How Sacrificial Anodes Avoid Over-Protection And Coating Blistering?
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cathodic Protection Background and Objectives
Cathodic protection has evolved significantly since its initial discovery in the early 19th century when Sir Humphry Davy successfully protected copper-clad naval vessels from corrosion using zinc and iron anodes. This groundbreaking work established the fundamental principles of sacrificial anode systems that continue to underpin modern corrosion prevention strategies. The technology has since expanded from maritime applications to become essential in protecting buried pipelines, storage tanks, offshore platforms, and various critical infrastructure components.
The core principle of cathodic protection involves creating an electrochemical cell where the metal structure becomes the cathode, thus preventing it from corroding. This is achieved either through impressed current systems or sacrificial anodes, with the latter being particularly valued for their simplicity and reliability in many applications. However, the evolution of this technology has revealed significant challenges, particularly regarding the delicate balance required to prevent both under-protection and over-protection.
Over-protection has emerged as a critical concern in modern cathodic protection systems, particularly when used in conjunction with protective coatings. When excessive cathodic current is applied, it can lead to hydrogen evolution at the protected surface, causing coating blistering, disbondment, and potentially accelerating corrosion in areas where the coating has been compromised. This phenomenon represents a significant technical challenge that has driven continuous innovation in the field.
The technical objectives in addressing over-protection and coating blistering focus on developing more precise control mechanisms for sacrificial anode systems. These include advanced anode materials with more predictable dissolution rates, improved design methodologies that account for varying environmental conditions, and sophisticated monitoring systems that can detect potential issues before they cause significant damage. The goal is to maintain protection potentials within the optimal range that prevents corrosion without generating excessive hydrogen.
Recent technological advancements have focused on "smart" cathodic protection systems that can automatically adjust protection levels based on real-time environmental and structural conditions. These systems represent the cutting edge of the field, incorporating sensors, data analytics, and sometimes AI-driven decision-making to optimize protection while minimizing adverse effects on coatings.
The industry continues to pursue research into the fundamental electrochemical processes at the metal-electrolyte interface, aiming to develop more comprehensive models that can predict the behavior of cathodic protection systems under various conditions. This research is essential for developing next-generation solutions that can provide reliable protection while avoiding the pitfalls of over-protection, particularly in increasingly complex and demanding applications.
The core principle of cathodic protection involves creating an electrochemical cell where the metal structure becomes the cathode, thus preventing it from corroding. This is achieved either through impressed current systems or sacrificial anodes, with the latter being particularly valued for their simplicity and reliability in many applications. However, the evolution of this technology has revealed significant challenges, particularly regarding the delicate balance required to prevent both under-protection and over-protection.
Over-protection has emerged as a critical concern in modern cathodic protection systems, particularly when used in conjunction with protective coatings. When excessive cathodic current is applied, it can lead to hydrogen evolution at the protected surface, causing coating blistering, disbondment, and potentially accelerating corrosion in areas where the coating has been compromised. This phenomenon represents a significant technical challenge that has driven continuous innovation in the field.
The technical objectives in addressing over-protection and coating blistering focus on developing more precise control mechanisms for sacrificial anode systems. These include advanced anode materials with more predictable dissolution rates, improved design methodologies that account for varying environmental conditions, and sophisticated monitoring systems that can detect potential issues before they cause significant damage. The goal is to maintain protection potentials within the optimal range that prevents corrosion without generating excessive hydrogen.
Recent technological advancements have focused on "smart" cathodic protection systems that can automatically adjust protection levels based on real-time environmental and structural conditions. These systems represent the cutting edge of the field, incorporating sensors, data analytics, and sometimes AI-driven decision-making to optimize protection while minimizing adverse effects on coatings.
The industry continues to pursue research into the fundamental electrochemical processes at the metal-electrolyte interface, aiming to develop more comprehensive models that can predict the behavior of cathodic protection systems under various conditions. This research is essential for developing next-generation solutions that can provide reliable protection while avoiding the pitfalls of over-protection, particularly in increasingly complex and demanding applications.
Market Analysis for Sacrificial Anode Systems
The global sacrificial anode systems market is experiencing steady growth, driven primarily by increasing infrastructure development in marine environments, oil and gas industries, and underground pipeline networks. Current market valuation stands at approximately 2.7 billion USD, with projections indicating a compound annual growth rate of 5.8% through 2028, according to recent industry analyses.
The market segmentation reveals distinct application sectors with varying growth trajectories. The offshore oil and gas segment currently dominates market share at roughly 38%, followed by shipping and marine applications at 27%, underground pipelines at 22%, and water treatment facilities at 13%. The remaining market share is distributed among various smaller applications including reinforced concrete structures and industrial equipment protection.
Geographically, the Asia-Pacific region leads market consumption, accounting for nearly 40% of global demand, with China and South Korea being the primary growth engines. North America follows at 25% market share, while Europe represents approximately 20% of the global market. The Middle East and Africa region, though currently smaller at 15%, is demonstrating the fastest growth rate due to extensive oil and gas infrastructure development.
A significant market trend is the increasing demand for advanced sacrificial anode systems that specifically address the over-protection and coating blistering issues. This demand is particularly pronounced in high-value offshore installations where coating damage can lead to substantial maintenance costs. Industry surveys indicate that approximately 30% of marine structure operators have reported coating blistering issues related to improper cathodic protection systems.
The competitive landscape features both established players and innovative newcomers. Traditional manufacturers focusing on zinc, aluminum, and magnesium anodes hold approximately 65% of the market, while companies offering integrated monitoring systems and controlled-potential solutions are gaining market share, currently at about 35% and growing at twice the rate of traditional segments.
Customer demand is increasingly shifting toward intelligent sacrificial anode systems that can self-regulate to prevent over-protection. Market research indicates willingness-to-pay premiums of up to 40% for systems that demonstrably reduce coating blistering while maintaining effective corrosion protection. This trend is particularly evident in marine and offshore applications where maintenance accessibility is limited and replacement costs are high.
Future market growth is expected to be driven by technological innovations addressing the precise control of protection potential, with particular emphasis on solutions that optimize the balance between adequate protection and avoiding hydrogen evolution that leads to coating damage.
The market segmentation reveals distinct application sectors with varying growth trajectories. The offshore oil and gas segment currently dominates market share at roughly 38%, followed by shipping and marine applications at 27%, underground pipelines at 22%, and water treatment facilities at 13%. The remaining market share is distributed among various smaller applications including reinforced concrete structures and industrial equipment protection.
Geographically, the Asia-Pacific region leads market consumption, accounting for nearly 40% of global demand, with China and South Korea being the primary growth engines. North America follows at 25% market share, while Europe represents approximately 20% of the global market. The Middle East and Africa region, though currently smaller at 15%, is demonstrating the fastest growth rate due to extensive oil and gas infrastructure development.
A significant market trend is the increasing demand for advanced sacrificial anode systems that specifically address the over-protection and coating blistering issues. This demand is particularly pronounced in high-value offshore installations where coating damage can lead to substantial maintenance costs. Industry surveys indicate that approximately 30% of marine structure operators have reported coating blistering issues related to improper cathodic protection systems.
The competitive landscape features both established players and innovative newcomers. Traditional manufacturers focusing on zinc, aluminum, and magnesium anodes hold approximately 65% of the market, while companies offering integrated monitoring systems and controlled-potential solutions are gaining market share, currently at about 35% and growing at twice the rate of traditional segments.
Customer demand is increasingly shifting toward intelligent sacrificial anode systems that can self-regulate to prevent over-protection. Market research indicates willingness-to-pay premiums of up to 40% for systems that demonstrably reduce coating blistering while maintaining effective corrosion protection. This trend is particularly evident in marine and offshore applications where maintenance accessibility is limited and replacement costs are high.
Future market growth is expected to be driven by technological innovations addressing the precise control of protection potential, with particular emphasis on solutions that optimize the balance between adequate protection and avoiding hydrogen evolution that leads to coating damage.
Current Challenges in Sacrificial Anode Technology
Despite significant advancements in cathodic protection systems, sacrificial anode technology continues to face several critical challenges that limit its effectiveness and efficiency. One of the most persistent issues is the difficulty in achieving optimal protection potential. When sacrificial anodes deliver excessive current, they can cause over-protection, leading to hydrogen evolution at the cathode surface. This phenomenon not only wastes protective current but also potentially damages protective coatings through hydrogen-induced blistering and disbondment.
The unpredictable performance of sacrificial anodes in varying environmental conditions presents another significant challenge. Factors such as water salinity, temperature fluctuations, and oxygen content can dramatically alter anode consumption rates and protection effectiveness. In freshwater or low-conductivity environments, the limited throwing power of sacrificial anodes often results in inadequate protection of remote structures, creating protection "shadows" where corrosion can accelerate.
Current anode materials face inherent limitations in their electrochemical properties. Zinc anodes, while effective in seawater, perform poorly in high-temperature environments and may become passive in certain conditions. Aluminum anodes, though offering higher capacity, can suffer from self-passivation issues that reduce their effectiveness over time. Magnesium anodes provide strong driving voltage but often deliver excessive current that accelerates their consumption and increases the risk of over-protection.
The design and placement of sacrificial anodes present ongoing engineering challenges. Achieving uniform current distribution across complex structures remains difficult, particularly for internally protected systems such as storage tanks and pipelines. Computational models for predicting optimal anode placement and consumption rates still lack sufficient accuracy in complex real-world scenarios.
Monitoring and maintenance of sacrificial anode systems pose practical challenges in field applications. Unlike impressed current systems, sacrificial anodes offer limited options for real-time monitoring and adjustment. Determining the remaining life of installed anodes typically requires physical inspection, which can be costly and disruptive, especially for submerged or buried structures.
Economic and sustainability concerns also present growing challenges. The rising costs of anode materials, particularly high-purity aluminum and zinc alloys, impact the cost-effectiveness of sacrificial anode systems for large-scale applications. Additionally, the environmental impact of anode dissolution products is receiving increased regulatory scrutiny, particularly in sensitive marine ecosystems.
The unpredictable performance of sacrificial anodes in varying environmental conditions presents another significant challenge. Factors such as water salinity, temperature fluctuations, and oxygen content can dramatically alter anode consumption rates and protection effectiveness. In freshwater or low-conductivity environments, the limited throwing power of sacrificial anodes often results in inadequate protection of remote structures, creating protection "shadows" where corrosion can accelerate.
Current anode materials face inherent limitations in their electrochemical properties. Zinc anodes, while effective in seawater, perform poorly in high-temperature environments and may become passive in certain conditions. Aluminum anodes, though offering higher capacity, can suffer from self-passivation issues that reduce their effectiveness over time. Magnesium anodes provide strong driving voltage but often deliver excessive current that accelerates their consumption and increases the risk of over-protection.
The design and placement of sacrificial anodes present ongoing engineering challenges. Achieving uniform current distribution across complex structures remains difficult, particularly for internally protected systems such as storage tanks and pipelines. Computational models for predicting optimal anode placement and consumption rates still lack sufficient accuracy in complex real-world scenarios.
Monitoring and maintenance of sacrificial anode systems pose practical challenges in field applications. Unlike impressed current systems, sacrificial anodes offer limited options for real-time monitoring and adjustment. Determining the remaining life of installed anodes typically requires physical inspection, which can be costly and disruptive, especially for submerged or buried structures.
Economic and sustainability concerns also present growing challenges. The rising costs of anode materials, particularly high-purity aluminum and zinc alloys, impact the cost-effectiveness of sacrificial anode systems for large-scale applications. Additionally, the environmental impact of anode dissolution products is receiving increased regulatory scrutiny, particularly in sensitive marine ecosystems.
Current Solutions for Over-Protection Prevention
01 Mechanisms of coating blistering due to cathodic over-protection
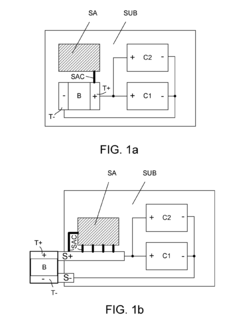
When sacrificial anodes provide excessive cathodic protection, hydrogen evolution can occur at the protected metal surface. This hydrogen gas can accumulate beneath protective coatings, causing them to blister and delaminate. The excessive negative potential from over-protection accelerates water electrolysis, producing hydrogen that cannot diffuse through the coating fast enough, resulting in pressure buildup and coating failure.- Mechanisms of coating blistering due to cathodic over-protection: Excessive cathodic protection from sacrificial anodes can lead to coating blistering through several mechanisms. When the protective potential becomes too negative, water molecules can be reduced to hydrogen at the metal surface. This hydrogen generation can cause pressure buildup beneath coatings, leading to delamination and blistering. Additionally, the high alkalinity created at the metal-coating interface due to hydroxyl ion formation can degrade certain coating types, further contributing to adhesion loss and blistering.
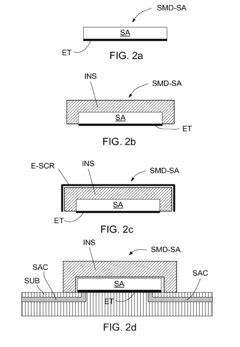
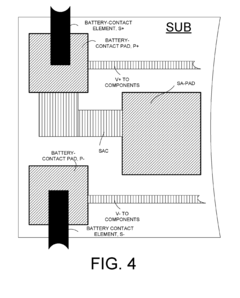
- Optimized anode design and placement to prevent over-protection: Proper design and strategic placement of sacrificial anodes are crucial to prevent over-protection. This includes calculating the optimal number, size, and distribution of anodes based on the structure's surface area and environmental conditions. Specialized anode geometries and configurations can ensure more uniform current distribution, preventing localized areas of excessive protection. Some systems incorporate distance-based placement strategies to maintain appropriate protection levels across the entire protected structure while avoiding over-protection near the anodes.
- Monitoring and control systems for cathodic protection: Advanced monitoring and control systems help prevent over-protection by continuously measuring protection potentials and adjusting current output accordingly. These systems can include reference electrodes, potential measurement devices, and automated control circuits that maintain protection within optimal ranges. Remote monitoring capabilities allow for real-time data collection and analysis, enabling prompt intervention when protection levels approach values that could cause coating damage. Some systems incorporate alarm features that activate when protection potentials exceed predetermined thresholds.
- Coating formulations resistant to cathodic disbondment: Specialized coating formulations have been developed to resist the effects of cathodic over-protection. These coatings incorporate alkali-resistant polymers and additives that maintain adhesion even in high-pH environments created by excessive cathodic protection. Some formulations include hydrogen-permeable components that allow generated hydrogen to dissipate rather than accumulate beneath the coating. Multi-layer systems with specific barrier properties can also help prevent the migration of water and hydroxyl ions to the metal-coating interface, reducing the risk of blistering.
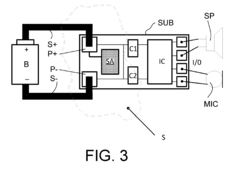
- Hybrid protection systems to balance protection levels: Hybrid protection systems combine sacrificial anodes with impressed current cathodic protection (ICCP) or other methods to achieve more precise control over protection potentials. These systems can incorporate adjustable resistors or diodes to limit current from sacrificial anodes when protection levels approach the over-protection threshold. Some designs include sacrificial anodes with self-regulating properties that reduce current output as the protection potential becomes more negative. By balancing different protection methods, these systems maintain adequate corrosion prevention while minimizing the risk of coating damage from over-protection.
02 Control systems for optimizing cathodic protection levels
Advanced control systems can be implemented to monitor and regulate the potential provided by sacrificial anodes, preventing over-protection. These systems typically include reference electrodes, potential monitoring devices, and control circuits that can adjust the current output. By maintaining the protected structure's potential within an optimal range, these systems prevent hydrogen evolution while ensuring adequate corrosion protection.Expand Specific Solutions03 Coating formulations resistant to cathodic disbondment
Specialized coating formulations can be developed to resist the effects of cathodic over-protection. These coatings typically feature enhanced adhesion properties, hydrogen permeability to allow gas diffusion, and chemical resistance to alkaline conditions that develop at the cathode. Multi-layer systems with specific barrier properties can also be employed to minimize water penetration while allowing hydrogen to escape.Expand Specific Solutions04 Anode design and placement to prevent over-protection
The design and strategic placement of sacrificial anodes can help prevent localized over-protection. This includes calculating appropriate anode sizes, using distributed anode systems rather than concentrated ones, and maintaining optimal distances between anodes and protected surfaces. Proper anode distribution ensures more uniform current distribution, reducing hotspots of excessive cathodic protection.Expand Specific Solutions05 Testing and monitoring methods for cathodic protection systems
Regular testing and monitoring of cathodic protection systems are essential to prevent over-protection. This includes potential surveys, coating adhesion tests, and inspection for early signs of blistering. Advanced monitoring techniques such as electrochemical impedance spectroscopy can detect changes in coating properties before visible damage occurs. Implementing these methods allows for timely adjustments to the protection system.Expand Specific Solutions
Leading Manufacturers and Service Providers
The sacrificial anode protection market is currently in a growth phase, with increasing applications in marine, oil and gas, and infrastructure sectors. The global cathodic protection market, which includes sacrificial anodes, is projected to reach approximately $7.5 billion by 2026. Technologically, the field is evolving from traditional zinc, aluminum, and magnesium anodes toward more sophisticated solutions addressing over-protection and coating blistering challenges. Companies like Naval Research Laboratory and Galvotec Alloys lead innovation in anode design and composition, while China National Offshore Oil Corp. and Diamond Offshore Drilling represent major end-users implementing advanced protection systems. Research institutions such as Ocean University of China and Kyushu University are developing next-generation solutions focusing on precise potential control and environmentally friendly formulations to prevent hydrogen evolution that causes coating damage.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has pioneered advanced sacrificial anode systems that incorporate controlled-release technology to prevent over-protection. Their approach utilizes specially formulated zinc-aluminum-magnesium alloys with precise additions of rare earth elements that modify the electrochemical behavior of the anodes. These anodes feature a multi-layer structure with varying compositions throughout the anode body, creating a graduated protection effect that becomes less aggressive as the structure reaches optimal protection levels. The laboratory has developed proprietary surface treatment processes that create a semi-passive film on the anode surface, which regulates dissolution rates based on the surrounding electrolyte conditions. This film becomes more resistant to dissolution as the protected structure's potential becomes more negative, effectively creating a self-limiting mechanism. Additionally, their research has led to the development of composite anodes with embedded reference electrodes that enable the anode to "sense" the protection level and adjust its output accordingly through controlled galvanic coupling.
Strengths: Cutting-edge research capabilities have produced highly sophisticated anode systems with superior control characteristics. Their solutions incorporate advanced materials science principles not available in commercial systems. Weaknesses: Technologies may be primarily developed for military applications and might not be widely available for commercial use. Implementation costs likely higher than conventional systems.
Vetco Gray Scandinavia AS
Technical Solution: Vetco Gray Scandinavia has developed an advanced sacrificial anode system specifically designed for subsea oil and gas infrastructure that prevents over-protection through innovative alloy formulation and geometric design. Their proprietary aluminum-zinc-indium-silicon alloy composition features carefully controlled impurity levels to maintain consistent electrochemical behavior throughout the anode's service life. The company's anodes incorporate a tapered design with varying cross-sectional areas that naturally reduces current output as the anode depletes, preventing the initial high current output that often leads to over-protection. Additionally, Vetco Gray's system utilizes specialized anode brackets with integrated dielectric shields that control current distribution patterns, directing protection current away from coated areas susceptible to blistering. Their anodes undergo a proprietary pre-conditioning process that eliminates the initial high-current "break-in" period common with new sacrificial anodes, ensuring stable protection levels from installation. For critical applications, they've developed dual-alloy anodes with an outer layer designed for controlled dissolution and an inner core that maintains stable long-term protection.
Strengths: Specialized expertise in subsea and offshore applications with solutions specifically engineered for harsh deepwater environments. Their systems provide exceptionally stable protection potentials even under changing environmental conditions. Weaknesses: Solutions are primarily targeted at high-value offshore assets and may be cost-prohibitive for simpler applications. System design requires detailed engineering analysis specific to each protected structure.
Key Innovations in Anode Design and Materials
Corrosion sensor
PatentInactiveUS6902661B2
Innovation
- A tank corrosion monitoring system that includes Silver/Silver Chloride reference half-cells and an instrumented sacrificial anode to measure electrochemical potential and current output, allowing for objective assessment of corrosion levels and coating degradation, reducing the need for manned inspections by providing data for condition-based maintenance.
Use of a sacrificial anode for corrosion protection of a portable device, e.g. a hearing aid
PatentInactiveEP2348141A1
Innovation
- Incorporating a sacrificial anode, typically made of Zn, Al, or Cu, which is less noble than the device's components, to be mounted on the substrate, allowing controlled corrosion at a specific location, thereby protecting critical parts from galvanic corrosion.
Environmental Impact of Sacrificial Anode Systems
Sacrificial anode systems, while effective for corrosion protection, present several environmental considerations that must be addressed in their implementation and lifecycle management. The dissolution of sacrificial anodes releases metal ions into the surrounding environment, particularly zinc, aluminum, and magnesium compounds. These metals, when accumulated in marine sediments and water columns, can potentially alter local ecosystems and impact aquatic organisms through bioaccumulation processes.
The rate of anode consumption directly correlates with environmental impact severity. Over-protection scenarios not only waste resources but amplify metal discharge into environments. Modern cathodic protection systems increasingly incorporate monitoring technologies to optimize anode consumption rates, thereby minimizing unnecessary metal dissolution while maintaining effective protection levels.
Regulatory frameworks worldwide have begun addressing these environmental concerns. The International Maritime Organization (IMO) has established guidelines for ships, while various national environmental protection agencies have implemented standards for offshore structures and coastal installations. These regulations typically focus on permissible metal release rates and mandate regular environmental impact assessments.
Alternative environmentally-friendly anode materials represent a significant area of research and development. Low-toxicity alloys with reduced environmental persistence are being engineered to provide comparable protection while minimizing ecological footprint. These innovations include biodegradable components and alloys designed to release less harmful compounds during their operational lifecycle.
Proper disposal and recycling of spent anodes present another environmental challenge. When sacrificial anodes reach end-of-life, they contain residual metals that require appropriate handling. Established recycling pathways can recover valuable materials and prevent improper disposal that might otherwise contribute to environmental contamination.
The interaction between protective coatings and sacrificial anodes also carries environmental implications. When blistering occurs due to over-protection, coating materials may delaminate and enter the environment as microplastics or chemical compounds. Optimized protection systems that prevent such degradation therefore offer dual environmental benefits: extended infrastructure lifespan and reduced pollution from coating failures.
Long-term environmental monitoring programs around major installations using sacrificial anodes have provided valuable data on cumulative effects. These studies indicate that while localized metal concentration increases are measurable, proper system design and maintenance can keep environmental impacts within acceptable parameters for most marine and freshwater applications.
The rate of anode consumption directly correlates with environmental impact severity. Over-protection scenarios not only waste resources but amplify metal discharge into environments. Modern cathodic protection systems increasingly incorporate monitoring technologies to optimize anode consumption rates, thereby minimizing unnecessary metal dissolution while maintaining effective protection levels.
Regulatory frameworks worldwide have begun addressing these environmental concerns. The International Maritime Organization (IMO) has established guidelines for ships, while various national environmental protection agencies have implemented standards for offshore structures and coastal installations. These regulations typically focus on permissible metal release rates and mandate regular environmental impact assessments.
Alternative environmentally-friendly anode materials represent a significant area of research and development. Low-toxicity alloys with reduced environmental persistence are being engineered to provide comparable protection while minimizing ecological footprint. These innovations include biodegradable components and alloys designed to release less harmful compounds during their operational lifecycle.
Proper disposal and recycling of spent anodes present another environmental challenge. When sacrificial anodes reach end-of-life, they contain residual metals that require appropriate handling. Established recycling pathways can recover valuable materials and prevent improper disposal that might otherwise contribute to environmental contamination.
The interaction between protective coatings and sacrificial anodes also carries environmental implications. When blistering occurs due to over-protection, coating materials may delaminate and enter the environment as microplastics or chemical compounds. Optimized protection systems that prevent such degradation therefore offer dual environmental benefits: extended infrastructure lifespan and reduced pollution from coating failures.
Long-term environmental monitoring programs around major installations using sacrificial anodes have provided valuable data on cumulative effects. These studies indicate that while localized metal concentration increases are measurable, proper system design and maintenance can keep environmental impacts within acceptable parameters for most marine and freshwater applications.
Standards and Testing Protocols for Cathodic Protection
The standardization of cathodic protection systems is critical for ensuring effective corrosion prevention while avoiding over-protection and coating blistering. International standards such as NACE SP0169, ISO 15589, and DNV-RP-B401 provide comprehensive guidelines for the design, installation, and monitoring of sacrificial anode systems. These standards establish acceptable potential ranges that prevent both under-protection (insufficient corrosion control) and over-protection (which can lead to hydrogen evolution and coating damage).
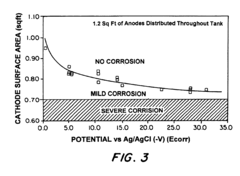
Testing protocols for sacrificial anode systems typically involve potential measurements using reference electrodes, with copper/copper sulfate (Cu/CuSO₄) electrodes for soil applications and silver/silver chloride (Ag/AgCl) electrodes for marine environments. The standards specify that protection potentials should generally be maintained between -850 mV and -1200 mV versus Cu/CuSO₄ for steel structures, with more negative values potentially causing hydrogen evolution and subsequent coating blistering.
Performance verification tests are mandated by these standards to ensure the sacrificial anodes provide adequate protection without causing detrimental effects. These include current output tests, which measure the electrical current delivered by anodes under simulated service conditions, and accelerated life tests that evaluate long-term performance and degradation patterns.
Quality control procedures outlined in these standards include chemical composition analysis of anode materials, dimensional inspections, and electrochemical capacity tests. For example, NACE TM0190 specifies procedures for measuring the electrochemical efficiency of aluminum, zinc, and magnesium anodes, ensuring they meet minimum performance requirements while avoiding excessive current output that could lead to over-protection.
Environmental considerations are increasingly incorporated into modern standards, with requirements for monitoring potential environmental impacts and ensuring compliance with local regulations. This includes guidelines for anode material selection based on environmental sensitivity of the installation area.
Monitoring requirements established by these standards typically include regular potential surveys, visual inspections of coatings for signs of blistering or degradation, and current measurement to verify that protection levels remain within the specified range. NACE SP0104 specifically addresses the monitoring of cathodic protection systems and provides guidance on measurement frequency and documentation requirements.
Certification and compliance documentation are essential components of these standards, requiring detailed records of system design, installation parameters, and ongoing monitoring results to demonstrate adherence to established protection criteria while avoiding the risks associated with over-protection.
Testing protocols for sacrificial anode systems typically involve potential measurements using reference electrodes, with copper/copper sulfate (Cu/CuSO₄) electrodes for soil applications and silver/silver chloride (Ag/AgCl) electrodes for marine environments. The standards specify that protection potentials should generally be maintained between -850 mV and -1200 mV versus Cu/CuSO₄ for steel structures, with more negative values potentially causing hydrogen evolution and subsequent coating blistering.
Performance verification tests are mandated by these standards to ensure the sacrificial anodes provide adequate protection without causing detrimental effects. These include current output tests, which measure the electrical current delivered by anodes under simulated service conditions, and accelerated life tests that evaluate long-term performance and degradation patterns.
Quality control procedures outlined in these standards include chemical composition analysis of anode materials, dimensional inspections, and electrochemical capacity tests. For example, NACE TM0190 specifies procedures for measuring the electrochemical efficiency of aluminum, zinc, and magnesium anodes, ensuring they meet minimum performance requirements while avoiding excessive current output that could lead to over-protection.
Environmental considerations are increasingly incorporated into modern standards, with requirements for monitoring potential environmental impacts and ensuring compliance with local regulations. This includes guidelines for anode material selection based on environmental sensitivity of the installation area.
Monitoring requirements established by these standards typically include regular potential surveys, visual inspections of coatings for signs of blistering or degradation, and current measurement to verify that protection levels remain within the specified range. NACE SP0104 specifically addresses the monitoring of cathodic protection systems and provides guidance on measurement frequency and documentation requirements.
Certification and compliance documentation are essential components of these standards, requiring detailed records of system design, installation parameters, and ongoing monitoring results to demonstrate adherence to established protection criteria while avoiding the risks associated with over-protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!