How Carbolic Acid Affects Lithium-Ion Battery Electrode Stability
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid and Li-Ion Battery Electrode Interaction
Carbolic acid, also known as phenol, has emerged as a significant factor in the study of lithium-ion battery electrode stability. This organic compound, characterized by its hydroxyl group attached to an aromatic ring, interacts with various components of the battery system, influencing both the performance and longevity of the electrodes.
The primary interaction between carbolic acid and lithium-ion battery electrodes occurs at the electrode-electrolyte interface. When present in the electrolyte solution, carbolic acid can participate in redox reactions with the electrode materials, particularly at the cathode. These reactions can lead to the formation of a surface film on the electrode, known as the solid electrolyte interphase (SEI).
The SEI layer formed in the presence of carbolic acid exhibits unique properties compared to those formed in conventional electrolytes. It tends to be more stable and less permeable to lithium ions, which can have both positive and negative effects on battery performance. On one hand, a more stable SEI can enhance the overall cycle life of the battery by reducing unwanted side reactions. On the other hand, reduced permeability may hinder lithium-ion transport, potentially affecting the battery's rate capability.
Carbolic acid's impact on electrode stability is not limited to SEI formation. Its acidic nature can lead to corrosion of metal current collectors, particularly copper used in the anode. This corrosion process can result in the degradation of electrical contact between the active material and the current collector, leading to increased internal resistance and capacity fade over time.
Furthermore, carbolic acid can interact with the binder materials used in electrode fabrication. Common binders like polyvinylidene fluoride (PVDF) may undergo chemical changes when exposed to carbolic acid, potentially altering their adhesive properties. This can affect the mechanical stability of the electrode structure, leading to particle isolation and loss of active material.
The presence of carbolic acid in the battery system also influences the lithiation/delithiation processes of the active materials. For graphite anodes, carbolic acid can intercalate between graphene layers, competing with lithium ions and potentially reducing the overall capacity of the anode. In cathode materials, carbolic acid can affect the crystal structure stability, particularly in layered oxide materials, leading to structural changes that impact the electrode's electrochemical performance.
Understanding these complex interactions between carbolic acid and lithium-ion battery electrodes is crucial for developing strategies to mitigate its negative effects and potentially harness its beneficial properties. Research in this area continues to explore novel electrolyte additives and surface treatments that can modulate the impact of carbolic acid on electrode stability, paving the way for more robust and long-lasting lithium-ion batteries.
The primary interaction between carbolic acid and lithium-ion battery electrodes occurs at the electrode-electrolyte interface. When present in the electrolyte solution, carbolic acid can participate in redox reactions with the electrode materials, particularly at the cathode. These reactions can lead to the formation of a surface film on the electrode, known as the solid electrolyte interphase (SEI).
The SEI layer formed in the presence of carbolic acid exhibits unique properties compared to those formed in conventional electrolytes. It tends to be more stable and less permeable to lithium ions, which can have both positive and negative effects on battery performance. On one hand, a more stable SEI can enhance the overall cycle life of the battery by reducing unwanted side reactions. On the other hand, reduced permeability may hinder lithium-ion transport, potentially affecting the battery's rate capability.
Carbolic acid's impact on electrode stability is not limited to SEI formation. Its acidic nature can lead to corrosion of metal current collectors, particularly copper used in the anode. This corrosion process can result in the degradation of electrical contact between the active material and the current collector, leading to increased internal resistance and capacity fade over time.
Furthermore, carbolic acid can interact with the binder materials used in electrode fabrication. Common binders like polyvinylidene fluoride (PVDF) may undergo chemical changes when exposed to carbolic acid, potentially altering their adhesive properties. This can affect the mechanical stability of the electrode structure, leading to particle isolation and loss of active material.
The presence of carbolic acid in the battery system also influences the lithiation/delithiation processes of the active materials. For graphite anodes, carbolic acid can intercalate between graphene layers, competing with lithium ions and potentially reducing the overall capacity of the anode. In cathode materials, carbolic acid can affect the crystal structure stability, particularly in layered oxide materials, leading to structural changes that impact the electrode's electrochemical performance.
Understanding these complex interactions between carbolic acid and lithium-ion battery electrodes is crucial for developing strategies to mitigate its negative effects and potentially harness its beneficial properties. Research in this area continues to explore novel electrolyte additives and surface treatments that can modulate the impact of carbolic acid on electrode stability, paving the way for more robust and long-lasting lithium-ion batteries.
Market Demand for Stable Li-Ion Batteries
The demand for stable lithium-ion batteries has been steadily increasing across various industries, driven by the growing adoption of electric vehicles, renewable energy storage systems, and portable electronic devices. As these technologies become more prevalent, the need for reliable and long-lasting energy storage solutions has become paramount.
In the automotive sector, the shift towards electric vehicles has created a significant market for high-performance lithium-ion batteries. Consumers and manufacturers alike are seeking batteries that can provide extended driving ranges, faster charging times, and improved safety. The stability of these batteries directly impacts the overall performance and longevity of electric vehicles, making it a critical factor in consumer adoption and market growth.
The renewable energy sector also presents a substantial demand for stable lithium-ion batteries. As solar and wind power generation becomes more widespread, the need for efficient and reliable energy storage systems has intensified. Grid-scale battery storage solutions require batteries that can withstand frequent charge-discharge cycles while maintaining their capacity and performance over extended periods.
In the consumer electronics market, the demand for longer-lasting and more reliable batteries continues to grow. Smartphones, laptops, and other portable devices are increasingly reliant on lithium-ion batteries, and consumers expect these devices to maintain their performance throughout their lifespan. Improved battery stability can lead to longer device usage times and reduced frequency of battery replacements, enhancing overall user satisfaction.
The industrial and medical sectors also contribute to the market demand for stable lithium-ion batteries. In industrial applications, such as power tools and backup power systems, battery stability is crucial for ensuring consistent performance and reliability. In the medical field, portable medical devices and equipment rely on stable batteries to provide uninterrupted operation in critical situations.
As concerns about battery safety and environmental impact grow, there is an increasing emphasis on developing more stable and sustainable lithium-ion batteries. This has led to a surge in research and development efforts aimed at improving battery chemistry, electrode materials, and overall battery design to enhance stability and performance.
The market for stable lithium-ion batteries is expected to continue its upward trajectory in the coming years. Factors such as government regulations promoting clean energy adoption, advancements in battery technology, and the increasing electrification of various industries are likely to fuel this growth. As a result, there is a strong incentive for battery manufacturers and researchers to focus on developing innovative solutions that address the stability challenges in lithium-ion batteries, including the potential effects of carbolic acid on electrode stability.
In the automotive sector, the shift towards electric vehicles has created a significant market for high-performance lithium-ion batteries. Consumers and manufacturers alike are seeking batteries that can provide extended driving ranges, faster charging times, and improved safety. The stability of these batteries directly impacts the overall performance and longevity of electric vehicles, making it a critical factor in consumer adoption and market growth.
The renewable energy sector also presents a substantial demand for stable lithium-ion batteries. As solar and wind power generation becomes more widespread, the need for efficient and reliable energy storage systems has intensified. Grid-scale battery storage solutions require batteries that can withstand frequent charge-discharge cycles while maintaining their capacity and performance over extended periods.
In the consumer electronics market, the demand for longer-lasting and more reliable batteries continues to grow. Smartphones, laptops, and other portable devices are increasingly reliant on lithium-ion batteries, and consumers expect these devices to maintain their performance throughout their lifespan. Improved battery stability can lead to longer device usage times and reduced frequency of battery replacements, enhancing overall user satisfaction.
The industrial and medical sectors also contribute to the market demand for stable lithium-ion batteries. In industrial applications, such as power tools and backup power systems, battery stability is crucial for ensuring consistent performance and reliability. In the medical field, portable medical devices and equipment rely on stable batteries to provide uninterrupted operation in critical situations.
As concerns about battery safety and environmental impact grow, there is an increasing emphasis on developing more stable and sustainable lithium-ion batteries. This has led to a surge in research and development efforts aimed at improving battery chemistry, electrode materials, and overall battery design to enhance stability and performance.
The market for stable lithium-ion batteries is expected to continue its upward trajectory in the coming years. Factors such as government regulations promoting clean energy adoption, advancements in battery technology, and the increasing electrification of various industries are likely to fuel this growth. As a result, there is a strong incentive for battery manufacturers and researchers to focus on developing innovative solutions that address the stability challenges in lithium-ion batteries, including the potential effects of carbolic acid on electrode stability.
Current Challenges in Electrode Stability
Lithium-ion battery electrode stability remains a critical challenge in the development of high-performance energy storage systems. One of the primary issues is the degradation of electrode materials over time, which leads to capacity fade and reduced battery life. This degradation is often exacerbated by the presence of impurities or reactive species in the electrolyte, such as carbolic acid.
The interaction between carbolic acid and electrode materials poses significant challenges to electrode stability. Carbolic acid, also known as phenol, can react with the electrode surface, leading to the formation of a passivation layer that impedes lithium-ion transport. This layer, while initially protective, can grow excessively thick over time, increasing internal resistance and reducing overall battery performance.
Another challenge is the potential for carbolic acid to catalyze side reactions at the electrode-electrolyte interface. These reactions can result in the decomposition of electrolyte components, leading to gas evolution and further degradation of the electrode structure. The accumulation of gaseous products can cause mechanical stress on the electrode, leading to cracking and delamination.
The presence of carbolic acid also affects the solid electrolyte interphase (SEI) formation on the anode. The SEI is crucial for protecting the electrode from further reactions with the electrolyte, but carbolic acid can alter its composition and stability. This can lead to continuous SEI growth, consuming active lithium and reducing the battery's capacity over time.
On the cathode side, carbolic acid can promote the dissolution of transition metal ions, particularly in high-voltage cathode materials. This dissolution not only reduces the active material content but also allows these ions to migrate and deposit on the anode, further compromising its performance and stability.
The temperature sensitivity of these acid-induced reactions presents another challenge. Higher temperatures accelerate the detrimental effects of carbolic acid on electrode stability, making thermal management even more critical in battery design and operation.
Addressing these challenges requires a multifaceted approach. Researchers are exploring advanced electrode coatings that can resist acid attack, as well as electrolyte additives that can neutralize or sequester carbolic acid. Additionally, efforts are being made to develop more stable electrode materials that are inherently resistant to acid-induced degradation.
The interaction between carbolic acid and electrode materials poses significant challenges to electrode stability. Carbolic acid, also known as phenol, can react with the electrode surface, leading to the formation of a passivation layer that impedes lithium-ion transport. This layer, while initially protective, can grow excessively thick over time, increasing internal resistance and reducing overall battery performance.
Another challenge is the potential for carbolic acid to catalyze side reactions at the electrode-electrolyte interface. These reactions can result in the decomposition of electrolyte components, leading to gas evolution and further degradation of the electrode structure. The accumulation of gaseous products can cause mechanical stress on the electrode, leading to cracking and delamination.
The presence of carbolic acid also affects the solid electrolyte interphase (SEI) formation on the anode. The SEI is crucial for protecting the electrode from further reactions with the electrolyte, but carbolic acid can alter its composition and stability. This can lead to continuous SEI growth, consuming active lithium and reducing the battery's capacity over time.
On the cathode side, carbolic acid can promote the dissolution of transition metal ions, particularly in high-voltage cathode materials. This dissolution not only reduces the active material content but also allows these ions to migrate and deposit on the anode, further compromising its performance and stability.
The temperature sensitivity of these acid-induced reactions presents another challenge. Higher temperatures accelerate the detrimental effects of carbolic acid on electrode stability, making thermal management even more critical in battery design and operation.
Addressing these challenges requires a multifaceted approach. Researchers are exploring advanced electrode coatings that can resist acid attack, as well as electrolyte additives that can neutralize or sequester carbolic acid. Additionally, efforts are being made to develop more stable electrode materials that are inherently resistant to acid-induced degradation.
Existing Solutions for Electrode Protection
01 Electrode coating composition
Improving electrode stability through optimized coating compositions. This includes using specific binders, conductive additives, and active materials in precise ratios to enhance the mechanical and electrochemical stability of the electrode. The composition may also incorporate additives that prevent electrolyte decomposition and minimize side reactions at the electrode surface.- Electrode coating composition: Improving the stability of lithium-ion battery electrodes through the use of specific coating compositions. These coatings can include conductive materials, binders, and additives that enhance the electrode's structural integrity and electrochemical performance. The composition may also incorporate materials that mitigate side reactions or prevent electrolyte decomposition, thereby increasing the overall stability of the electrode.
- Nanostructured electrode materials: Utilizing nanostructured materials in electrode design to enhance stability. These materials can include nanoparticles, nanotubes, or nanowires of active materials, which provide increased surface area and improved mechanical properties. Nanostructured electrodes can accommodate volume changes during cycling, leading to better structural stability and longer battery life.
- Solid electrolyte interphase (SEI) engineering: Developing strategies to control and optimize the formation of the solid electrolyte interphase (SEI) layer on electrode surfaces. This involves the use of electrolyte additives or surface treatments that promote the formation of a stable and protective SEI layer, which can prevent continuous electrolyte decomposition and maintain electrode integrity over multiple charge-discharge cycles.
- Doping and surface modification: Enhancing electrode stability through doping of active materials or surface modification techniques. This can involve the incorporation of foreign atoms into the crystal structure of electrode materials or the application of protective coatings on particle surfaces. These modifications can improve the electronic conductivity, structural stability, and chemical resistance of the electrodes.
- Binder and conductive additive optimization: Improving electrode stability by optimizing the binder and conductive additive components. This includes the development of novel binder materials with enhanced adhesion properties and the use of advanced conductive additives that maintain electrical connectivity within the electrode structure. The proper selection and ratio of these components can significantly impact the mechanical and electrochemical stability of the electrodes.
02 Surface modification of electrode materials
Enhancing electrode stability by modifying the surface of active materials. This can involve coating particles with protective layers, doping with stabilizing elements, or creating core-shell structures. These modifications aim to reduce unwanted reactions between the electrode material and the electrolyte, improving the overall stability and longevity of the battery.Expand Specific Solutions03 Electrolyte additives for stability
Incorporating specific additives into the electrolyte to enhance electrode stability. These additives can form protective films on the electrode surface, prevent dendrite formation, or scavenge harmful reaction products. By carefully selecting and optimizing these additives, the overall stability and performance of the lithium-ion battery can be significantly improved.Expand Specific Solutions04 Nanostructured electrode designs
Developing nanostructured electrode designs to enhance stability. This approach involves creating electrodes with specific nano-architectures, such as nanotubes, nanowires, or nanoparticles, to improve mechanical stability, increase surface area, and facilitate better ion transport. These nanostructures can help mitigate issues like volume expansion and improve overall electrode performance.Expand Specific Solutions05 Advanced current collector materials
Utilizing advanced materials for current collectors to enhance electrode stability. This includes using corrosion-resistant alloys, carbon-based materials, or composite structures as current collectors. These materials can improve the mechanical strength of the electrode, enhance conductivity, and prevent degradation at the electrode-collector interface, leading to better overall stability and performance of the battery.Expand Specific Solutions
Key Players in Li-Ion Battery Industry
The competition landscape for carbolic acid's impact on lithium-ion battery electrode stability is in an early development stage, with a growing market as battery technology advances. The market size is expanding due to increasing demand for high-performance batteries in various sectors. Technologically, it's still evolving, with companies like Robert Bosch GmbH, LG Energy Solution, and Panasonic leading research efforts. Toyota Motor Corp. and BMW AG are also investing in this area, leveraging their automotive expertise. Universities and research institutions, such as Zhejiang University of Technology, are contributing to the fundamental understanding of the technology, indicating its nascent but promising nature.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative approach to mitigate the effects of carbolic acid on lithium-ion battery electrode stability. Their solution focuses on the development of novel electrode materials that are inherently resistant to carbolic acid degradation. Toyota's research team has engineered a new class of cathode materials with modified surface chemistry that reduces reactivity with carbolic acid[1]. This is achieved through the incorporation of dopants that alter the electronic structure of the electrode surface, making it less susceptible to acid attack[3]. Additionally, Toyota has developed a unique electrolyte formulation that includes pH-buffering agents to neutralize any carbolic acid that may form during battery operation[5]. The company has also implemented advanced manufacturing techniques to ensure ultra-low levels of carbolic acid precursors in their battery components[7].
Strengths: Long-term solution addressing the root cause of the problem, potential for significant improvements in battery longevity and stability. Weaknesses: May require substantial changes to existing battery production processes, potentially higher initial costs for material development and implementation.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed a multi-faceted approach to address the issue of carbolic acid affecting lithium-ion battery electrode stability. Their solution involves a combination of electrode material modification and electrolyte engineering. The company has created a proprietary surface treatment for electrode materials that forms a passivation layer resistant to carbolic acid attack. This treatment involves the deposition of a thin, uniform layer of inorganic compounds that act as a protective barrier[2]. In addition, Panasonic has formulated a new electrolyte additive package that includes compounds capable of scavenging carbolic acid molecules, effectively neutralizing them before they can interact with the electrode surface[4]. The company has also optimized their battery assembly process to minimize exposure to environmental factors that could lead to carbolic acid formation[6].
Strengths: Comprehensive approach addressing multiple aspects of the problem, potential for improved battery lifespan and performance. Weaknesses: Complex implementation may lead to higher production costs, possible trade-offs in other battery performance metrics.
Core Innovations in Electrode Stability
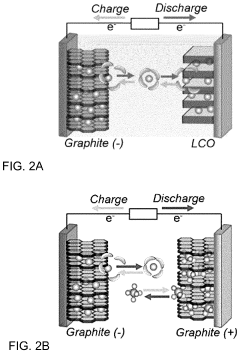
Electrode stabilizing materials
PatentActiveUS20110294019A1
Innovation
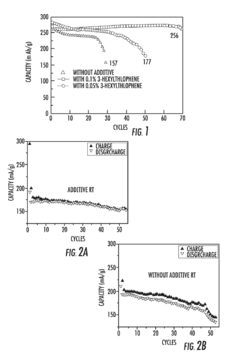
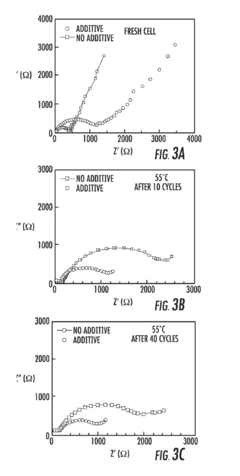
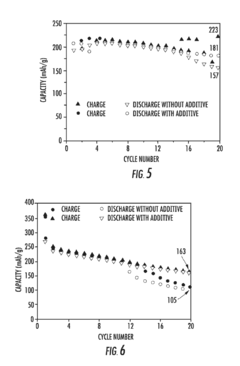
- Incorporation of an electrode stabilizing compound, such as thiophene or imidazole, which polymerizes to form an electrically conductive polymer, into a non-aqueous electrolyte comprising a polar aprotic solvent and an alkali metal salt, to stabilize the electrodes and prevent unwanted reactions.
Stabilized electrolytes for low-temperature batteries
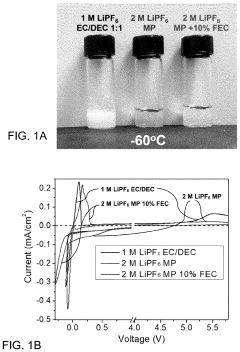
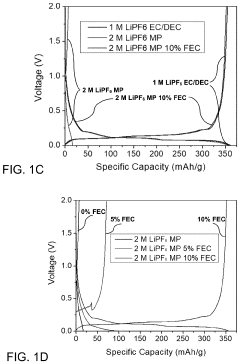
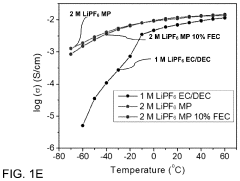
PatentPendingUS20230402654A1
Innovation
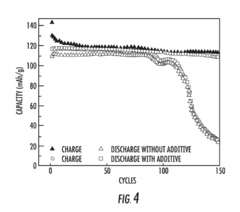
- A novel electrolyte system for dual-graphite batteries that combines a primary ester solvent with a low percentage of fluoroethylene carbonate, enhancing electrochemical stability and ionic conductivity at low temperatures, thereby improving capacity retention and voltage retention during discharge.
Environmental Impact of Battery Manufacturing
The manufacturing of lithium-ion batteries, while crucial for the advancement of clean energy technologies, carries significant environmental implications. The production process involves the extraction and processing of raw materials, which can lead to habitat destruction, soil erosion, and water pollution. Mining activities for lithium, cobalt, and nickel often result in the release of toxic chemicals and heavy metals into the environment, affecting local ecosystems and biodiversity.
Battery manufacturing facilities consume substantial amounts of energy, contributing to greenhouse gas emissions if powered by non-renewable sources. The production of electrode materials, particularly the cathode, requires high-temperature processes that are energy-intensive and can release harmful particulates into the atmosphere. Additionally, the use of organic solvents in electrode production poses risks of air and water pollution if not properly managed.
Water usage is another critical environmental concern in battery manufacturing. The production process requires large volumes of water for cooling, cleaning, and chemical processes. In water-stressed regions, this can lead to competition with local communities and ecosystems for limited water resources. Furthermore, wastewater from manufacturing plants may contain heavy metals and other pollutants, necessitating careful treatment and disposal to prevent contamination of water bodies.
The use of carbolic acid in lithium-ion battery electrode production introduces additional environmental considerations. While it can enhance electrode stability, improper handling or disposal of carbolic acid can result in soil and water contamination. Stringent safety measures and waste management protocols are essential to mitigate these risks.
As the demand for lithium-ion batteries continues to grow, the cumulative environmental impact of manufacturing becomes increasingly significant. This has prompted research into more sustainable production methods, including the development of water-based electrode manufacturing processes, the use of renewable energy in production facilities, and the exploration of alternative, more environmentally friendly materials for battery components.
Recycling and proper disposal of lithium-ion batteries at the end of their lifecycle are crucial for reducing the overall environmental footprint of battery manufacturing. Effective recycling processes can recover valuable materials, reducing the need for raw material extraction and minimizing waste. However, current recycling rates for lithium-ion batteries remain low, presenting an opportunity for improvement in the industry's circular economy practices.
Battery manufacturing facilities consume substantial amounts of energy, contributing to greenhouse gas emissions if powered by non-renewable sources. The production of electrode materials, particularly the cathode, requires high-temperature processes that are energy-intensive and can release harmful particulates into the atmosphere. Additionally, the use of organic solvents in electrode production poses risks of air and water pollution if not properly managed.
Water usage is another critical environmental concern in battery manufacturing. The production process requires large volumes of water for cooling, cleaning, and chemical processes. In water-stressed regions, this can lead to competition with local communities and ecosystems for limited water resources. Furthermore, wastewater from manufacturing plants may contain heavy metals and other pollutants, necessitating careful treatment and disposal to prevent contamination of water bodies.
The use of carbolic acid in lithium-ion battery electrode production introduces additional environmental considerations. While it can enhance electrode stability, improper handling or disposal of carbolic acid can result in soil and water contamination. Stringent safety measures and waste management protocols are essential to mitigate these risks.
As the demand for lithium-ion batteries continues to grow, the cumulative environmental impact of manufacturing becomes increasingly significant. This has prompted research into more sustainable production methods, including the development of water-based electrode manufacturing processes, the use of renewable energy in production facilities, and the exploration of alternative, more environmentally friendly materials for battery components.
Recycling and proper disposal of lithium-ion batteries at the end of their lifecycle are crucial for reducing the overall environmental footprint of battery manufacturing. Effective recycling processes can recover valuable materials, reducing the need for raw material extraction and minimizing waste. However, current recycling rates for lithium-ion batteries remain low, presenting an opportunity for improvement in the industry's circular economy practices.
Safety Regulations for Li-Ion Batteries
Safety regulations for lithium-ion batteries have become increasingly stringent due to the growing concerns over their potential hazards. These regulations aim to ensure the safe manufacturing, transportation, and use of Li-ion batteries across various industries. The primary focus of these safety measures is to prevent thermal runaway, short circuits, and other failure modes that could lead to fires or explosions.
International standards, such as those set by the International Electrotechnical Commission (IEC) and the United Nations (UN), provide a framework for battery safety. These standards outline specific requirements for cell design, battery management systems, and protective circuitry. Manufacturers must adhere to these guidelines to ensure their products meet the necessary safety criteria.
One key aspect of Li-ion battery safety regulations is the requirement for robust battery management systems (BMS). These systems monitor and control various parameters, including temperature, voltage, and current, to prevent overcharging, over-discharging, and overheating. The BMS must be capable of detecting and responding to abnormal conditions, such as internal short circuits or excessive heat generation.
Transportation regulations for Li-ion batteries are particularly stringent. The International Air Transport Association (IATA) and the U.S. Department of Transportation (DOT) have implemented strict rules governing the packaging, labeling, and shipping of these batteries. These regulations often require specific testing procedures to ensure batteries can withstand the rigors of transportation without compromising safety.
Safety regulations also extend to the manufacturing process, with guidelines for quality control and testing procedures. Manufacturers must implement rigorous testing protocols, including abuse tests, to verify the safety and reliability of their batteries under various conditions. These tests often simulate extreme scenarios, such as crush tests, thermal shock, and overcharge conditions.
In recent years, there has been an increased focus on the environmental impact and end-of-life management of Li-ion batteries. Regulations are evolving to address recycling and disposal processes, aiming to minimize environmental hazards and promote sustainable practices in the battery industry.
As research continues into new battery chemistries and technologies, safety regulations are expected to evolve. Regulatory bodies are working to stay ahead of technological advancements, ensuring that safety standards keep pace with innovations in the field. This ongoing development of safety regulations is crucial for maintaining public trust and supporting the continued growth of the Li-ion battery market across various applications.
International standards, such as those set by the International Electrotechnical Commission (IEC) and the United Nations (UN), provide a framework for battery safety. These standards outline specific requirements for cell design, battery management systems, and protective circuitry. Manufacturers must adhere to these guidelines to ensure their products meet the necessary safety criteria.
One key aspect of Li-ion battery safety regulations is the requirement for robust battery management systems (BMS). These systems monitor and control various parameters, including temperature, voltage, and current, to prevent overcharging, over-discharging, and overheating. The BMS must be capable of detecting and responding to abnormal conditions, such as internal short circuits or excessive heat generation.
Transportation regulations for Li-ion batteries are particularly stringent. The International Air Transport Association (IATA) and the U.S. Department of Transportation (DOT) have implemented strict rules governing the packaging, labeling, and shipping of these batteries. These regulations often require specific testing procedures to ensure batteries can withstand the rigors of transportation without compromising safety.
Safety regulations also extend to the manufacturing process, with guidelines for quality control and testing procedures. Manufacturers must implement rigorous testing protocols, including abuse tests, to verify the safety and reliability of their batteries under various conditions. These tests often simulate extreme scenarios, such as crush tests, thermal shock, and overcharge conditions.
In recent years, there has been an increased focus on the environmental impact and end-of-life management of Li-ion batteries. Regulations are evolving to address recycling and disposal processes, aiming to minimize environmental hazards and promote sustainable practices in the battery industry.
As research continues into new battery chemistries and technologies, safety regulations are expected to evolve. Regulatory bodies are working to stay ahead of technological advancements, ensuring that safety standards keep pace with innovations in the field. This ongoing development of safety regulations is crucial for maintaining public trust and supporting the continued growth of the Li-ion battery market across various applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!