Sacrificial Anode For Ballast/Potable Water: Passivation Risks, Chlorination And QA
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sacrificial Anode Technology Background and Objectives
Sacrificial anodes have been utilized for corrosion protection in marine and industrial environments for over a century, with significant technological advancements occurring since the 1950s. These galvanic protection systems operate on the principle of preferential corrosion, where a more electrochemically active metal corrodes instead of the protected structure. In ballast and potable water systems specifically, sacrificial anodes play a crucial role in extending infrastructure lifespan and maintaining water quality.
The evolution of sacrificial anode technology has progressed from simple zinc and aluminum applications to sophisticated alloy formulations designed for specific environments. For ballast water systems, which experience cyclical wet/dry conditions and varying salinity levels, specialized anode compositions have been developed to maintain consistent protection across these changing conditions. Similarly, potable water system anodes have evolved to meet increasingly stringent health and safety regulations while still providing effective corrosion protection.
Current technological trends focus on optimizing anode performance while addressing key challenges including passivation phenomena, where protective oxide layers form on anodes and reduce their effectiveness. This passivation risk is particularly pronounced in potable water systems where chlorination treatments can accelerate the formation of these passive layers. Research indicates that chlorine concentrations as low as 0.5 ppm can significantly impact anode performance, creating a complex relationship between water treatment and corrosion protection systems.
The primary technical objectives for advancing sacrificial anode technology in these applications include developing alloy compositions resistant to passivation, establishing reliable quality assurance methodologies for anode performance prediction, and understanding the complex electrochemical interactions between anodes and chlorinated environments. Additionally, there is growing interest in creating environmentally friendly anode formulations that minimize the release of potentially harmful metals into water systems while maintaining protective capabilities.
Recent innovations have explored the incorporation of rare earth elements and transition metals to enhance anode performance and resistance to passivation. These advancements aim to extend anode lifespan by 30-50% compared to traditional formulations, representing a significant improvement in maintenance intervals and system reliability. Computational modeling of electrochemical processes has also emerged as a valuable tool for predicting anode behavior under various operating conditions.
The technical trajectory suggests movement toward "smart" sacrificial anode systems that incorporate monitoring capabilities to provide real-time data on protection levels and remaining anode life. This integration with digital infrastructure management systems represents the next frontier in corrosion protection technology for water systems, potentially revolutionizing maintenance practices and system reliability.
The evolution of sacrificial anode technology has progressed from simple zinc and aluminum applications to sophisticated alloy formulations designed for specific environments. For ballast water systems, which experience cyclical wet/dry conditions and varying salinity levels, specialized anode compositions have been developed to maintain consistent protection across these changing conditions. Similarly, potable water system anodes have evolved to meet increasingly stringent health and safety regulations while still providing effective corrosion protection.
Current technological trends focus on optimizing anode performance while addressing key challenges including passivation phenomena, where protective oxide layers form on anodes and reduce their effectiveness. This passivation risk is particularly pronounced in potable water systems where chlorination treatments can accelerate the formation of these passive layers. Research indicates that chlorine concentrations as low as 0.5 ppm can significantly impact anode performance, creating a complex relationship between water treatment and corrosion protection systems.
The primary technical objectives for advancing sacrificial anode technology in these applications include developing alloy compositions resistant to passivation, establishing reliable quality assurance methodologies for anode performance prediction, and understanding the complex electrochemical interactions between anodes and chlorinated environments. Additionally, there is growing interest in creating environmentally friendly anode formulations that minimize the release of potentially harmful metals into water systems while maintaining protective capabilities.
Recent innovations have explored the incorporation of rare earth elements and transition metals to enhance anode performance and resistance to passivation. These advancements aim to extend anode lifespan by 30-50% compared to traditional formulations, representing a significant improvement in maintenance intervals and system reliability. Computational modeling of electrochemical processes has also emerged as a valuable tool for predicting anode behavior under various operating conditions.
The technical trajectory suggests movement toward "smart" sacrificial anode systems that incorporate monitoring capabilities to provide real-time data on protection levels and remaining anode life. This integration with digital infrastructure management systems represents the next frontier in corrosion protection technology for water systems, potentially revolutionizing maintenance practices and system reliability.
Market Analysis for Marine and Potable Water Protection Systems
The global market for marine and potable water protection systems is experiencing significant growth, driven by increasing awareness of corrosion issues and stricter regulations regarding water quality and vessel maintenance. The sacrificial anode sector within this market is projected to reach $1.2 billion by 2027, growing at a compound annual growth rate of 5.8% from 2022.
Maritime applications represent the largest segment, accounting for approximately 62% of the total market share. This dominance stems from the extensive use of sacrificial anodes in ships, offshore platforms, and port facilities to protect underwater metal structures from galvanic corrosion. The commercial shipping sector alone constitutes nearly 40% of maritime applications due to the critical need to extend vessel lifespan and reduce maintenance costs.
Potable water system protection represents a smaller but rapidly growing segment with 15-18% annual growth, particularly in developed regions with aging water infrastructure. Municipal water systems are increasingly adopting sacrificial anode technology to protect storage tanks and distribution networks, driven by public health concerns and infrastructure longevity requirements.
Geographically, Asia-Pacific leads the market with 38% share, primarily due to extensive shipbuilding activities in China, South Korea, and Japan. North America follows at 27%, with strong demand from both maritime and municipal water treatment sectors. Europe accounts for 24% of the market, characterized by stringent environmental regulations driving adoption of advanced protection systems.
Key customer segments include shipbuilding and repair yards (31%), offshore energy companies (18%), municipal water authorities (16%), industrial facilities (14%), and commercial building operators (11%). The remaining 10% comprises various smaller applications including recreational marine vessels and specialized water treatment facilities.
Demand drivers vary by segment but commonly include regulatory compliance requirements, increasing infrastructure lifespans, cost reduction initiatives, and growing awareness of water quality issues. The market for passivation-resistant anodes is growing at twice the rate of traditional products, indicating strong customer interest in solutions addressing the specific technical challenges highlighted in the research focus.
Price sensitivity differs significantly between segments, with municipal water authorities demonstrating higher price sensitivity than offshore energy operators who prioritize performance and reliability over initial cost considerations. This market dynamic creates opportunities for tiered product offerings with varying performance-to-price ratios targeting specific customer segments.
Maritime applications represent the largest segment, accounting for approximately 62% of the total market share. This dominance stems from the extensive use of sacrificial anodes in ships, offshore platforms, and port facilities to protect underwater metal structures from galvanic corrosion. The commercial shipping sector alone constitutes nearly 40% of maritime applications due to the critical need to extend vessel lifespan and reduce maintenance costs.
Potable water system protection represents a smaller but rapidly growing segment with 15-18% annual growth, particularly in developed regions with aging water infrastructure. Municipal water systems are increasingly adopting sacrificial anode technology to protect storage tanks and distribution networks, driven by public health concerns and infrastructure longevity requirements.
Geographically, Asia-Pacific leads the market with 38% share, primarily due to extensive shipbuilding activities in China, South Korea, and Japan. North America follows at 27%, with strong demand from both maritime and municipal water treatment sectors. Europe accounts for 24% of the market, characterized by stringent environmental regulations driving adoption of advanced protection systems.
Key customer segments include shipbuilding and repair yards (31%), offshore energy companies (18%), municipal water authorities (16%), industrial facilities (14%), and commercial building operators (11%). The remaining 10% comprises various smaller applications including recreational marine vessels and specialized water treatment facilities.
Demand drivers vary by segment but commonly include regulatory compliance requirements, increasing infrastructure lifespans, cost reduction initiatives, and growing awareness of water quality issues. The market for passivation-resistant anodes is growing at twice the rate of traditional products, indicating strong customer interest in solutions addressing the specific technical challenges highlighted in the research focus.
Price sensitivity differs significantly between segments, with municipal water authorities demonstrating higher price sensitivity than offshore energy operators who prioritize performance and reliability over initial cost considerations. This market dynamic creates opportunities for tiered product offerings with varying performance-to-price ratios targeting specific customer segments.
Current Challenges in Sacrificial Anode Implementation
Despite the widespread use of sacrificial anodes in marine environments, their implementation in ballast and potable water systems faces several significant challenges. The primary concern is passivation, where protective oxide layers form on the anode surface, effectively preventing the intended galvanic protection. This phenomenon is particularly problematic in potable water systems where lower conductivity and varying water chemistry can accelerate passivation rates, rendering anodes ineffective long before their material is consumed.
Chlorination treatments, while necessary for biological control in water systems, introduce additional complications. Chlorine compounds can interact with anode materials, particularly aluminum and zinc alloys, altering their electrochemical properties and dissolution rates. Research indicates that chlorine concentrations above certain thresholds can significantly reduce anode efficiency through formation of complex chloride compounds on the anode surface, creating barriers to electron transfer.
Water flow dynamics present another challenge, as uneven flow patterns can lead to localized protection zones and leave other areas vulnerable to corrosion. This is especially problematic in ballast tanks with complex geometries where achieving uniform protection is technically difficult. Studies show that areas with restricted water circulation often experience accelerated corrosion despite the presence of sacrificial anodes in the system.
Temperature fluctuations in both ballast and potable water systems further complicate anode performance. Higher temperatures generally accelerate anode consumption rates while potentially changing the composition of protective films forming on protected surfaces. Conversely, lower temperatures can reduce anode efficiency by decreasing the conductivity of the electrolyte and slowing ion migration.
Quality assurance presents perhaps the most pressing operational challenge. Current testing methodologies for anode performance often rely on simplified laboratory conditions that fail to replicate the complex, dynamic environments of actual water systems. This disconnect leads to unpredictable field performance and premature system failures. Additionally, there is a lack of standardized in-situ monitoring techniques that can reliably assess anode condition and remaining service life without system disruption.
Material inconsistencies in anode manufacturing further compound these issues. Variations in alloy composition, even within acceptable industry standards, can significantly impact electrochemical properties and dissolution behavior. Research indicates that trace impurities can catalyze unwanted side reactions, particularly in the presence of chlorinated compounds, leading to unpredictable protection outcomes.
Chlorination treatments, while necessary for biological control in water systems, introduce additional complications. Chlorine compounds can interact with anode materials, particularly aluminum and zinc alloys, altering their electrochemical properties and dissolution rates. Research indicates that chlorine concentrations above certain thresholds can significantly reduce anode efficiency through formation of complex chloride compounds on the anode surface, creating barriers to electron transfer.
Water flow dynamics present another challenge, as uneven flow patterns can lead to localized protection zones and leave other areas vulnerable to corrosion. This is especially problematic in ballast tanks with complex geometries where achieving uniform protection is technically difficult. Studies show that areas with restricted water circulation often experience accelerated corrosion despite the presence of sacrificial anodes in the system.
Temperature fluctuations in both ballast and potable water systems further complicate anode performance. Higher temperatures generally accelerate anode consumption rates while potentially changing the composition of protective films forming on protected surfaces. Conversely, lower temperatures can reduce anode efficiency by decreasing the conductivity of the electrolyte and slowing ion migration.
Quality assurance presents perhaps the most pressing operational challenge. Current testing methodologies for anode performance often rely on simplified laboratory conditions that fail to replicate the complex, dynamic environments of actual water systems. This disconnect leads to unpredictable field performance and premature system failures. Additionally, there is a lack of standardized in-situ monitoring techniques that can reliably assess anode condition and remaining service life without system disruption.
Material inconsistencies in anode manufacturing further compound these issues. Variations in alloy composition, even within acceptable industry standards, can significantly impact electrochemical properties and dissolution behavior. Research indicates that trace impurities can catalyze unwanted side reactions, particularly in the presence of chlorinated compounds, leading to unpredictable protection outcomes.
Current Solutions for Passivation Prevention
01 Prevention of passivation in sacrificial anodes
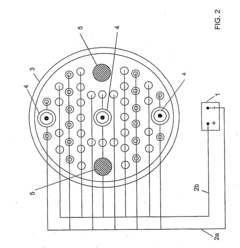
Various methods are employed to prevent passivation in sacrificial anodes, which can reduce their effectiveness in cathodic protection systems. These methods include surface treatments, alloying with specific elements, and mechanical designs that help maintain active surfaces. Prevention of passivation ensures continuous galvanic protection and extends the operational life of the anode by maintaining the electrochemical activity necessary for corrosion protection.- Prevention of sacrificial anode passivation: Various methods are employed to prevent passivation of sacrificial anodes, which occurs when a layer forms on the anode surface inhibiting its electrochemical activity. These methods include mechanical cleaning systems, chemical treatments, and design modifications that disrupt oxide film formation. Preventing passivation ensures continued cathodic protection and extends the operational life of the sacrificial anode systems in corrosive environments.
- Sacrificial anode materials and composition: The composition of sacrificial anodes significantly affects their resistance to passivation. Anodes are typically made from metals such as zinc, aluminum, or magnesium with specific alloying elements added to improve performance. These alloying elements can include indium, mercury, or gallium which help break down passive films. The material selection depends on the specific environment where cathodic protection is needed, with different compositions optimized for seawater, soil, or freshwater applications.
- Activation methods for sacrificial anodes: Sacrificial anodes often require activation to overcome initial passivation and ensure immediate protection. Activation methods include chemical etching, mechanical abrasion, or electrical treatments that remove passive layers and expose fresh, reactive metal. Some anodes incorporate self-activating features that automatically break down passive films when installed. These activation techniques are crucial for ensuring the anode begins providing cathodic protection immediately upon installation in the protected system.
- Environmental factors affecting anode passivation: Environmental conditions significantly impact sacrificial anode passivation. Factors such as temperature, pH, oxygen concentration, water chemistry, and flow rates can either accelerate or inhibit passivation processes. In certain environments, such as high-temperature or high-pH conditions, passivation occurs more rapidly. Understanding these environmental influences allows for better design and selection of anode systems to maintain effective cathodic protection despite challenging conditions.
- Monitoring and control systems for anode passivation: Advanced monitoring and control systems are implemented to detect and address sacrificial anode passivation. These systems may include potential measurement devices, current output monitors, and automated cleaning mechanisms. Some designs incorporate remote monitoring capabilities that alert operators when passivation is occurring. Intelligent control systems can adjust protection parameters or activate cleaning mechanisms when signs of passivation are detected, ensuring continuous cathodic protection of the protected structure.
02 Composition of sacrificial anodes to control passivation
The composition of sacrificial anodes can be specifically engineered to control or minimize passivation effects. By incorporating certain alloying elements such as indium, silicon, or mercury into aluminum, zinc, or magnesium anodes, the formation of passive oxide layers can be disrupted. These specialized compositions ensure that the anode remains electrochemically active throughout its service life, providing consistent cathodic protection to the protected structure.Expand Specific Solutions03 Monitoring and control systems for sacrificial anode passivation
Advanced monitoring and control systems are developed to detect and manage passivation in sacrificial anodes. These systems may include sensors that measure electrical potential, current output, or impedance to identify when passivation is occurring. Some systems incorporate automated cleaning mechanisms or electrical pulses to break down passive layers when detected. Real-time monitoring allows for immediate intervention before protection effectiveness is compromised.Expand Specific Solutions04 Environmental factors affecting sacrificial anode passivation
Environmental conditions significantly impact the passivation behavior of sacrificial anodes. Factors such as water chemistry, temperature, pH levels, oxygen concentration, and flow rates can accelerate or inhibit the formation of passive films. Understanding these environmental influences is crucial for selecting appropriate anode materials and designing effective cathodic protection systems for specific operating environments to minimize passivation issues.Expand Specific Solutions05 Structural designs to minimize passivation effects
Innovative structural designs of sacrificial anodes can help minimize passivation effects. These designs may include increased surface area configurations, special exposure patterns, or physical barriers that prevent the accumulation of passivating films. Some designs incorporate self-cleaning features or utilize strategic positioning to ensure continuous exposure to the electrolyte. These structural innovations help maintain the electrochemical efficiency of the anode throughout its service life.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The sacrificial anode market for ballast and potable water systems is currently in a growth phase, driven by increasing maritime regulations and water quality standards. The market size is estimated to be expanding at a moderate rate, with significant potential in marine and industrial applications. Technologically, the field is moderately mature but evolving to address passivation challenges and chlorination effects. Leading players include Volvo Penta, which brings marine engineering expertise; Galvotec Alloys, specializing in anode manufacturing; Vector Corrosion Technologies, offering advanced corrosion mitigation solutions; and BAC Corrosion Control, providing comprehensive protection systems. Research institutions like Suzhou Nuclear Power Research Institute and IIT Bombay are advancing quality assurance methodologies, while companies like Magnesium Elektron and Höganäs AB contribute specialized metallurgical knowledge for improved anode formulations.
Magnesium Elektron Ltd.
Technical Solution: Magnesium Elektron has pioneered advanced magnesium-based sacrificial anode technology specifically engineered for ballast and potable water systems. Their proprietary AZ63 and AM50 magnesium alloys incorporate precise amounts of aluminum and zinc to optimize performance in low-conductivity environments typical of potable water systems. The company has developed specialized heat treatment processes that modify the microstructure of their anodes to resist passivation even under chlorinated conditions. Their research has demonstrated that controlled addition of rare earth elements to magnesium alloys can significantly reduce the formation of passive films in chlorinated environments. Magnesium Elektron's quality assurance program includes electrochemical impedance spectroscopy (EIS) testing to verify anode performance under simulated service conditions, ensuring consistent protection across varying water chemistries. Their anodes undergo rigorous testing including accelerated aging in chlorinated environments to predict long-term performance and establish replacement intervals for different water treatment regimes.
Strengths: Their magnesium-based anodes provide higher driving potential compared to zinc or aluminum alternatives, making them effective in low-conductivity water. Their proprietary alloy formulations show enhanced resistance to passivation in chlorinated environments. Weaknesses: Magnesium anodes typically have higher consumption rates than other materials, potentially requiring more frequent replacement. Their higher reactivity can sometimes lead to excessive hydrogen evolution in confined spaces.
BAC Corrosion Control Ltd
Technical Solution: BAC Corrosion Control has developed comprehensive sacrificial anode systems specifically designed for ballast and potable water environments. Their technology centers on zinc-aluminum-indium alloys that maintain electrochemical activity even in chlorinated water conditions. BAC's research has focused on understanding the passivation mechanisms in treated water systems, leading to the development of their ProTech™ series anodes with specialized surface activation treatments that prevent oxide film formation. Their innovation includes proprietary manufacturing processes that control the microstructure of the anodes to maintain consistent current output even under varying chlorine levels. BAC has pioneered remote monitoring systems that track anode performance in real-time, allowing for predictive maintenance before passivation occurs. Their quality assurance methodology incorporates chemical composition analysis, metallographic examination, and electrochemical testing to ensure consistent performance across production batches. BAC's research has demonstrated that their specialized zinc alloys maintain effectiveness in water conductivity as low as 100 μS/cm while meeting potable water safety standards.
Strengths: Their integrated approach combines optimized alloy formulations with monitoring technology to prevent unexpected protection failures. Their anodes are specifically engineered to resist passivation in chlorinated environments while maintaining potable water safety compliance. Weaknesses: Their systems may require more complex installation and monitoring compared to traditional sacrificial anodes, potentially increasing initial costs. Performance can still be affected by extreme variations in water chemistry or treatment regimens.
Key Research on Chlorination Effects on Anode Performance
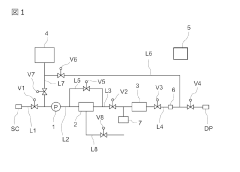
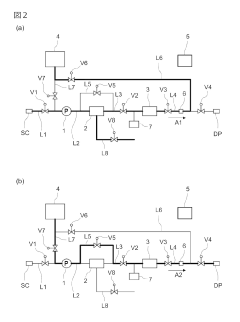
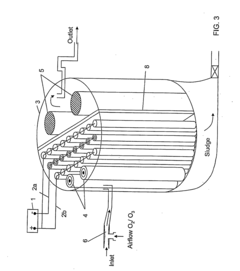
Ballast water treatment installation
PatentInactiveJP2018095949A
Innovation
- A sacrificial anode is positioned downstream of the microorganism-killing means, such as an ultraviolet reactor, to prevent the eluted metals from adhering to the reactor and degrading its performance, and a control system is used to fill the reactor with fresh water after operations to separate the sacrificial anode.
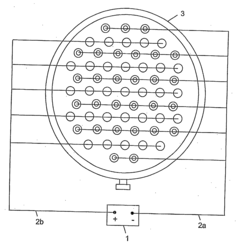
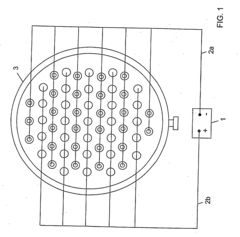
Method and apparatus for treating water or wastewater or the like
PatentInactiveUS20090242424A1
Innovation
- The method employs electro-physical precipitation using a sacrificial anode that slowly dissolves, generating reactive radicals and optimizing electrode spacing for efficient electrolysis, reducing the need for chemicals and energy consumption.
Regulatory Standards for Water System Protection
The regulatory landscape governing water system protection is extensive and multifaceted, particularly concerning sacrificial anodes in ballast and potable water systems. International Maritime Organization (IMO) regulations, specifically the Ballast Water Management Convention, establish stringent requirements for ballast water treatment systems, including specifications for materials used in cathodic protection systems to prevent corrosion while maintaining water quality.
For potable water systems, the World Health Organization (WHO) Guidelines for Drinking-water Quality provide comprehensive standards regarding acceptable levels of metals that may leach from sacrificial anodes. These guidelines establish maximum contaminant levels for zinc, aluminum, and magnesium—common materials in sacrificial anodes—ensuring these elements remain below harmful thresholds in drinking water.
In the United States, the Environmental Protection Agency (EPA) enforces the Safe Drinking Water Act, which includes specific provisions for materials in contact with potable water. The NSF/ANSI 61 standard is particularly relevant, as it evaluates the safety of products that come into contact with drinking water, including sacrificial anodes, by assessing potential leaching of contaminants.
The European Union's Drinking Water Directive (98/83/EC) similarly regulates materials in contact with drinking water, establishing limits for metal ions and requiring certification for components used in water systems. The directive specifically addresses passivation concerns and mandates regular testing protocols to monitor water quality when cathodic protection systems are employed.
Maritime classification societies, including Lloyd's Register, American Bureau of Shipping (ABS), and Det Norske Veritas (DNV), have developed technical standards specifically addressing sacrificial anode installation, maintenance, and performance in marine environments. These standards include specifications for anode composition, installation procedures, and inspection protocols to ensure effective corrosion protection without compromising water quality.
ISO 15589-2 provides international standards for cathodic protection of offshore structures, including guidelines for sacrificial anode systems in marine environments. This standard addresses material selection criteria, design considerations, and quality assurance methodologies specifically relevant to water systems exposed to chlorination treatments.
Compliance with these regulations requires comprehensive documentation, regular water quality testing, and certification of materials. Manufacturers of sacrificial anodes must provide detailed composition analyses, performance data, and leaching studies to demonstrate regulatory compliance. Additionally, operators must implement monitoring programs to detect potential passivation issues or elevated metal concentrations resulting from anode degradation.
For potable water systems, the World Health Organization (WHO) Guidelines for Drinking-water Quality provide comprehensive standards regarding acceptable levels of metals that may leach from sacrificial anodes. These guidelines establish maximum contaminant levels for zinc, aluminum, and magnesium—common materials in sacrificial anodes—ensuring these elements remain below harmful thresholds in drinking water.
In the United States, the Environmental Protection Agency (EPA) enforces the Safe Drinking Water Act, which includes specific provisions for materials in contact with potable water. The NSF/ANSI 61 standard is particularly relevant, as it evaluates the safety of products that come into contact with drinking water, including sacrificial anodes, by assessing potential leaching of contaminants.
The European Union's Drinking Water Directive (98/83/EC) similarly regulates materials in contact with drinking water, establishing limits for metal ions and requiring certification for components used in water systems. The directive specifically addresses passivation concerns and mandates regular testing protocols to monitor water quality when cathodic protection systems are employed.
Maritime classification societies, including Lloyd's Register, American Bureau of Shipping (ABS), and Det Norske Veritas (DNV), have developed technical standards specifically addressing sacrificial anode installation, maintenance, and performance in marine environments. These standards include specifications for anode composition, installation procedures, and inspection protocols to ensure effective corrosion protection without compromising water quality.
ISO 15589-2 provides international standards for cathodic protection of offshore structures, including guidelines for sacrificial anode systems in marine environments. This standard addresses material selection criteria, design considerations, and quality assurance methodologies specifically relevant to water systems exposed to chlorination treatments.
Compliance with these regulations requires comprehensive documentation, regular water quality testing, and certification of materials. Manufacturers of sacrificial anodes must provide detailed composition analyses, performance data, and leaching studies to demonstrate regulatory compliance. Additionally, operators must implement monitoring programs to detect potential passivation issues or elevated metal concentrations resulting from anode degradation.
Quality Control Methodologies for Anode Manufacturing
Quality control in sacrificial anode manufacturing represents a critical component in ensuring the reliability and effectiveness of corrosion protection systems for ballast and potable water applications. Comprehensive quality assurance begins with raw material verification, where incoming metals and alloys undergo rigorous chemical composition analysis using techniques such as X-ray fluorescence spectroscopy and inductively coupled plasma mass spectrometry to confirm compliance with industry standards like ASTM B843 for aluminum anodes or ASTM B418 for zinc anodes.
Manufacturing process controls constitute the next critical phase, incorporating statistical process control (SPC) methodologies to monitor key variables including temperature, pressure, and casting time. Real-time monitoring systems equipped with automated alerts help maintain process parameters within established tolerance limits, while advanced casting techniques such as continuous monitoring of molten metal purity ensure consistent microstructure development essential for optimal electrochemical performance.
Physical and dimensional inspection represents another fundamental quality control measure, utilizing coordinate measuring machines (CMMs) and 3D scanning technologies to verify critical dimensions with tolerances typically maintained within ±0.5mm. Surface quality assessment includes visual inspection for casting defects and advanced techniques such as dye penetrant testing to identify subsurface flaws that could compromise anode performance or accelerate passivation.
Electrochemical performance testing forms perhaps the most crucial aspect of quality control, with potentiodynamic polarization tests measuring the anode's current capacity and operating potential. Accelerated life testing in simulated environments provides valuable data on long-term performance, while electrochemical impedance spectroscopy (EIS) helps identify potential passivation tendencies—particularly important for systems exposed to chlorinated water.
Documentation and traceability systems complete the quality control framework, with each anode batch assigned unique identification codes linked to manufacturing parameters, raw material sources, and test results. This comprehensive approach enables full product lifecycle tracking and facilitates rapid response to any field performance issues that may arise during service.
Implementation of these methodologies requires integration of automated inspection systems with manufacturing processes, creating a closed-loop quality control system that continuously monitors and adjusts production parameters based on real-time data analysis, ultimately ensuring consistent protection against corrosion in critical water systems.
Manufacturing process controls constitute the next critical phase, incorporating statistical process control (SPC) methodologies to monitor key variables including temperature, pressure, and casting time. Real-time monitoring systems equipped with automated alerts help maintain process parameters within established tolerance limits, while advanced casting techniques such as continuous monitoring of molten metal purity ensure consistent microstructure development essential for optimal electrochemical performance.
Physical and dimensional inspection represents another fundamental quality control measure, utilizing coordinate measuring machines (CMMs) and 3D scanning technologies to verify critical dimensions with tolerances typically maintained within ±0.5mm. Surface quality assessment includes visual inspection for casting defects and advanced techniques such as dye penetrant testing to identify subsurface flaws that could compromise anode performance or accelerate passivation.
Electrochemical performance testing forms perhaps the most crucial aspect of quality control, with potentiodynamic polarization tests measuring the anode's current capacity and operating potential. Accelerated life testing in simulated environments provides valuable data on long-term performance, while electrochemical impedance spectroscopy (EIS) helps identify potential passivation tendencies—particularly important for systems exposed to chlorinated water.
Documentation and traceability systems complete the quality control framework, with each anode batch assigned unique identification codes linked to manufacturing parameters, raw material sources, and test results. This comprehensive approach enables full product lifecycle tracking and facilitates rapid response to any field performance issues that may arise during service.
Implementation of these methodologies requires integration of automated inspection systems with manufacturing processes, creating a closed-loop quality control system that continuously monitors and adjusts production parameters based on real-time data analysis, ultimately ensuring consistent protection against corrosion in critical water systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!