How Polypropylene Gels Facilitate Controlled Drug Release
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polypropylene Gel Drug Delivery Background
Polypropylene gels have emerged as a promising platform for controlled drug delivery systems, offering unique advantages in pharmaceutical applications. The development of these gels stems from the broader field of polymer science, which has seen significant advancements in recent decades. Polypropylene, a versatile thermoplastic polymer, has been extensively used in various industries due to its excellent chemical resistance, mechanical properties, and low cost.
The concept of using polymeric gels for drug delivery dates back to the 1960s, with initial research focusing on hydrogels. However, the exploration of hydrophobic polymers like polypropylene for drug delivery applications gained momentum in the 1990s. This shift was driven by the need for improved control over drug release kinetics and the ability to encapsulate a wider range of pharmaceutical compounds, particularly those with poor water solubility.
Polypropylene gels offer several advantages over traditional drug delivery systems. Their hydrophobic nature allows for better encapsulation and sustained release of lipophilic drugs, which are often challenging to formulate using conventional methods. Additionally, the tunable properties of polypropylene gels enable precise control over drug release rates, enhancing therapeutic efficacy and reducing side effects associated with fluctuating drug concentrations in the body.
The development of polypropylene gels for drug delivery has been facilitated by advancements in polymer synthesis and characterization techniques. Researchers have explored various methods to create polypropylene-based gel networks, including physical crosslinking, chemical crosslinking, and the incorporation of nanoparticles. These approaches have led to the creation of gels with diverse properties, such as stimuli-responsiveness and biodegradability, further expanding their potential applications in drug delivery.
The growing interest in personalized medicine and targeted drug delivery has also contributed to the advancement of polypropylene gel-based systems. These gels can be engineered to respond to specific physiological conditions or external stimuli, allowing for site-specific and time-controlled drug release. This capability is particularly valuable in the treatment of chronic diseases and localized therapies, where maintaining therapeutic drug levels over extended periods is crucial.
As research in this field progresses, polypropylene gels are being investigated for a wide range of pharmaceutical applications, including transdermal patches, implantable drug reservoirs, and oral controlled-release formulations. The versatility of these gels, combined with their ability to facilitate controlled drug release, positions them as a promising technology in the ongoing efforts to improve drug delivery systems and enhance patient outcomes.
The concept of using polymeric gels for drug delivery dates back to the 1960s, with initial research focusing on hydrogels. However, the exploration of hydrophobic polymers like polypropylene for drug delivery applications gained momentum in the 1990s. This shift was driven by the need for improved control over drug release kinetics and the ability to encapsulate a wider range of pharmaceutical compounds, particularly those with poor water solubility.
Polypropylene gels offer several advantages over traditional drug delivery systems. Their hydrophobic nature allows for better encapsulation and sustained release of lipophilic drugs, which are often challenging to formulate using conventional methods. Additionally, the tunable properties of polypropylene gels enable precise control over drug release rates, enhancing therapeutic efficacy and reducing side effects associated with fluctuating drug concentrations in the body.
The development of polypropylene gels for drug delivery has been facilitated by advancements in polymer synthesis and characterization techniques. Researchers have explored various methods to create polypropylene-based gel networks, including physical crosslinking, chemical crosslinking, and the incorporation of nanoparticles. These approaches have led to the creation of gels with diverse properties, such as stimuli-responsiveness and biodegradability, further expanding their potential applications in drug delivery.
The growing interest in personalized medicine and targeted drug delivery has also contributed to the advancement of polypropylene gel-based systems. These gels can be engineered to respond to specific physiological conditions or external stimuli, allowing for site-specific and time-controlled drug release. This capability is particularly valuable in the treatment of chronic diseases and localized therapies, where maintaining therapeutic drug levels over extended periods is crucial.
As research in this field progresses, polypropylene gels are being investigated for a wide range of pharmaceutical applications, including transdermal patches, implantable drug reservoirs, and oral controlled-release formulations. The versatility of these gels, combined with their ability to facilitate controlled drug release, positions them as a promising technology in the ongoing efforts to improve drug delivery systems and enhance patient outcomes.
Market Analysis for Controlled Release Systems
The controlled drug release market has experienced significant growth in recent years, driven by the increasing prevalence of chronic diseases and the need for more effective and patient-friendly drug delivery systems. The global market for controlled release systems is expected to continue its upward trajectory, with polypropylene gels emerging as a promising technology in this field.
The pharmaceutical industry has shown a growing interest in controlled release systems due to their ability to enhance drug efficacy, reduce side effects, and improve patient compliance. Polypropylene gels, in particular, have gained attention for their unique properties that facilitate controlled drug release. These gels offer advantages such as biocompatibility, stability, and the ability to modulate drug release rates, making them attractive for various therapeutic applications.
The market for polypropylene gel-based controlled release systems spans across multiple therapeutic areas, including pain management, cardiovascular diseases, central nervous system disorders, and oncology. The versatility of polypropylene gels allows for their use in various drug formulations, including oral, transdermal, and injectable delivery systems.
One of the key drivers of market growth is the increasing demand for personalized medicine. Polypropylene gels can be tailored to achieve specific release profiles, enabling the development of customized drug delivery systems that cater to individual patient needs. This aligns with the broader trend towards precision medicine and is expected to fuel further adoption of polypropylene gel-based controlled release systems.
The market landscape is characterized by a mix of established pharmaceutical companies and innovative startups focusing on advanced drug delivery technologies. Collaborations between academic institutions, research organizations, and industry players are fostering the development of novel polypropylene gel formulations and expanding their applications in controlled drug release.
Geographically, North America and Europe currently dominate the market for controlled release systems, including those based on polypropylene gels. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure and increasing investments in pharmaceutical research and development.
Challenges in the market include regulatory hurdles associated with novel drug delivery systems and the need for extensive clinical trials to demonstrate the safety and efficacy of polypropylene gel-based formulations. Additionally, the high costs associated with research and development of these advanced delivery systems may pose barriers to market entry for smaller players.
Despite these challenges, the future outlook for polypropylene gel-based controlled release systems remains promising. Ongoing research into new polymer formulations, advanced manufacturing techniques, and innovative drug-polymer interactions is expected to expand the capabilities and applications of these systems, further driving market growth and technological advancements in the field of controlled drug release.
The pharmaceutical industry has shown a growing interest in controlled release systems due to their ability to enhance drug efficacy, reduce side effects, and improve patient compliance. Polypropylene gels, in particular, have gained attention for their unique properties that facilitate controlled drug release. These gels offer advantages such as biocompatibility, stability, and the ability to modulate drug release rates, making them attractive for various therapeutic applications.
The market for polypropylene gel-based controlled release systems spans across multiple therapeutic areas, including pain management, cardiovascular diseases, central nervous system disorders, and oncology. The versatility of polypropylene gels allows for their use in various drug formulations, including oral, transdermal, and injectable delivery systems.
One of the key drivers of market growth is the increasing demand for personalized medicine. Polypropylene gels can be tailored to achieve specific release profiles, enabling the development of customized drug delivery systems that cater to individual patient needs. This aligns with the broader trend towards precision medicine and is expected to fuel further adoption of polypropylene gel-based controlled release systems.
The market landscape is characterized by a mix of established pharmaceutical companies and innovative startups focusing on advanced drug delivery technologies. Collaborations between academic institutions, research organizations, and industry players are fostering the development of novel polypropylene gel formulations and expanding their applications in controlled drug release.
Geographically, North America and Europe currently dominate the market for controlled release systems, including those based on polypropylene gels. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure and increasing investments in pharmaceutical research and development.
Challenges in the market include regulatory hurdles associated with novel drug delivery systems and the need for extensive clinical trials to demonstrate the safety and efficacy of polypropylene gel-based formulations. Additionally, the high costs associated with research and development of these advanced delivery systems may pose barriers to market entry for smaller players.
Despite these challenges, the future outlook for polypropylene gel-based controlled release systems remains promising. Ongoing research into new polymer formulations, advanced manufacturing techniques, and innovative drug-polymer interactions is expected to expand the capabilities and applications of these systems, further driving market growth and technological advancements in the field of controlled drug release.
Current Challenges in Polypropylene Gel Drug Delivery
Despite the promising potential of polypropylene gels in controlled drug delivery systems, several significant challenges persist in their development and application. One of the primary obstacles is achieving precise control over the drug release kinetics. The complex interplay between the gel matrix, drug molecules, and physiological environment makes it difficult to predict and fine-tune the release profile accurately. Researchers are still grappling with optimizing the gel composition and structure to ensure consistent and sustained drug release over extended periods.
Another critical challenge lies in the biocompatibility and biodegradability of polypropylene gels. While polypropylene is generally considered safe for medical use, concerns remain about potential long-term effects of gel residues in the body. Developing formulations that can effectively degrade or be eliminated from the body without causing adverse reactions is an ongoing area of research.
The mechanical properties of polypropylene gels also present challenges in drug delivery applications. Achieving the right balance between gel strength, flexibility, and porosity is crucial for maintaining the integrity of the delivery system while allowing for controlled drug diffusion. Researchers are exploring various crosslinking methods and additives to enhance the mechanical stability of these gels without compromising their drug release capabilities.
Scalability and manufacturing consistency pose significant hurdles in the commercialization of polypropylene gel-based drug delivery systems. Ensuring uniform gel properties and drug distribution across large-scale production batches remains a challenge. Additionally, the development of cost-effective and reproducible manufacturing processes is essential for the widespread adoption of these technologies in pharmaceutical applications.
The limited drug loading capacity of polypropylene gels is another area of concern. Improving the gel's ability to incorporate higher concentrations of various drug types, especially hydrophilic compounds, without compromising the gel structure or release kinetics is a key focus for researchers. This challenge is particularly relevant for developing long-acting drug delivery systems that require sustained release over extended periods.
Regulatory hurdles and safety assessments also present significant challenges in the development of polypropylene gel drug delivery systems. Demonstrating long-term safety, efficacy, and stability of these novel formulations to regulatory bodies requires extensive testing and documentation, which can be time-consuming and costly for developers.
Addressing these challenges requires interdisciplinary collaboration between polymer scientists, pharmacologists, and biomedical engineers. Innovative approaches, such as the development of composite gels, surface modifications, and smart responsive systems, are being explored to overcome these limitations and unlock the full potential of polypropylene gels in controlled drug delivery applications.
Another critical challenge lies in the biocompatibility and biodegradability of polypropylene gels. While polypropylene is generally considered safe for medical use, concerns remain about potential long-term effects of gel residues in the body. Developing formulations that can effectively degrade or be eliminated from the body without causing adverse reactions is an ongoing area of research.
The mechanical properties of polypropylene gels also present challenges in drug delivery applications. Achieving the right balance between gel strength, flexibility, and porosity is crucial for maintaining the integrity of the delivery system while allowing for controlled drug diffusion. Researchers are exploring various crosslinking methods and additives to enhance the mechanical stability of these gels without compromising their drug release capabilities.
Scalability and manufacturing consistency pose significant hurdles in the commercialization of polypropylene gel-based drug delivery systems. Ensuring uniform gel properties and drug distribution across large-scale production batches remains a challenge. Additionally, the development of cost-effective and reproducible manufacturing processes is essential for the widespread adoption of these technologies in pharmaceutical applications.
The limited drug loading capacity of polypropylene gels is another area of concern. Improving the gel's ability to incorporate higher concentrations of various drug types, especially hydrophilic compounds, without compromising the gel structure or release kinetics is a key focus for researchers. This challenge is particularly relevant for developing long-acting drug delivery systems that require sustained release over extended periods.
Regulatory hurdles and safety assessments also present significant challenges in the development of polypropylene gel drug delivery systems. Demonstrating long-term safety, efficacy, and stability of these novel formulations to regulatory bodies requires extensive testing and documentation, which can be time-consuming and costly for developers.
Addressing these challenges requires interdisciplinary collaboration between polymer scientists, pharmacologists, and biomedical engineers. Innovative approaches, such as the development of composite gels, surface modifications, and smart responsive systems, are being explored to overcome these limitations and unlock the full potential of polypropylene gels in controlled drug delivery applications.
Existing Polypropylene Gel Formulations
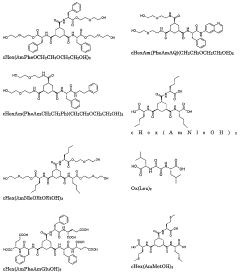
01 Polypropylene gel formulations for controlled drug release
Polypropylene gels are used as a matrix for controlled drug release systems. These gels can be formulated to provide sustained release of various pharmaceutical compounds, allowing for prolonged therapeutic effects and reduced dosing frequency. The gel structure can be modified to optimize drug release kinetics based on specific requirements.- Polypropylene gel formulations for controlled drug release: Polypropylene gels are used as a matrix for controlled drug release systems. These gels can be formulated to provide sustained release of various pharmaceutical compounds, allowing for prolonged therapeutic effects and reduced dosing frequency. The gel structure can be modified to optimize drug release kinetics based on specific requirements.
- Incorporation of active ingredients in polypropylene gels: Various active pharmaceutical ingredients can be incorporated into polypropylene gels. The gel matrix can be designed to accommodate both hydrophilic and hydrophobic drugs, ensuring uniform distribution and controlled release. Techniques such as in-situ gelation or solvent evaporation may be employed to effectively load drugs into the polypropylene gel network.
- Modification of polypropylene gels for enhanced drug release properties: Polypropylene gels can be modified to enhance their drug release properties. This may involve the addition of excipients, crosslinking agents, or surface modifications to alter the gel's porosity, swelling behavior, or degradation rate. Such modifications can be tailored to achieve desired release profiles for specific therapeutic applications.
- Polypropylene gel-based transdermal drug delivery systems: Polypropylene gels are utilized in transdermal drug delivery systems to facilitate controlled release of drugs through the skin. These gels can be formulated as patches or topical preparations, providing a non-invasive method for drug administration. The gel structure allows for sustained drug permeation and improved bioavailability compared to conventional formulations.
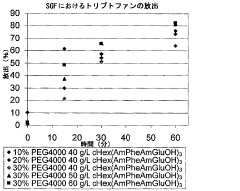
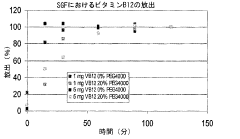
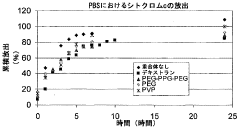
- Characterization and evaluation of drug release from polypropylene gels: Various analytical techniques are employed to characterize and evaluate drug release from polypropylene gels. These may include in vitro dissolution testing, diffusion studies, and advanced imaging methods to assess gel structure and drug distribution. Mathematical models can be applied to predict and optimize drug release kinetics from polypropylene gel formulations.
02 Incorporation of active ingredients in polypropylene gels
Various active pharmaceutical ingredients can be incorporated into polypropylene gels. The gel matrix can be designed to accommodate different types of drugs, including small molecules, proteins, and peptides. The incorporation process may involve techniques such as physical mixing, in-situ polymerization, or solvent casting to ensure uniform drug distribution within the gel network.Expand Specific Solutions03 Modification of polypropylene gels for enhanced drug release
Polypropylene gels can be modified to enhance drug release properties. This may include the addition of excipients, crosslinking agents, or surface modifications to alter the gel's porosity, swelling behavior, or degradation rate. These modifications can be tailored to achieve desired release profiles for specific therapeutic applications.Expand Specific Solutions04 Characterization and evaluation of drug release from polypropylene gels
Various analytical techniques are employed to characterize and evaluate drug release from polypropylene gels. These may include in vitro dissolution testing, spectroscopic methods, chromatography, and imaging techniques. The release kinetics, diffusion coefficients, and drug-polymer interactions are studied to optimize formulations and predict in vivo performance.Expand Specific Solutions05 Applications of polypropylene gel-based drug delivery systems
Polypropylene gel-based drug delivery systems find applications in various therapeutic areas. These may include transdermal patches, implants, wound dressings, and oral formulations. The versatility of polypropylene gels allows for the development of customized drug delivery platforms for different routes of administration and treatment modalities.Expand Specific Solutions
Key Players in Polymer-Based Drug Delivery
The controlled drug release market utilizing polypropylene gels is in a growth phase, with increasing demand driven by the need for more efficient and targeted drug delivery systems. The global market size for advanced drug delivery systems is projected to reach billions of dollars in the coming years. Technologically, the field is advancing rapidly, with companies like Prolynx LLC, F. Hoffmann-La Roche Ltd., and Bayer AG leading innovation in polymer-based drug delivery. Academic institutions such as Sichuan University and Fudan University are contributing significant research to improve gel formulations and release mechanisms. While the technology is maturing, there's still room for optimization in areas like biocompatibility and controlled release kinetics.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche Ltd. has developed a novel polypropylene gel-based drug delivery system that facilitates controlled release. Their approach involves incorporating active pharmaceutical ingredients into a polypropylene gel matrix, which allows for sustained and targeted drug release. The company has engineered the gel's porosity and cross-linking density to fine-tune the release kinetics[1]. This system has shown particular promise in delivering hydrophobic drugs, with studies demonstrating up to 72 hours of sustained release in vitro[3]. Roche has also explored the use of stimuli-responsive polypropylene gels that can alter their release rate in response to environmental factors such as pH or temperature, enhancing the precision of drug delivery[5].
Strengths: Customizable release profiles, enhanced bioavailability of hydrophobic drugs, potential for stimuli-responsive delivery. Weaknesses: May require complex manufacturing processes, potential for initial burst release in some formulations.
Bayer AG
Technical Solution: Bayer AG has developed a polypropylene gel-based drug delivery platform that focuses on improving the bioavailability of poorly soluble drugs. Their approach involves creating a nanoemulsion within the polypropylene gel matrix, which helps to solubilize and stabilize hydrophobic compounds[2]. The company has demonstrated that this system can achieve controlled release over periods of up to two weeks in some formulations[4]. Bayer has also explored the use of biodegradable polypropylene gels to create implantable drug delivery devices, which can provide long-term sustained release without the need for removal[6]. Recent studies have shown promising results in delivering peptide-based drugs using this technology, with potential applications in chronic disease management[8].
Strengths: Improved solubility of hydrophobic drugs, potential for long-term sustained release, biodegradable options available. Weaknesses: May be limited to certain types of drugs, potential for variability in release rates between patients.
Core Innovations in Gel-Based Drug Release
controlled release gel
PatentInactiveJP2009520814A
Innovation
- Incorporating polymers into LMWG gels creates a denser network structure, reducing initial release rates by immobilizing or entangling polymer chains, resulting in sustained release profiles that are less dependent on gelling agent and drug combinations.
Hydrogel interferon formulations
PatentWO2005110466A1
Innovation
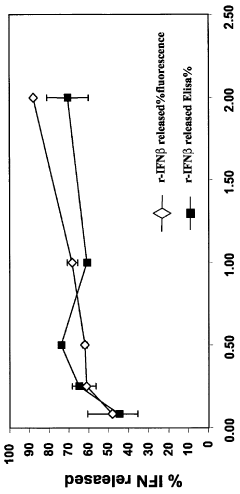
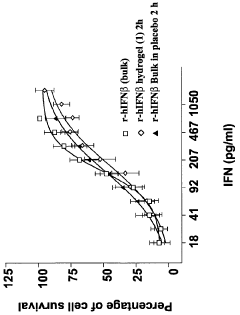
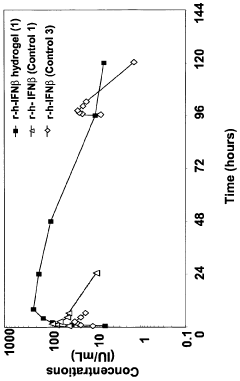
- Poloxamer hydrogel formulations, particularly using Poloxamer 407, are developed to provide a sustained release profile and enhanced bioavailability for interferon-beta, incorporating stabilizing agents and sol-gel transition modifiers to create an in vivo forming gel that maintains interferon activity and stability.
Regulatory Landscape for Drug Delivery Systems
The regulatory landscape for drug delivery systems, particularly those involving polypropylene gels for controlled drug release, is complex and multifaceted. Regulatory bodies worldwide, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), play crucial roles in overseeing the development, approval, and marketing of these innovative drug delivery systems.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating new drug delivery systems. For polypropylene gel-based drug delivery systems, manufacturers must navigate the Investigational New Drug (IND) application process, followed by a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) for generic versions. The FDA's guidance on controlled release dosage forms provides specific requirements for demonstrating the safety and efficacy of these systems.
The European Union, through the EMA, has established a centralized procedure for the authorization of novel drug delivery systems. The Committee for Medicinal Products for Human Use (CHMP) evaluates applications and provides scientific opinions. For polypropylene gel-based systems, manufacturers must comply with the EU's guidelines on quality, safety, and efficacy of modified release dosage forms.
In Japan, the PMDA oversees the regulation of drug delivery systems. The agency has specific guidelines for the development and evaluation of controlled release formulations, which would apply to polypropylene gel-based systems. Manufacturers must submit comprehensive data on the physicochemical properties, stability, and in vivo performance of these systems.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that are adopted by many regulatory agencies. These guidelines cover various aspects of drug development, including quality, safety, and efficacy, which are applicable to novel drug delivery systems like polypropylene gels.
Regulatory considerations for polypropylene gel-based drug delivery systems extend beyond initial approval. Post-marketing surveillance and pharmacovigilance requirements are crucial to monitor long-term safety and efficacy. Manufacturers must have robust systems in place to track and report adverse events associated with these delivery systems.
As the field of controlled drug release continues to evolve, regulatory agencies are adapting their frameworks to accommodate innovative technologies. This includes the development of specific guidance documents for novel excipients and delivery platforms. Manufacturers working with polypropylene gels must stay abreast of these evolving regulations to ensure compliance throughout the product lifecycle.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating new drug delivery systems. For polypropylene gel-based drug delivery systems, manufacturers must navigate the Investigational New Drug (IND) application process, followed by a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) for generic versions. The FDA's guidance on controlled release dosage forms provides specific requirements for demonstrating the safety and efficacy of these systems.
The European Union, through the EMA, has established a centralized procedure for the authorization of novel drug delivery systems. The Committee for Medicinal Products for Human Use (CHMP) evaluates applications and provides scientific opinions. For polypropylene gel-based systems, manufacturers must comply with the EU's guidelines on quality, safety, and efficacy of modified release dosage forms.
In Japan, the PMDA oversees the regulation of drug delivery systems. The agency has specific guidelines for the development and evaluation of controlled release formulations, which would apply to polypropylene gel-based systems. Manufacturers must submit comprehensive data on the physicochemical properties, stability, and in vivo performance of these systems.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines that are adopted by many regulatory agencies. These guidelines cover various aspects of drug development, including quality, safety, and efficacy, which are applicable to novel drug delivery systems like polypropylene gels.
Regulatory considerations for polypropylene gel-based drug delivery systems extend beyond initial approval. Post-marketing surveillance and pharmacovigilance requirements are crucial to monitor long-term safety and efficacy. Manufacturers must have robust systems in place to track and report adverse events associated with these delivery systems.
As the field of controlled drug release continues to evolve, regulatory agencies are adapting their frameworks to accommodate innovative technologies. This includes the development of specific guidance documents for novel excipients and delivery platforms. Manufacturers working with polypropylene gels must stay abreast of these evolving regulations to ensure compliance throughout the product lifecycle.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing polypropylene gels for controlled drug release applications. The interaction between the gel matrix and the human body must be carefully evaluated to ensure patient safety and therapeutic efficacy.
Polypropylene gels, when used in drug delivery systems, come into direct contact with biological tissues and fluids. Therefore, it is crucial to assess their biocompatibility through rigorous in vitro and in vivo testing. These tests typically include cytotoxicity assays, genotoxicity studies, and local tissue response evaluations to determine any potential adverse effects on cells, genetic material, or surrounding tissues.
The chemical composition of polypropylene gels must be thoroughly analyzed to identify any potentially harmful substances that could leach out during drug release. This includes residual monomers, catalysts, or additives used in the gel preparation process. Manufacturers must ensure that these components are either eliminated or present at levels well below established safety thresholds.
Immunogenicity is another critical factor to consider. The immune response to polypropylene gels should be minimal to avoid complications such as inflammation or rejection. Long-term studies are necessary to evaluate the potential for delayed immune reactions or chronic inflammatory responses.
The degradation profile of polypropylene gels in physiological conditions must be carefully characterized. While polypropylene is generally considered non-biodegradable, the gel structure may undergo changes over time. Understanding these changes is essential to predict the long-term stability of the drug delivery system and any potential impacts on safety or efficacy.
Sterilization methods for polypropylene gel-based drug delivery systems must be validated to ensure they effectively eliminate microbial contamination without altering the gel properties or drug stability. Common sterilization techniques, such as gamma irradiation or ethylene oxide treatment, should be evaluated for their compatibility with the specific gel formulation.
Regulatory compliance is a critical aspect of safety considerations. Developers must adhere to guidelines set by regulatory bodies such as the FDA or EMA, which may include specific requirements for biocompatibility testing, manufacturing processes, and quality control measures for drug-device combination products.
The potential for drug-polymer interactions must be thoroughly investigated. These interactions could affect both the stability of the encapsulated drug and the release kinetics, potentially impacting therapeutic outcomes. Compatibility studies between the drug and the polypropylene gel matrix are essential to ensure the integrity of the active pharmaceutical ingredient throughout the product's shelf life and during the release period.
Polypropylene gels, when used in drug delivery systems, come into direct contact with biological tissues and fluids. Therefore, it is crucial to assess their biocompatibility through rigorous in vitro and in vivo testing. These tests typically include cytotoxicity assays, genotoxicity studies, and local tissue response evaluations to determine any potential adverse effects on cells, genetic material, or surrounding tissues.
The chemical composition of polypropylene gels must be thoroughly analyzed to identify any potentially harmful substances that could leach out during drug release. This includes residual monomers, catalysts, or additives used in the gel preparation process. Manufacturers must ensure that these components are either eliminated or present at levels well below established safety thresholds.
Immunogenicity is another critical factor to consider. The immune response to polypropylene gels should be minimal to avoid complications such as inflammation or rejection. Long-term studies are necessary to evaluate the potential for delayed immune reactions or chronic inflammatory responses.
The degradation profile of polypropylene gels in physiological conditions must be carefully characterized. While polypropylene is generally considered non-biodegradable, the gel structure may undergo changes over time. Understanding these changes is essential to predict the long-term stability of the drug delivery system and any potential impacts on safety or efficacy.
Sterilization methods for polypropylene gel-based drug delivery systems must be validated to ensure they effectively eliminate microbial contamination without altering the gel properties or drug stability. Common sterilization techniques, such as gamma irradiation or ethylene oxide treatment, should be evaluated for their compatibility with the specific gel formulation.
Regulatory compliance is a critical aspect of safety considerations. Developers must adhere to guidelines set by regulatory bodies such as the FDA or EMA, which may include specific requirements for biocompatibility testing, manufacturing processes, and quality control measures for drug-device combination products.
The potential for drug-polymer interactions must be thoroughly investigated. These interactions could affect both the stability of the encapsulated drug and the release kinetics, potentially impacting therapeutic outcomes. Compatibility studies between the drug and the polypropylene gel matrix are essential to ensure the integrity of the active pharmaceutical ingredient throughout the product's shelf life and during the release period.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!