GO nanoparticles on ecosystems and human health are not yet fully understood, necessitating further research and regulatory considerations.
Lastly, the integration of GO into existing electrocatalytic systems and devices presents technical challenges. Issues such as uniform dispersion, adhesion to substrates, and compatibility with other materials in composite structures need to be addressed to fully leverage GO's potential in practical applications. Overcoming these integration challenges is crucial for the development of efficient and durable GO-based electrocatalytic systems.
Existing Solutions
01 Graphene oxide-based electrocatalysts for energy conversion
Graphene oxide is utilized as a base material for developing efficient electrocatalysts in various energy conversion applications. These catalysts exhibit enhanced performance in processes such as fuel cells, water splitting, and CO2 reduction due to their high surface area, excellent conductivity, and tunable electronic properties.- Graphene oxide-based electrocatalysts for energy conversion: Graphene oxide is used as a base material for developing efficient electrocatalysts for various energy conversion processes. These catalysts show enhanced performance in applications such as fuel cells, water splitting, and metal-air batteries due to their high surface area and excellent electrical conductivity.
- Synthesis methods for graphene oxide-based electrocatalysts: Various synthesis methods are employed to produce graphene oxide-based electrocatalysts with improved catalytic activity. These methods include chemical reduction, hydrothermal treatment, and doping with heteroatoms or metal nanoparticles to enhance the electrocatalytic properties of graphene oxide.
- Graphene oxide composites for electrocatalysis: Composite materials combining graphene oxide with other materials such as metal oxides, conductive polymers, or carbon nanotubes are developed to improve electrocatalytic performance. These composites exhibit synergistic effects, leading to enhanced catalytic activity and stability in various electrochemical reactions.
- Applications of graphene oxide electrocatalysts in sensors: Graphene oxide-based electrocatalysts are utilized in the development of highly sensitive and selective electrochemical sensors. These sensors find applications in environmental monitoring, biomedical diagnostics, and industrial process control due to their excellent electrocatalytic properties and large surface area.
- Functionalization of graphene oxide for improved electrocatalysis: Various functionalization techniques are employed to modify the surface of graphene oxide, enhancing its electrocatalytic properties. These modifications include the introduction of specific functional groups, defects, or heteroatoms to tailor the electronic structure and catalytic activity of graphene oxide for specific electrochemical reactions.
02 Synthesis and modification of graphene oxide for electrocatalysis
Various methods are employed to synthesize and modify graphene oxide for electrocatalytic applications. These include chemical exfoliation, reduction processes, and functionalization techniques to tailor the material's properties for specific catalytic reactions.Expand Specific Solutions03 Graphene oxide-metal hybrid electrocatalysts
Hybrid electrocatalysts combining graphene oxide with metal nanoparticles or compounds are developed to enhance catalytic activity and selectivity. These composites leverage the synergistic effects between graphene oxide and metals to improve overall performance in electrochemical reactions.Expand Specific Solutions04 Graphene oxide-based sensors and electrodes
Graphene oxide is utilized in the development of highly sensitive electrochemical sensors and electrodes. These materials exhibit excellent electron transfer properties and can be functionalized for specific analyte detection, making them suitable for various sensing applications.Expand Specific Solutions05 Graphene oxide in energy storage applications
Graphene oxide-based materials are employed in energy storage devices such as supercapacitors and batteries. The unique properties of graphene oxide, including high surface area and conductivity, contribute to improved energy storage capacity and cycling stability in these applications.Expand Specific Solutions
Key Industry Players
The graphene oxide electrocatalysis market is in a growth phase, with increasing research and commercial interest. The global market size for graphene-based products is projected to expand significantly in the coming years. Technologically, graphene oxide for electrocatalysis is advancing rapidly, with various degrees of maturity across applications. Leading academic institutions like MIT, University of Manchester, and Zhejiang University are driving fundamental research, while companies such as LG Chem and Toray Industries are focusing on commercial applications. Collaboration between academia and industry, exemplified by partnerships involving CNRS and Baoshan Iron & Steel, is accelerating technological progress. The field is highly competitive, with players from diverse geographical regions contributing to innovation and market development.
The University of Manchester
LG Chem Ltd.
Core Innovations
- Electrochemically exfoliated phosphated graphene (EEP) is synthesized using inorganic salt solutions, particularly diammonium phosphate, to introduce phosphorous-containing functional groups, improving thermal stability and catalytic activity, with applications in high-temperature electrochemical technologies.
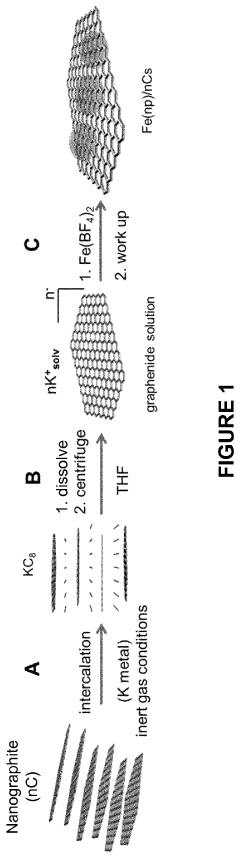
- A method involving the dissolution of nanographite intercalation compounds in aprotic organic solvents, followed by reaction with metal salts or complexes under inert conditions, without a reducing agent, to form nanographene-supported metal and/or metal oxide nanoparticles, allowing for precise control over particle size and distribution.
Environmental Impact
The environmental impact of graphene oxide (GO) in electrocatalysis is a critical consideration for its widespread adoption and sustainable implementation. GO's unique properties, while beneficial for enhancing electrocatalytic performance, also raise concerns about its potential effects on ecosystems and human health.
One of the primary environmental advantages of using GO in electrocatalysis is its potential to improve the efficiency of various electrochemical processes, including water splitting, fuel cells, and CO2 reduction. By enhancing catalytic activity and reducing the need for precious metal catalysts, GO-based electrocatalysts can contribute to more sustainable energy production and storage systems. This could lead to reduced greenhouse gas emissions and a decreased reliance on fossil fuels, positively impacting climate change mitigation efforts.
However, the production and use of GO also present environmental challenges. The synthesis of GO often involves harsh chemicals and energy-intensive processes, which can result in the generation of hazardous waste and increased carbon footprint. Efforts are being made to develop greener synthesis methods, such as electrochemical exfoliation or mechanochemical approaches, to minimize these environmental impacts.
The potential release of GO nanoparticles into the environment during production, use, or disposal is another concern. Due to their small size and unique surface properties, GO nanoparticles may interact with biological systems in ways that are not yet fully understood. Studies have shown that GO can accumulate in aquatic organisms and potentially disrupt ecosystem functions. Long-term exposure effects on human health and the environment are still being investigated, necessitating careful handling and disposal protocols.
On the other hand, GO's high surface area and adsorption capabilities offer potential environmental remediation applications. GO-based materials have shown promise in removing heavy metals, organic pollutants, and other contaminants from water and soil. This dual nature of GO – as both a potential environmental risk and a tool for environmental cleanup – highlights the complexity of its environmental impact assessment.
The recyclability and end-of-life management of GO-based electrocatalysts are also important considerations. While GO itself is not biodegradable, research is ongoing to develop methods for recovering and recycling GO from spent catalysts. Successful implementation of these recycling strategies could significantly reduce the environmental footprint of GO-enhanced electrocatalysis systems.
As research in this field progresses, it is crucial to conduct comprehensive life cycle assessments of GO-based electrocatalysts to fully understand their environmental implications. This includes evaluating the entire production chain, from raw material extraction to disposal or recycling. Such assessments will help inform policy decisions and guide the development of more environmentally friendly GO production and application methods in electrocatalysis.
Scalability Assessment
Scalability assessment is a critical factor in evaluating the potential of graphene oxide for enhanced electrocatalysis. The production of graphene oxide at industrial scales presents both opportunities and challenges that must be carefully considered.
Current manufacturing processes for graphene oxide typically involve chemical exfoliation methods, such as the modified Hummers' method. While these techniques can produce high-quality graphene oxide, they often face limitations in terms of scalability and environmental impact. The use of strong oxidizing agents and acids in these processes raises concerns about waste management and production costs at larger scales.
Recent advancements in production techniques have shown promise for improving scalability. Electrochemical exfoliation methods, for instance, offer a more environmentally friendly approach with potential for continuous production. These methods can produce graphene oxide with controlled oxidation levels and reduced impurities, which is crucial for electrocatalytic applications.
The scalability of graphene oxide production also depends on the availability and cost of raw materials. Graphite, the primary precursor, is relatively abundant and inexpensive. However, the quality of graphite can significantly impact the properties of the resulting graphene oxide, necessitating careful sourcing and quality control measures as production scales up.
Another important aspect of scalability is the ability to maintain consistent quality across large-scale production batches. Variations in oxidation degree, sheet size, and defect density can significantly affect the electrocatalytic performance of graphene oxide. Implementing robust quality control protocols and standardization measures will be essential for ensuring reproducibility in industrial-scale production.
The integration of graphene oxide into existing manufacturing processes for electrocatalysts presents additional scalability considerations. Developing efficient methods for incorporating graphene oxide into composite materials or depositing it onto electrode surfaces at large scales will be crucial for its widespread adoption in electrocatalysis applications.
Economic factors also play a significant role in scalability assessment. While the production costs of graphene oxide have decreased in recent years, further reductions will be necessary to compete with established electrocatalyst materials. Economies of scale and process optimizations can contribute to cost reductions, but substantial investments in research and development may be required to achieve economic viability at industrial scales.
In conclusion, while graphene oxide shows great promise for enhanced electrocatalysis, several challenges must be addressed to achieve scalability. Advancements in production techniques, quality control measures, and cost-effective integration methods will be key to realizing the full potential of graphene oxide in large-scale electrocatalytic applications.