How Biocompatible Polypropylene Affects Medical Implants
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocompatible PP in Medical Implants: Background and Objectives
Polypropylene (PP) has been a cornerstone material in the medical industry for decades, particularly in the realm of implantable devices. Its journey in medical applications began in the mid-20th century when researchers recognized its potential for biocompatibility and versatility. As medical technology advanced, so did the need for materials that could safely interact with the human body for extended periods.
The evolution of biocompatible PP in medical implants has been driven by the increasing demand for minimally invasive procedures and long-term implantable devices. From surgical meshes to cardiovascular stents, PP has found its way into various medical applications due to its unique properties. These include chemical resistance, flexibility, and the ability to be sterilized without degradation.
However, the use of PP in medical implants has not been without challenges. Early applications faced issues such as material degradation and adverse tissue reactions. These setbacks spurred extensive research into enhancing PP's biocompatibility, leading to significant advancements in polymer science and surface modification techniques.
The primary objective of current research in biocompatible PP for medical implants is to optimize its interaction with biological systems while maintaining its beneficial mechanical properties. This involves developing PP variants with improved surface characteristics, reduced inflammatory responses, and enhanced integration with surrounding tissues.
Another crucial goal is to extend the lifespan of PP-based implants, addressing concerns about long-term stability and potential complications. Researchers are exploring ways to minimize oxidation and degradation of PP in vivo, which can lead to implant failure or adverse reactions over time.
Furthermore, there is a growing focus on tailoring PP properties for specific medical applications. This includes developing PP composites that combine the base material's advantages with additional functionalities, such as controlled drug release or improved imaging visibility.
As we look to the future, the aim is to create "smart" PP-based implants that can adapt to the body's environment, potentially even promoting tissue regeneration. This ambitious objective requires interdisciplinary collaboration between material scientists, bioengineers, and medical professionals to push the boundaries of what is possible with biocompatible PP in medical implants.
The evolution of biocompatible PP in medical implants has been driven by the increasing demand for minimally invasive procedures and long-term implantable devices. From surgical meshes to cardiovascular stents, PP has found its way into various medical applications due to its unique properties. These include chemical resistance, flexibility, and the ability to be sterilized without degradation.
However, the use of PP in medical implants has not been without challenges. Early applications faced issues such as material degradation and adverse tissue reactions. These setbacks spurred extensive research into enhancing PP's biocompatibility, leading to significant advancements in polymer science and surface modification techniques.
The primary objective of current research in biocompatible PP for medical implants is to optimize its interaction with biological systems while maintaining its beneficial mechanical properties. This involves developing PP variants with improved surface characteristics, reduced inflammatory responses, and enhanced integration with surrounding tissues.
Another crucial goal is to extend the lifespan of PP-based implants, addressing concerns about long-term stability and potential complications. Researchers are exploring ways to minimize oxidation and degradation of PP in vivo, which can lead to implant failure or adverse reactions over time.
Furthermore, there is a growing focus on tailoring PP properties for specific medical applications. This includes developing PP composites that combine the base material's advantages with additional functionalities, such as controlled drug release or improved imaging visibility.
As we look to the future, the aim is to create "smart" PP-based implants that can adapt to the body's environment, potentially even promoting tissue regeneration. This ambitious objective requires interdisciplinary collaboration between material scientists, bioengineers, and medical professionals to push the boundaries of what is possible with biocompatible PP in medical implants.
Market Analysis for Biocompatible Medical Implants
The market for biocompatible medical implants has experienced significant growth in recent years, driven by an aging global population, increasing prevalence of chronic diseases, and advancements in medical technology. Biocompatible polypropylene, a versatile and cost-effective material, has emerged as a key player in this expanding market.
The global medical implants market is projected to reach substantial value in the coming years, with biocompatible materials playing a crucial role in this growth. Polypropylene, known for its excellent biocompatibility and mechanical properties, is widely used in various medical implant applications, including hernia meshes, sutures, and cardiovascular devices.
Demographic trends are a major factor driving market demand. As the world's population ages, there is an increasing need for medical implants to address age-related health issues. Additionally, the rising incidence of chronic diseases and lifestyle-related health problems has led to a greater demand for implantable medical devices.
Technological advancements in implant design and manufacturing processes have also contributed to market growth. Innovations in surface modification techniques and the development of composite materials have improved the performance and longevity of polypropylene-based implants, expanding their potential applications.
Geographically, North America and Europe currently dominate the biocompatible medical implants market, owing to well-established healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare access and rising disposable incomes.
The market for biocompatible polypropylene implants faces some challenges, including stringent regulatory requirements and concerns about long-term implant performance. However, ongoing research and development efforts are addressing these issues, focusing on enhancing the material's properties and exploring new applications.
In terms of market segmentation, orthopedic implants represent the largest share of the biocompatible medical implants market, followed by cardiovascular implants and dental implants. Polypropylene finds extensive use in soft tissue repair applications, particularly in hernia meshes and surgical sutures.
Looking ahead, the market for biocompatible polypropylene implants is expected to continue its growth trajectory. Factors such as increasing healthcare expenditure, growing awareness about advanced medical treatments, and ongoing technological innovations are likely to drive market expansion. Additionally, the trend towards personalized medicine and 3D-printed implants presents new opportunities for polypropylene-based solutions in the medical implant market.
The global medical implants market is projected to reach substantial value in the coming years, with biocompatible materials playing a crucial role in this growth. Polypropylene, known for its excellent biocompatibility and mechanical properties, is widely used in various medical implant applications, including hernia meshes, sutures, and cardiovascular devices.
Demographic trends are a major factor driving market demand. As the world's population ages, there is an increasing need for medical implants to address age-related health issues. Additionally, the rising incidence of chronic diseases and lifestyle-related health problems has led to a greater demand for implantable medical devices.
Technological advancements in implant design and manufacturing processes have also contributed to market growth. Innovations in surface modification techniques and the development of composite materials have improved the performance and longevity of polypropylene-based implants, expanding their potential applications.
Geographically, North America and Europe currently dominate the biocompatible medical implants market, owing to well-established healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare access and rising disposable incomes.
The market for biocompatible polypropylene implants faces some challenges, including stringent regulatory requirements and concerns about long-term implant performance. However, ongoing research and development efforts are addressing these issues, focusing on enhancing the material's properties and exploring new applications.
In terms of market segmentation, orthopedic implants represent the largest share of the biocompatible medical implants market, followed by cardiovascular implants and dental implants. Polypropylene finds extensive use in soft tissue repair applications, particularly in hernia meshes and surgical sutures.
Looking ahead, the market for biocompatible polypropylene implants is expected to continue its growth trajectory. Factors such as increasing healthcare expenditure, growing awareness about advanced medical treatments, and ongoing technological innovations are likely to drive market expansion. Additionally, the trend towards personalized medicine and 3D-printed implants presents new opportunities for polypropylene-based solutions in the medical implant market.
Current Status and Challenges in Biocompatible PP Technology
The current status of biocompatible polypropylene (PP) technology for medical implants is characterized by significant advancements, yet it still faces several challenges. Biocompatible PP has gained traction in the medical field due to its excellent mechanical properties, chemical resistance, and cost-effectiveness. However, its inherent hydrophobicity and lack of bioactivity have limited its widespread adoption in certain implant applications.
Recent developments have focused on surface modification techniques to enhance the biocompatibility of PP. Plasma treatment, grafting, and coating methods have shown promising results in improving cell adhesion and proliferation on PP surfaces. These modifications aim to create a more hydrophilic surface and introduce functional groups that promote better integration with surrounding tissues.
Despite these advancements, ensuring long-term stability of surface modifications remains a significant challenge. The durability of modified surfaces under physiological conditions and their resistance to degradation over time are critical factors that require further investigation. Additionally, the potential for these modifications to alter the bulk properties of PP, such as mechanical strength or flexibility, must be carefully considered.
Another major challenge lies in achieving consistent and uniform surface modifications across complex implant geometries. Current techniques often struggle to provide homogeneous treatment on intricate shapes or porous structures, which can lead to variability in implant performance and biocompatibility.
The biocompatibility of PP is also influenced by the presence of additives and processing aids used during manufacturing. Leaching of these substances can potentially cause adverse reactions in the body. Developing PP formulations with biocompatible additives or finding ways to minimize leaching is an ongoing area of research.
Infection prevention remains a critical challenge for PP implants. While some progress has been made in incorporating antimicrobial agents into PP, balancing long-term antimicrobial efficacy with maintaining the material's mechanical properties and avoiding potential cytotoxicity is still a complex issue.
Regulatory hurdles present another significant challenge in the development and commercialization of biocompatible PP implants. Stringent approval processes and the need for extensive clinical trials can slow down innovation and market entry for new PP-based medical devices.
Looking ahead, the field of biocompatible PP technology is moving towards more advanced surface engineering techniques, such as nanopatterning and biomimetic approaches. These methods aim to create surfaces that more closely mimic natural tissue environments, potentially leading to better implant integration and reduced foreign body responses.
Recent developments have focused on surface modification techniques to enhance the biocompatibility of PP. Plasma treatment, grafting, and coating methods have shown promising results in improving cell adhesion and proliferation on PP surfaces. These modifications aim to create a more hydrophilic surface and introduce functional groups that promote better integration with surrounding tissues.
Despite these advancements, ensuring long-term stability of surface modifications remains a significant challenge. The durability of modified surfaces under physiological conditions and their resistance to degradation over time are critical factors that require further investigation. Additionally, the potential for these modifications to alter the bulk properties of PP, such as mechanical strength or flexibility, must be carefully considered.
Another major challenge lies in achieving consistent and uniform surface modifications across complex implant geometries. Current techniques often struggle to provide homogeneous treatment on intricate shapes or porous structures, which can lead to variability in implant performance and biocompatibility.
The biocompatibility of PP is also influenced by the presence of additives and processing aids used during manufacturing. Leaching of these substances can potentially cause adverse reactions in the body. Developing PP formulations with biocompatible additives or finding ways to minimize leaching is an ongoing area of research.
Infection prevention remains a critical challenge for PP implants. While some progress has been made in incorporating antimicrobial agents into PP, balancing long-term antimicrobial efficacy with maintaining the material's mechanical properties and avoiding potential cytotoxicity is still a complex issue.
Regulatory hurdles present another significant challenge in the development and commercialization of biocompatible PP implants. Stringent approval processes and the need for extensive clinical trials can slow down innovation and market entry for new PP-based medical devices.
Looking ahead, the field of biocompatible PP technology is moving towards more advanced surface engineering techniques, such as nanopatterning and biomimetic approaches. These methods aim to create surfaces that more closely mimic natural tissue environments, potentially leading to better implant integration and reduced foreign body responses.
Existing Applications of Biocompatible PP in Medical Implants
01 Surface modification of polypropylene for biocompatibility
Various techniques are employed to modify the surface of polypropylene to enhance its biocompatibility. These methods include plasma treatment, chemical grafting, and coating with biocompatible materials. Such modifications can improve cell adhesion, reduce inflammatory responses, and increase the material's suitability for medical applications.- Surface modification of polypropylene for biocompatibility: Various techniques are employed to modify the surface of polypropylene to enhance its biocompatibility. These methods include plasma treatment, chemical grafting, and coating with biocompatible materials. Such modifications can improve cell adhesion, reduce inflammatory responses, and increase the material's suitability for medical applications.
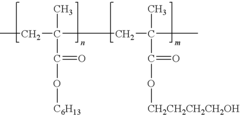
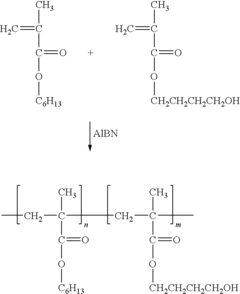
- Biocompatible polypropylene composites: Polypropylene is combined with other biocompatible materials to create composite structures with enhanced properties. These composites may incorporate materials such as hydroxyapatite, collagen, or other polymers to improve biocompatibility, mechanical strength, and tissue integration for various medical applications, including implants and tissue engineering scaffolds.
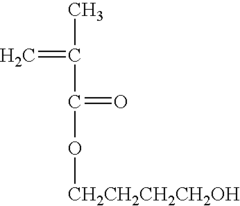
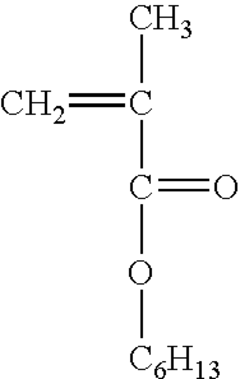
- Polypropylene-based drug delivery systems: Biocompatible polypropylene is utilized in the development of drug delivery systems. These systems can be designed as implants, microspheres, or nanoparticles that gradually release therapeutic agents. The polymer's properties are tailored to control drug release rates and enhance the overall efficacy of the treatment.
- Sterilization methods for biocompatible polypropylene: Various sterilization techniques are developed and optimized for biocompatible polypropylene medical devices. These methods aim to effectively sterilize the material while maintaining its structural integrity and biocompatibility. Techniques may include gamma irradiation, ethylene oxide treatment, or novel sterilization approaches specifically designed for polypropylene-based medical products.
- Biocompatible polypropylene for tissue engineering scaffolds: Polypropylene is engineered to serve as a biocompatible scaffold material for tissue engineering applications. These scaffolds are designed with specific porosity, mechanical properties, and surface characteristics to support cell growth, differentiation, and tissue formation. The material may be further functionalized to enhance cell attachment and promote tissue regeneration.
02 Biocompatible polypropylene composites
Polypropylene is combined with other biocompatible materials to create composite structures with enhanced properties. These composites may incorporate natural fibers, bioactive agents, or other polymers to improve mechanical strength, biodegradability, or specific biological interactions. Such composites find applications in tissue engineering and medical device manufacturing.Expand Specific Solutions03 Polypropylene-based biomedical implants
Biocompatible polypropylene is used to create various medical implants, including surgical meshes, sutures, and scaffolds for tissue regeneration. These implants are designed to minimize foreign body reactions while providing necessary mechanical support. Innovations focus on optimizing the material's properties for specific anatomical locations and functions.Expand Specific Solutions04 Sterilization methods for biocompatible polypropylene
Developing effective sterilization techniques for polypropylene medical devices is crucial to maintain biocompatibility and material integrity. Methods such as ethylene oxide treatment, gamma irradiation, and novel plasma-based approaches are explored to ensure sterility without compromising the material's properties or biocompatibility.Expand Specific Solutions05 Biocompatible polypropylene in drug delivery systems
Polypropylene is utilized in the development of drug delivery systems due to its biocompatibility and versatile processing capabilities. These systems include microparticles, nanofibers, and controlled-release devices that can be tailored to deliver therapeutic agents over extended periods. The material's properties are optimized to enhance drug loading, release kinetics, and overall efficacy.Expand Specific Solutions
Key Players in Biocompatible PP and Medical Implant Industry
The biocompatible polypropylene market for medical implants is in a growth phase, driven by increasing demand for advanced medical devices. The market size is expanding due to rising healthcare expenditure and an aging population. Technologically, the field is advancing rapidly, with companies like Tepha, Inc. and Medtronic Vascular, Inc. leading innovation in biocompatible materials. Tepha's focus on polyhydroxyalkanoates and Medtronic's extensive product range demonstrate the industry's maturity. Research institutions such as Wuhan University of Technology and the Agency for Science, Technology & Research are contributing to technological advancements, indicating a collaborative ecosystem between academia and industry. The competitive landscape is diverse, with both established medical device manufacturers and specialized biomaterial companies vying for market share.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has developed a biocompatible polypropylene-based material for medical implants, particularly focusing on cardiovascular applications. Their approach involves surface modification of polypropylene to enhance its biocompatibility and reduce the risk of inflammation or rejection. The company has implemented a proprietary coating technology that incorporates anti-thrombogenic agents into the polypropylene surface, significantly reducing the risk of blood clot formation on implanted devices [1]. Additionally, Medtronic has explored the use of nanostructured polypropylene surfaces to promote endothelialization, which can improve the long-term performance and integration of cardiovascular implants [3].
Strengths: Enhanced biocompatibility, reduced thrombogenicity, improved long-term performance. Weaknesses: Potential for increased manufacturing costs, need for extensive clinical trials to validate long-term safety and efficacy.
Covidien Pte Ltd.
Technical Solution: Covidien has developed a novel approach to utilizing biocompatible polypropylene in medical implants, particularly for hernia repair and soft tissue reconstruction. Their technology involves creating a composite mesh that combines polypropylene with absorbable materials, providing both strength and biocompatibility. The company's proprietary manufacturing process results in a lightweight, macroporous structure that promotes rapid tissue ingrowth and reduces the risk of infection [2]. Covidien has also incorporated anti-adhesion coatings on one side of their polypropylene meshes to prevent unwanted tissue attachment, particularly important in intraperitoneal applications [4]. Recent developments include the integration of antimicrobial agents into the polypropylene fibers to further reduce the risk of post-operative infections [5].
Strengths: Improved tissue integration, reduced risk of adhesions and infections, versatile applications. Weaknesses: Higher production costs, potential for long-term foreign body response in some patients.
Key Innovations in Biocompatible PP for Medical Use
Biocompatible Polymers for Coating or Fabricating Implantable Medical Devices
PatentActiveUS20100247597A1
Innovation
- Development of biocompatible polymers comprising hydroxyalkyl methacrylate and acrylate monomers, specifically 4-hydroxybutyl methacrylate and hexyl methacrylate, for coating medical devices, which provide controlled drug release and improved biocompatibility, reducing the risk of adverse reactions.
Biocompatible polymers for coating or fabricating implantable medical devices
PatentInactiveEP2413987A2
Innovation
- Development of biocompatible polymers comprising hydroxyalkyl methacrylate and acrylate monomers, such as 4-hydroxybutyl methacrylate and hexyl methacrylate, for coating or fabricating implantable medical devices, which provide controlled drug release and improved biocompatibility by minimizing immune reactions and cytotoxicity.
Regulatory Framework for Biocompatible Medical Implants
The regulatory framework for biocompatible medical implants is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of devices using materials like biocompatible polypropylene. At the forefront of this framework is the U.S. Food and Drug Administration (FDA), which has established stringent guidelines for the development, testing, and approval of medical implants.
The FDA's regulatory process for medical implants typically involves a premarket approval (PMA) application, which requires extensive clinical data to demonstrate the safety and effectiveness of the device. For biocompatible polypropylene implants, manufacturers must provide comprehensive documentation on the material's properties, biocompatibility testing results, and long-term performance data.
In addition to the FDA, international regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have their own sets of regulations and approval processes. These agencies often collaborate and harmonize their standards to facilitate global market access for medical implants.
The International Organization for Standardization (ISO) has developed several standards specifically addressing the biocompatibility of medical devices, including ISO 10993. This standard provides a framework for evaluating the biological safety of medical devices, including those made from biocompatible polypropylene. Manufacturers must demonstrate compliance with these standards as part of the regulatory approval process.
Regulatory requirements also extend to the manufacturing processes of biocompatible polypropylene implants. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of the regulatory framework, ensuring consistent production of high-quality, safe devices.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to monitor the performance of their implants after market release and report any adverse events or complications to the relevant regulatory authorities. This ongoing surveillance helps identify potential long-term issues and informs future regulatory decisions.
As the field of biocompatible materials advances, regulatory agencies continually update their guidelines to address new technologies and emerging safety concerns. This dynamic nature of the regulatory framework necessitates ongoing engagement between manufacturers, researchers, and regulatory bodies to ensure that biocompatible polypropylene implants meet the highest standards of safety and efficacy.
The FDA's regulatory process for medical implants typically involves a premarket approval (PMA) application, which requires extensive clinical data to demonstrate the safety and effectiveness of the device. For biocompatible polypropylene implants, manufacturers must provide comprehensive documentation on the material's properties, biocompatibility testing results, and long-term performance data.
In addition to the FDA, international regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have their own sets of regulations and approval processes. These agencies often collaborate and harmonize their standards to facilitate global market access for medical implants.
The International Organization for Standardization (ISO) has developed several standards specifically addressing the biocompatibility of medical devices, including ISO 10993. This standard provides a framework for evaluating the biological safety of medical devices, including those made from biocompatible polypropylene. Manufacturers must demonstrate compliance with these standards as part of the regulatory approval process.
Regulatory requirements also extend to the manufacturing processes of biocompatible polypropylene implants. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of the regulatory framework, ensuring consistent production of high-quality, safe devices.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to monitor the performance of their implants after market release and report any adverse events or complications to the relevant regulatory authorities. This ongoing surveillance helps identify potential long-term issues and informs future regulatory decisions.
As the field of biocompatible materials advances, regulatory agencies continually update their guidelines to address new technologies and emerging safety concerns. This dynamic nature of the regulatory framework necessitates ongoing engagement between manufacturers, researchers, and regulatory bodies to ensure that biocompatible polypropylene implants meet the highest standards of safety and efficacy.
Long-term Safety and Efficacy Studies of Biocompatible PP Implants
Long-term safety and efficacy studies of biocompatible polypropylene (PP) implants are crucial for understanding the impact of this material on medical devices and patient outcomes. These studies typically span several years, allowing researchers to assess the durability, biocompatibility, and potential long-term effects of PP implants in various medical applications.
One of the primary focuses of these studies is the evaluation of tissue integration and host response over extended periods. Researchers examine the interface between the PP implant and surrounding tissues, monitoring for signs of inflammation, fibrosis, or adverse reactions. This involves periodic imaging studies, histological analyses, and immunological assessments to track changes in tissue architecture and cellular responses.
The mechanical properties of PP implants are also closely monitored throughout the study duration. This includes assessing the material's strength, flexibility, and resistance to degradation under physiological conditions. Researchers employ various mechanical testing methods, such as tensile strength tests and fatigue analysis, to determine if the implants maintain their structural integrity and functionality over time.
Biocompatibility is a critical aspect of these long-term studies. Scientists evaluate the potential for PP implants to elicit allergic reactions, promote infection, or induce systemic toxicity. This involves comprehensive blood work, immunological profiling, and microbiological assessments to detect any signs of adverse effects on the host's immune system or overall health.
The studies also focus on the potential for PP implants to affect surrounding tissues and organs. This includes examining any changes in local blood flow, tissue oxygenation, or organ function that may be attributed to the presence of the implant. Advanced imaging techniques, such as MRI and CT scans, are often employed to visualize the implant's position and its interaction with adjacent anatomical structures.
Patient-reported outcomes are an integral part of these long-term studies. Researchers collect data on pain levels, quality of life, and functional improvements associated with the PP implants. This information is crucial for assessing the overall efficacy of the implants and their impact on patient well-being over extended periods.
Additionally, these studies often include a comparative analysis with other implant materials or treatment modalities. This helps in positioning biocompatible PP implants within the broader context of medical interventions, highlighting their potential advantages or limitations compared to alternative options.
One of the primary focuses of these studies is the evaluation of tissue integration and host response over extended periods. Researchers examine the interface between the PP implant and surrounding tissues, monitoring for signs of inflammation, fibrosis, or adverse reactions. This involves periodic imaging studies, histological analyses, and immunological assessments to track changes in tissue architecture and cellular responses.
The mechanical properties of PP implants are also closely monitored throughout the study duration. This includes assessing the material's strength, flexibility, and resistance to degradation under physiological conditions. Researchers employ various mechanical testing methods, such as tensile strength tests and fatigue analysis, to determine if the implants maintain their structural integrity and functionality over time.
Biocompatibility is a critical aspect of these long-term studies. Scientists evaluate the potential for PP implants to elicit allergic reactions, promote infection, or induce systemic toxicity. This involves comprehensive blood work, immunological profiling, and microbiological assessments to detect any signs of adverse effects on the host's immune system or overall health.
The studies also focus on the potential for PP implants to affect surrounding tissues and organs. This includes examining any changes in local blood flow, tissue oxygenation, or organ function that may be attributed to the presence of the implant. Advanced imaging techniques, such as MRI and CT scans, are often employed to visualize the implant's position and its interaction with adjacent anatomical structures.
Patient-reported outcomes are an integral part of these long-term studies. Researchers collect data on pain levels, quality of life, and functional improvements associated with the PP implants. This information is crucial for assessing the overall efficacy of the implants and their impact on patient well-being over extended periods.
Additionally, these studies often include a comparative analysis with other implant materials or treatment modalities. This helps in positioning biocompatible PP implants within the broader context of medical interventions, highlighting their potential advantages or limitations compared to alternative options.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!