How Polypropylene Hydrogels Simplify Smart Medicine Delivery Systems
Polypropylene Hydrogels in Smart Medicine: Background and Objectives
Polypropylene hydrogels have emerged as a groundbreaking technology in the field of smart medicine delivery systems, revolutionizing the way drugs are administered and controlled within the human body. These innovative materials combine the versatility of polypropylene with the unique properties of hydrogels, creating a powerful platform for advanced drug delivery applications.
The development of polypropylene hydrogels can be traced back to the early 2000s when researchers began exploring the potential of combining synthetic polymers with hydrogel structures. This fusion aimed to address the limitations of traditional drug delivery methods, such as poor bioavailability, inconsistent release rates, and potential side effects due to systemic administration.
Over the past two decades, significant advancements have been made in the synthesis, characterization, and application of polypropylene hydrogels. These materials have demonstrated remarkable properties, including high water absorption capacity, excellent mechanical strength, and tunable degradation rates, making them ideal candidates for smart medicine delivery systems.
The primary objective of incorporating polypropylene hydrogels into smart medicine delivery systems is to achieve precise, controlled, and targeted drug release. By leveraging the unique characteristics of these materials, researchers aim to develop intelligent drug carriers that can respond to specific physiological stimuli, such as pH changes, temperature fluctuations, or the presence of certain enzymes.
One of the key advantages of polypropylene hydrogels in smart medicine delivery is their ability to simplify complex drug administration protocols. Traditional methods often require frequent dosing or invasive procedures, which can be burdensome for patients and healthcare providers. In contrast, polypropylene hydrogel-based systems offer the potential for sustained and controlled release, reducing the frequency of drug administration and improving patient compliance.
Furthermore, these advanced materials enable the development of multifunctional drug delivery platforms. By incorporating various active compounds, imaging agents, or sensing elements into the hydrogel matrix, researchers can create comprehensive therapeutic systems that not only deliver drugs but also monitor treatment progress and provide real-time feedback.
As we look towards the future, the integration of polypropylene hydrogels in smart medicine delivery systems holds immense promise for personalized and precision medicine. The ongoing research in this field aims to optimize the performance of these materials, enhance their biocompatibility, and expand their applications across a wide range of therapeutic areas, from cancer treatment to regenerative medicine.
Market Analysis for Smart Drug Delivery Systems
The smart drug delivery systems market is experiencing significant growth, driven by the increasing prevalence of chronic diseases, the need for targeted and controlled drug release, and advancements in nanotechnology and materials science. The global market for smart drug delivery systems is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages.
One of the key factors fueling market growth is the rising demand for personalized medicine and precision therapeutics. Smart drug delivery systems offer the potential to tailor treatments to individual patient needs, improving efficacy and reducing side effects. This aligns with the broader trend towards patient-centric healthcare and the push for more effective, targeted treatments.
The market is segmented by technology, including liposomes, nanoparticles, polymeric systems, and smart pills. Among these, nanoparticle-based systems are gaining significant traction due to their ability to overcome biological barriers and enhance drug bioavailability. The introduction of polypropylene hydrogels in smart medicine delivery systems represents a promising development in this space, offering potential advantages in terms of biocompatibility, controlled release, and ease of manufacturing.
Geographically, North America currently holds the largest market share, attributed to its advanced healthcare infrastructure, high R&D investments, and favorable regulatory environment. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare access, increasing chronic disease burden, and rising healthcare expenditure in countries like China and India.
The pharmaceutical and biotechnology industries are the primary end-users of smart drug delivery systems, with oncology emerging as a key application area. The ability of these systems to target cancer cells while minimizing damage to healthy tissues has led to increased adoption in cancer treatment protocols.
Despite the promising outlook, the market faces challenges such as high development costs, complex regulatory pathways, and concerns about long-term safety and efficacy. These factors may impact market growth and adoption rates, particularly in emerging economies.
Looking ahead, the integration of smart drug delivery systems with digital health technologies, such as wearable devices and artificial intelligence, is expected to create new opportunities for market expansion. This convergence could lead to more sophisticated, data-driven approaches to drug delivery and patient monitoring.
In conclusion, the market for smart drug delivery systems, including those utilizing polypropylene hydrogels, shows strong growth potential. The technology's ability to address unmet medical needs, coupled with ongoing research and development efforts, positions it as a key area of innovation in the pharmaceutical and healthcare industries.
Current Challenges in Hydrogel-Based Drug Delivery
Despite the promising potential of hydrogel-based drug delivery systems, several significant challenges persist in their development and implementation. One of the primary obstacles is achieving precise control over drug release kinetics. Hydrogels, by nature, exhibit complex swelling behaviors that can lead to unpredictable drug release profiles. This variability can result in suboptimal therapeutic outcomes, particularly for medications requiring strict dosage control.
Another critical challenge lies in maintaining the stability of hydrogels under physiological conditions. The human body's dynamic environment, with its fluctuating pH levels, enzymes, and mechanical stresses, can compromise the structural integrity of hydrogels. This instability may lead to premature degradation or unexpected changes in drug release rates, potentially causing ineffective treatment or adverse side effects.
The biocompatibility of hydrogels also presents ongoing concerns. While many hydrogels are generally considered biocompatible, long-term effects and potential immune responses to certain hydrogel materials remain areas of active research. Ensuring that hydrogels do not elicit unwanted biological reactions or interfere with normal physiological processes is crucial for their successful application in drug delivery.
Scalability and manufacturing consistency pose significant hurdles in the commercialization of hydrogel-based drug delivery systems. Producing hydrogels with uniform properties and drug loading at an industrial scale remains challenging. Variations in production processes can lead to batch-to-batch inconsistencies, affecting the reliability and efficacy of the final product.
Furthermore, the incorporation of active pharmaceutical ingredients (APIs) into hydrogels without compromising their stability or efficacy is a complex task. Some drugs may interact unfavorably with the hydrogel matrix, leading to reduced bioavailability or altered pharmacokinetics. Developing hydrogel formulations that can accommodate a wide range of drug types while maintaining their therapeutic properties is an ongoing challenge.
Lastly, the regulatory landscape for hydrogel-based drug delivery systems presents its own set of challenges. As relatively novel technologies, these systems often face rigorous scrutiny from regulatory bodies. Demonstrating long-term safety, efficacy, and quality consistency to meet regulatory standards can be a time-consuming and resource-intensive process, potentially slowing the path to market for innovative hydrogel-based therapies.
Existing Polypropylene Hydrogel Solutions for Drug Delivery
01 Molecular modeling and simulation of polypropylene hydrogels
Advanced computational techniques are used to model and simulate the behavior of polypropylene hydrogels at the molecular level. These methods help in understanding the structure, properties, and interactions of the hydrogels, allowing for more efficient design and optimization of materials for various applications.- Simplified modeling of polypropylene hydrogels: Advanced techniques for simplifying the modeling of polypropylene hydrogels, including computational methods to reduce complexity while maintaining accuracy. This approach enables more efficient simulation and analysis of hydrogel properties and behaviors.
- Optimization of polypropylene hydrogel synthesis: Methods for streamlining the synthesis process of polypropylene hydrogels, focusing on reducing steps and improving efficiency. This includes innovative techniques for crosslinking and polymerization that simplify production while maintaining desired properties.
- Simplified characterization techniques for hydrogels: Development of simplified methods for characterizing polypropylene hydrogels, including streamlined testing procedures for swelling ratio, mechanical properties, and chemical composition. These techniques aim to reduce time and resources required for analysis.
- Simplified application methods for polypropylene hydrogels: Innovative approaches to simplify the application of polypropylene hydrogels in various fields, such as biomedical engineering and environmental remediation. This includes user-friendly delivery systems and easy-to-use formulations for practical applications.
- Data visualization for polypropylene hydrogel research: Advanced data visualization techniques to simplify the interpretation and analysis of complex data related to polypropylene hydrogels. This includes the development of user-friendly interfaces and graphical representations to aid in research and development processes.
02 Simplified synthesis methods for polypropylene hydrogels
Researchers have developed streamlined processes for synthesizing polypropylene hydrogels, focusing on reducing complexity and improving efficiency. These methods may involve novel catalysts, reaction conditions, or precursor materials to simplify the production of hydrogels with desired properties.Expand Specific Solutions03 Characterization and analysis techniques for polypropylene hydrogels
Various analytical methods have been developed to characterize and evaluate the properties of polypropylene hydrogels. These techniques may include spectroscopic analysis, mechanical testing, and imaging methods to assess the structure, composition, and performance of the hydrogels.Expand Specific Solutions04 Applications of simplified polypropylene hydrogels
Simplified polypropylene hydrogels find applications in diverse fields such as biomedical engineering, drug delivery systems, and environmental remediation. The simplified structures and synthesis methods enable easier integration into various products and technologies.Expand Specific Solutions05 Optimization of polypropylene hydrogel properties
Research focuses on optimizing the properties of polypropylene hydrogels through various methods, including adjusting crosslinking density, incorporating additives, or modifying the polymer structure. These efforts aim to enhance characteristics such as mechanical strength, swelling behavior, and biocompatibility.Expand Specific Solutions
Key Players in Polypropylene Hydrogel Development
The development of polypropylene hydrogels for smart medicine delivery systems is in an early growth stage, with significant potential for market expansion. The global smart drug delivery market is projected to reach $91 billion by 2025, driven by increasing demand for targeted and controlled release therapies. While the technology is still evolving, several key players are making strides in this field. Academic institutions like Sichuan University, Rutgers, and Purdue Research Foundation are conducting foundational research, while companies such as Allergan and DURECT Corporation are focusing on practical applications. The involvement of diverse organizations, from universities to pharmaceutical firms, indicates a growing interest in this technology, though it is not yet fully mature or widely commercialized.
Sichuan University
Purdue Research Foundation
Core Innovations in Polypropylene Hydrogel Formulations
- The development of biodegradable polymer particle delivery compositions using polymers such as polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) that incorporate therapeutic agents into their backbone, allowing for time-controlled release through enzymatic and hydrolytic degradation, with specific chemical formulas and structures described.
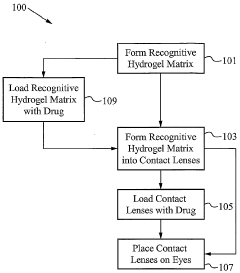
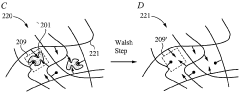
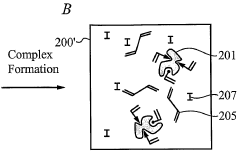
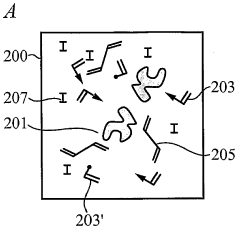
- The development of biomimetic recognitive polymeric hydrogel contact lenses that mimic biological recognition sites, allowing for enhanced drug loading and sustained release through complexing sites, using silicon-based or carbon-based cross-linking monomers and functional monomers that interact with bio-templates via electrostatic or hydrophobic interactions, enabling prolonged drug activity and reduced systemic absorption.
Regulatory Landscape for Hydrogel-Based Medical Devices
The regulatory landscape for hydrogel-based medical devices is complex and evolving, reflecting the innovative nature of these technologies and their potential impact on patient care. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of such devices. The FDA categorizes hydrogel-based medical devices based on their intended use and risk level, which determines the regulatory pathway they must follow.
For low-risk devices, such as certain wound dressings incorporating hydrogels, manufacturers may be able to pursue the 510(k) clearance process. This pathway requires demonstrating substantial equivalence to a predicate device already on the market. However, for more complex hydrogel-based systems, especially those involving drug delivery, the regulatory requirements are more stringent.
Novel hydrogel-based drug delivery systems often fall under the category of combination products, which involve both a device component and a drug component. These products are subject to additional scrutiny and may require a New Drug Application (NDA) or Biologics License Application (BLA) in addition to device-related submissions.
The European Union has its own regulatory framework for medical devices, governed by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations place a strong emphasis on clinical evidence and post-market surveillance, which can impact the development and approval process for hydrogel-based devices.
In both the US and EU, manufacturers must demonstrate the safety and efficacy of their hydrogel-based medical devices through rigorous clinical trials and quality management systems. This includes addressing potential risks such as biocompatibility, degradation, and unintended drug release.
Regulatory bodies are also increasingly focusing on the long-term safety and performance of implantable hydrogel-based devices. This has led to more stringent requirements for post-market surveillance and ongoing data collection to monitor device performance and patient outcomes over extended periods.
As the field of smart medicine delivery systems utilizing polypropylene hydrogels continues to advance, regulatory agencies are working to adapt their frameworks to keep pace with innovation. This includes developing new guidance documents and standards specific to hydrogel-based technologies and their unique characteristics.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of polypropylene hydrogels for smart medicine delivery systems. These advanced materials must undergo rigorous testing and evaluation to ensure their suitability for use in the human body.
One of the primary concerns is the potential for immune responses or inflammatory reactions when polypropylene hydrogels come into contact with biological tissues. Extensive in vitro and in vivo studies are necessary to assess the material's interaction with cells, proteins, and other biological components. These studies typically involve cytotoxicity assays, cell adhesion tests, and long-term implantation studies in animal models.
The degradation profile of polypropylene hydrogels is another critical factor to consider. As these materials are designed to release medications over time, it is essential to understand how they break down in the body and whether any degradation products could pose health risks. Researchers must carefully evaluate the hydrolysis and enzymatic degradation processes to ensure that all byproducts are non-toxic and can be safely eliminated from the body.
Mechanical properties of the hydrogels also play a role in their biocompatibility. The material should possess appropriate elasticity and strength to withstand physiological stresses without causing damage to surrounding tissues. Additionally, the hydrogel's swelling behavior in various biological environments must be thoroughly characterized to prevent any unintended complications due to excessive expansion or contraction.
The potential for microbial colonization on the hydrogel surface is another safety concern that requires attention. Antimicrobial properties may need to be incorporated into the hydrogel design to minimize the risk of infection, especially for long-term implantable devices.
Regulatory compliance is a crucial aspect of ensuring the safety of polypropylene hydrogels in medical applications. Developers must adhere to stringent guidelines set by regulatory bodies such as the FDA and EMA, which often require extensive preclinical and clinical trials before approval for human use.
Long-term effects of polypropylene hydrogels on the body must also be carefully monitored. This includes assessing the potential for chronic inflammation, fibrosis, or other adverse reactions that may not be immediately apparent in short-term studies. Post-market surveillance and long-term follow-up studies are essential to identify any unforeseen complications that may arise from prolonged use.
In conclusion, while polypropylene hydrogels offer promising solutions for smart medicine delivery systems, their successful implementation hinges on comprehensive biocompatibility and safety evaluations. Only through rigorous testing and continuous monitoring can these materials be confidently used to improve patient care and treatment outcomes.