Sacrificial Anode in Concrete: Chloride Exposure, Alkali Effects And Rebar CP
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sacrificial Anode Technology Background and Objectives
Sacrificial anode technology has evolved significantly since its inception in the early 20th century as a method to protect metal structures from corrosion. Initially developed for marine applications, this cathodic protection technique has been adapted for reinforced concrete structures since the 1970s. The technology leverages the principle of galvanic corrosion, where a more electrochemically active metal (the anode) corrodes preferentially to protect a more noble metal (the cathode, typically steel reinforcement in concrete).
The evolution of sacrificial anodes for concrete applications has seen several key developments, from simple zinc and aluminum anodes to more sophisticated alloy systems designed specifically for the high alkalinity environment of concrete. Recent advancements have focused on optimizing anode composition, geometry, and activation methods to enhance protection efficiency and longevity in various exposure conditions.
Current research trends indicate growing interest in understanding the complex interactions between sacrificial anodes and concrete environments, particularly under chloride exposure conditions. The presence of chloride ions, commonly from deicing salts or marine environments, significantly accelerates reinforcement corrosion and presents unique challenges for anode performance and service life prediction.
The alkaline environment of concrete (typically pH 12.5-13.5) creates additional complexities for sacrificial anode systems. This high pH can lead to passivation of certain anode materials, reducing their effectiveness over time. Understanding and mitigating these effects remains a critical research focus in the field.
The primary objective of current sacrificial anode technology research is to develop more efficient, long-lasting, and cost-effective systems for reinforced concrete structures exposed to aggressive environments. This includes optimizing anode composition and design for specific exposure conditions, improving installation techniques, and developing reliable methods for monitoring and predicting anode performance over time.
Additional research goals include understanding the relationship between chloride concentration, alkalinity levels, and anode performance; developing new alloy formulations with enhanced resistance to passivation; and creating comprehensive models that can accurately predict the service life of sacrificial anode systems under various environmental conditions.
The technology aims to address the growing global infrastructure deterioration crisis, where reinforced concrete structures are aging rapidly due to corrosion. With infrastructure replacement costs estimated in the trillions globally, effective and economical corrosion protection technologies like sacrificial anodes represent a critical area for continued innovation and development.
The evolution of sacrificial anodes for concrete applications has seen several key developments, from simple zinc and aluminum anodes to more sophisticated alloy systems designed specifically for the high alkalinity environment of concrete. Recent advancements have focused on optimizing anode composition, geometry, and activation methods to enhance protection efficiency and longevity in various exposure conditions.
Current research trends indicate growing interest in understanding the complex interactions between sacrificial anodes and concrete environments, particularly under chloride exposure conditions. The presence of chloride ions, commonly from deicing salts or marine environments, significantly accelerates reinforcement corrosion and presents unique challenges for anode performance and service life prediction.
The alkaline environment of concrete (typically pH 12.5-13.5) creates additional complexities for sacrificial anode systems. This high pH can lead to passivation of certain anode materials, reducing their effectiveness over time. Understanding and mitigating these effects remains a critical research focus in the field.
The primary objective of current sacrificial anode technology research is to develop more efficient, long-lasting, and cost-effective systems for reinforced concrete structures exposed to aggressive environments. This includes optimizing anode composition and design for specific exposure conditions, improving installation techniques, and developing reliable methods for monitoring and predicting anode performance over time.
Additional research goals include understanding the relationship between chloride concentration, alkalinity levels, and anode performance; developing new alloy formulations with enhanced resistance to passivation; and creating comprehensive models that can accurately predict the service life of sacrificial anode systems under various environmental conditions.
The technology aims to address the growing global infrastructure deterioration crisis, where reinforced concrete structures are aging rapidly due to corrosion. With infrastructure replacement costs estimated in the trillions globally, effective and economical corrosion protection technologies like sacrificial anodes represent a critical area for continued innovation and development.
Market Analysis for Concrete Corrosion Protection Systems
The global market for concrete corrosion protection systems has been experiencing steady growth, driven primarily by aging infrastructure and increasing awareness of the long-term economic benefits of corrosion prevention. The market size for concrete corrosion protection technologies was valued at approximately USD 6.7 billion in 2022, with projections indicating growth at a compound annual rate of 6.2% through 2030.
Sacrificial anode systems, particularly those designed for chloride-exposed environments, represent a significant segment within this market. These systems account for roughly 18% of the total concrete corrosion protection market, with higher adoption rates in coastal regions and areas with severe winter conditions where deicing salts are commonly used.
Regional analysis reveals that North America and Europe currently dominate the market for advanced concrete protection systems, collectively accounting for over 60% of global revenue. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading infrastructure development initiatives that increasingly incorporate corrosion protection measures from the design stage.
Market segmentation shows distinct trends across different application sectors. Marine structures represent the largest application segment for sacrificial anode systems, followed by highway infrastructure, parking structures, and industrial facilities. The bridge rehabilitation sector specifically has seen demand growth of nearly 8% annually as governments worldwide prioritize infrastructure maintenance.
Customer demand patterns indicate a growing preference for integrated corrosion protection solutions that combine sacrificial anodes with complementary technologies. End-users increasingly seek systems that offer extended service life, reduced maintenance requirements, and verifiable performance metrics. This trend has spurred innovation in monitoring technologies that can be embedded alongside protection systems.
Price sensitivity varies significantly by region and application. While initial installation costs remain a barrier to adoption in developing markets, lifecycle cost analysis increasingly favors early implementation of protection systems. Studies demonstrate that effective corrosion protection can reduce lifetime infrastructure costs by 25-30% compared to reactive maintenance approaches.
Market forecasts suggest that technological innovations addressing alkali effects in concrete and improving the efficiency of cathodic protection will drive the next wave of market growth. Specifically, systems optimized for high-alkalinity environments are projected to see above-average growth rates of 7-9% annually as research advances translate into commercial applications.
Sacrificial anode systems, particularly those designed for chloride-exposed environments, represent a significant segment within this market. These systems account for roughly 18% of the total concrete corrosion protection market, with higher adoption rates in coastal regions and areas with severe winter conditions where deicing salts are commonly used.
Regional analysis reveals that North America and Europe currently dominate the market for advanced concrete protection systems, collectively accounting for over 60% of global revenue. However, the Asia-Pacific region is emerging as the fastest-growing market, with China and India leading infrastructure development initiatives that increasingly incorporate corrosion protection measures from the design stage.
Market segmentation shows distinct trends across different application sectors. Marine structures represent the largest application segment for sacrificial anode systems, followed by highway infrastructure, parking structures, and industrial facilities. The bridge rehabilitation sector specifically has seen demand growth of nearly 8% annually as governments worldwide prioritize infrastructure maintenance.
Customer demand patterns indicate a growing preference for integrated corrosion protection solutions that combine sacrificial anodes with complementary technologies. End-users increasingly seek systems that offer extended service life, reduced maintenance requirements, and verifiable performance metrics. This trend has spurred innovation in monitoring technologies that can be embedded alongside protection systems.
Price sensitivity varies significantly by region and application. While initial installation costs remain a barrier to adoption in developing markets, lifecycle cost analysis increasingly favors early implementation of protection systems. Studies demonstrate that effective corrosion protection can reduce lifetime infrastructure costs by 25-30% compared to reactive maintenance approaches.
Market forecasts suggest that technological innovations addressing alkali effects in concrete and improving the efficiency of cathodic protection will drive the next wave of market growth. Specifically, systems optimized for high-alkalinity environments are projected to see above-average growth rates of 7-9% annually as research advances translate into commercial applications.
Current Challenges in Sacrificial Anode Technology
Despite significant advancements in sacrificial anode technology for reinforced concrete structures, several critical challenges persist that limit their widespread implementation and long-term effectiveness. The primary challenge involves the complex interaction between chloride exposure levels and anode performance. Current sacrificial anode systems demonstrate variable efficiency across different chloride concentration environments, with performance degradation occurring unpredictably in highly contaminated structures. This inconsistency makes it difficult to establish standardized application protocols across diverse infrastructure environments.
The alkaline environment of concrete presents another significant hurdle. Concrete typically maintains a pH level between 12.5-13.5, which can passivate both the sacrificial anodes and the steel reinforcement they are designed to protect. This passivation effect reduces the galvanic current output of zinc and aluminum-based anodes, diminishing their protective capacity over time. Research indicates that after initial activation, many commercial anodes experience a 40-60% reduction in current output within the first year of installation due to this alkaline passivation.
Connection reliability between the sacrificial anode and the reinforcement steel remains problematic. Current attachment methods, including mechanical fastening and conductive adhesives, often develop increased resistance over time due to corrosion products forming at the interface. This growing resistance progressively reduces the protective current flow, eventually rendering the cathodic protection system ineffective without visible indicators of failure.
Service life prediction represents another significant challenge. While manufacturers typically claim 10-25 year lifespans for sacrificial anodes in concrete, field performance data frequently shows premature depletion or passivation. The lack of reliable non-destructive monitoring techniques makes it difficult to assess remaining anode capacity without invasive testing, leading to either premature replacement or unexpected protection failure.
Cost-effectiveness concerns also hinder widespread adoption. The installation of sacrificial anodes increases initial construction costs by 15-30% compared to conventional reinforcement protection methods. Without standardized performance metrics and reliable life-cycle cost analyses, many infrastructure owners remain hesitant to invest in this technology despite its potential long-term benefits.
Environmental factors such as temperature fluctuations, moisture content variations, and oxygen availability further complicate anode performance prediction. These variables significantly influence the electrochemical reactions governing cathodic protection, yet current design methodologies inadequately account for these dynamic environmental conditions.
The alkaline environment of concrete presents another significant hurdle. Concrete typically maintains a pH level between 12.5-13.5, which can passivate both the sacrificial anodes and the steel reinforcement they are designed to protect. This passivation effect reduces the galvanic current output of zinc and aluminum-based anodes, diminishing their protective capacity over time. Research indicates that after initial activation, many commercial anodes experience a 40-60% reduction in current output within the first year of installation due to this alkaline passivation.
Connection reliability between the sacrificial anode and the reinforcement steel remains problematic. Current attachment methods, including mechanical fastening and conductive adhesives, often develop increased resistance over time due to corrosion products forming at the interface. This growing resistance progressively reduces the protective current flow, eventually rendering the cathodic protection system ineffective without visible indicators of failure.
Service life prediction represents another significant challenge. While manufacturers typically claim 10-25 year lifespans for sacrificial anodes in concrete, field performance data frequently shows premature depletion or passivation. The lack of reliable non-destructive monitoring techniques makes it difficult to assess remaining anode capacity without invasive testing, leading to either premature replacement or unexpected protection failure.
Cost-effectiveness concerns also hinder widespread adoption. The installation of sacrificial anodes increases initial construction costs by 15-30% compared to conventional reinforcement protection methods. Without standardized performance metrics and reliable life-cycle cost analyses, many infrastructure owners remain hesitant to invest in this technology despite its potential long-term benefits.
Environmental factors such as temperature fluctuations, moisture content variations, and oxygen availability further complicate anode performance prediction. These variables significantly influence the electrochemical reactions governing cathodic protection, yet current design methodologies inadequately account for these dynamic environmental conditions.
Existing Sacrificial Anode Solutions for Chloride Environments
01 Chloride exposure effects on sacrificial anodes
Chloride ions can significantly impact the performance of sacrificial anodes by accelerating corrosion rates. When exposed to chloride-rich environments, such as seawater or deicing salts, sacrificial anodes experience increased dissolution rates which can affect their protective capabilities and service life. The presence of chlorides can also lead to localized corrosion phenomena like pitting, which may compromise the uniform dissolution pattern necessary for effective cathodic protection.- Chloride exposure effects on sacrificial anodes: Chloride ions significantly affect the performance of sacrificial anodes in corrosion protection systems. High chloride concentrations can accelerate the dissolution rate of sacrificial anodes, particularly those made of zinc, aluminum, or magnesium alloys. This exposure can lead to increased anode consumption rates and potentially reduced service life. The presence of chlorides can also influence the formation and stability of protective films on the anode surface, affecting the overall efficiency of cathodic protection systems.
- Alkali effects on sacrificial anode performance: Alkaline environments significantly impact sacrificial anode behavior and effectiveness. In high pH conditions, certain anode materials may experience passivation, where a protective oxide layer forms on the surface, potentially reducing the anode's ability to provide adequate protection current. Conversely, controlled alkalinity can sometimes enhance anode performance by promoting more uniform dissolution patterns. The interaction between alkali conditions and specific anode alloy compositions is critical for optimizing cathodic protection systems in various industrial applications.
- Anode composition modifications for chloride and alkali resistance: Specialized alloy compositions can be formulated to enhance sacrificial anode performance in environments with high chloride concentrations and varying alkalinity. These modified compositions typically include carefully balanced amounts of activator elements such as silicon, iron, and indium that help maintain anode activity despite challenging conditions. Some advanced formulations incorporate rare earth elements or transition metals to improve dissolution characteristics and extend service life. These compositional modifications aim to prevent passivation while maintaining a stable and predictable electrochemical potential.
- Protective coatings and treatments for sacrificial anodes: Various protective treatments and coatings can be applied to sacrificial anodes to control their interaction with chloride-rich and alkaline environments. These treatments may include specialized activation processes, surface conditioning techniques, or selective application of semi-permeable coatings. Such approaches help to regulate the dissolution rate, prevent premature passivation, and ensure more uniform consumption of the anode material. These protective measures are particularly important in applications where anodes are exposed to fluctuating environmental conditions or where precise control of protection current is required.
- Monitoring and control systems for sacrificial anodes in variable environments: Advanced monitoring and control systems have been developed to optimize sacrificial anode performance in environments with varying chloride levels and pH conditions. These systems may include reference electrodes, potential measurement devices, and automated control mechanisms that adjust protection parameters based on environmental changes. Some systems incorporate remote monitoring capabilities, allowing for real-time assessment of anode consumption rates and protection effectiveness. These technological advances enable more efficient use of sacrificial anodes and help prevent both under-protection and over-protection scenarios in critical infrastructure applications.
02 Alkali effects on sacrificial anode performance
Alkaline environments significantly influence sacrificial anode behavior by affecting the formation and stability of protective films on the anode surface. High pH conditions can lead to passivation of certain anode materials, reducing their effectiveness as sacrificial elements. Conversely, controlled alkalinity can enhance the performance of some anode materials by promoting more uniform dissolution patterns and preventing localized corrosion. The interaction between alkali conditions and anode materials is critical for optimizing cathodic protection systems in various applications.Expand Specific Solutions03 Material composition for chloride-resistant sacrificial anodes
Specialized alloy compositions have been developed to enhance sacrificial anode performance in chloride-rich environments. These compositions typically include elements like aluminum, zinc, magnesium, and various activators or modifiers that improve the electrochemical properties of the anode. The precise balance of these elements determines the anode's resistance to chloride attack while maintaining sufficient electrochemical potential to provide effective cathodic protection. Advanced manufacturing techniques ensure uniform distribution of alloying elements, which is crucial for consistent performance in aggressive chloride environments.Expand Specific Solutions04 Protective coatings and barriers for sacrificial anodes
Protective coatings and barrier systems have been developed to control the interaction between sacrificial anodes and their environment, particularly in conditions with high chloride concentration or varying alkalinity. These coatings can regulate the dissolution rate of the anode, extend service life, and ensure more predictable performance. Semi-permeable membranes and selective barriers allow for controlled ion exchange while preventing excessive chloride attack. Some advanced systems incorporate pH buffers to maintain optimal conditions around the anode surface regardless of external environmental fluctuations.Expand Specific Solutions05 Monitoring and control systems for sacrificial anodes in variable environments
Advanced monitoring and control systems have been developed to optimize sacrificial anode performance in environments with fluctuating chloride levels and pH conditions. These systems typically include sensors that measure critical parameters such as current output, anode consumption rate, local chloride concentration, and pH levels. Integrated control mechanisms can adjust protection parameters in response to changing environmental conditions, ensuring consistent protection while maximizing anode lifespan. Some systems incorporate remote monitoring capabilities and predictive algorithms to anticipate maintenance needs and prevent protection failures.Expand Specific Solutions
Leading Companies in Sacrificial Anode Industry
The sacrificial anode technology for concrete structures in chloride environments is currently in a growth phase, with increasing adoption in infrastructure protection against corrosion. The global market for this technology is expanding steadily, estimated at approximately $300-400 million annually with projected growth rates of 5-7%. Technical maturity varies across applications, with companies demonstrating different specialization levels. Industry leaders like Industrie De Nora and BAC Corrosion Control have developed advanced commercial systems, while research institutions such as Qingdao University of Technology and Xiamen University are advancing fundamental understanding of alkali effects and chloride interactions. Companies like Structural Group and CCCC Fourth Harbor Engineering are implementing field applications, bridging the gap between laboratory research and practical deployment in marine and transportation infrastructure.
Industrie De Nora SpA
Technical Solution: De Nora has developed advanced sacrificial anode systems specifically designed for reinforced concrete structures exposed to chloride environments. Their technology utilizes high-purity zinc, aluminum, and magnesium alloy anodes with proprietary activator pastes that maintain performance even in high-alkalinity concrete environments. The company's LIDA® sacrificial anode system incorporates distributed anodes connected directly to the reinforcement, creating localized protective cells that prevent corrosion initiation. Their research has demonstrated that these systems can effectively maintain protection even when chloride levels exceed critical thresholds (>0.4% by weight of cement). De Nora's technology includes specialized formulations that account for alkali effects in concrete, ensuring consistent galvanic current output across varying pH levels. Their systems have been documented to provide 10-15 years of protection in severe marine environments without external power requirements.
Strengths: Specialized activator pastes overcome concrete's high alkalinity limitations; proven long-term performance in marine environments; requires no external power source or monitoring systems. Weaknesses: Higher initial installation costs compared to traditional methods; effectiveness can diminish in extremely dry concrete conditions; requires careful installation to ensure proper electrical connectivity.
BAC Corrosion Control Ltd
Technical Solution: BAC Corrosion Control has pioneered the Galvashield® XP sacrificial anode system specifically engineered for chloride-contaminated concrete structures. Their technology employs zinc-based anodes with a specialized alkaline cementitious matrix that maintains anode activity despite concrete's high pH environment. The system creates a protective galvanic current that mitigates the effects of chloride ions on steel reinforcement. BAC's research has demonstrated that their anodes can deliver effective protection even when chloride concentrations reach 3-4 times the corrosion threshold. Their technology incorporates proprietary activation methods that ensure consistent performance across varying concrete alkalinity levels (pH 12-14). The company has developed installation techniques that maximize the protective current distribution, with documented field performance showing 15-20 year protection periods in aggressive marine environments. BAC's systems have been extensively tested under accelerated aging conditions, confirming their ability to maintain protective current outputs despite alkali migration from the concrete to the anode interface.
Strengths: Specialized alkaline matrix ensures consistent performance in high-pH concrete; proven long-term effectiveness in chloride-rich environments; requires no external power or monitoring. Weaknesses: Performance can be affected by extremely dry concrete conditions; higher material costs compared to traditional repair methods; requires proper design calculations based on steel surface area.
Key Technical Innovations in Anode-Rebar CP Systems
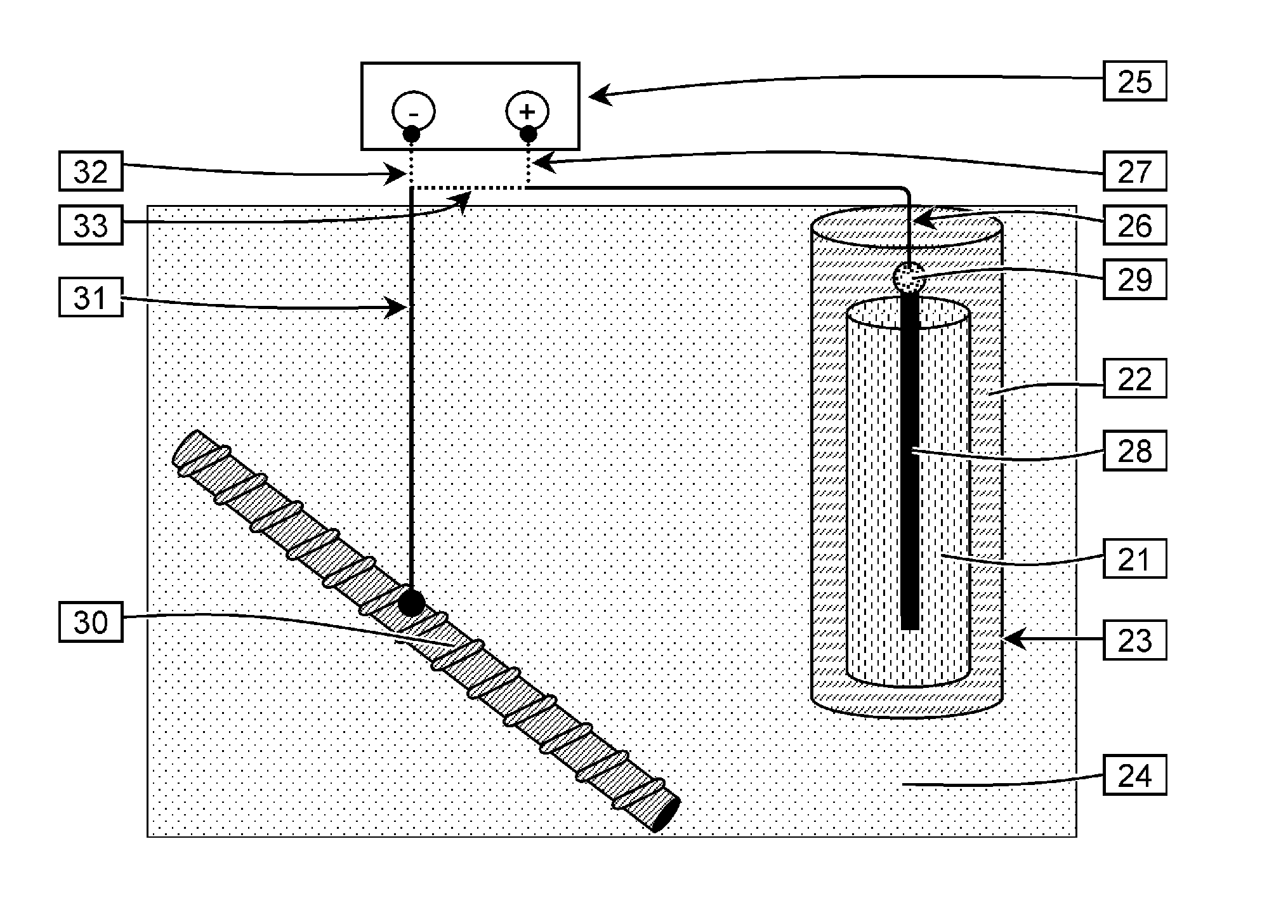
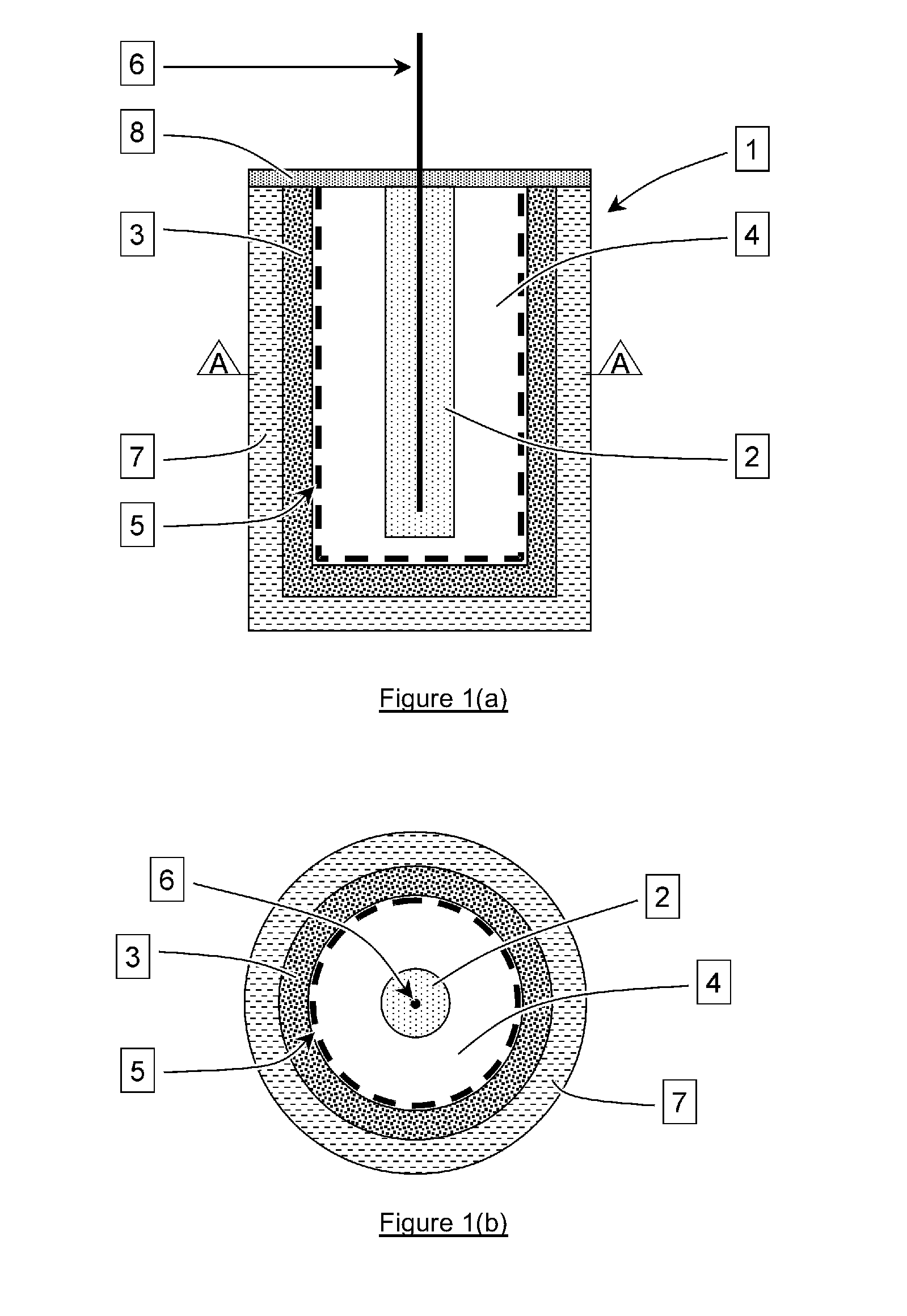
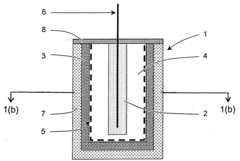
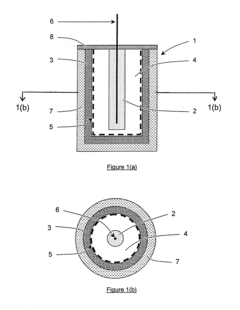
Sacrificial anode and treatment of concrete
PatentActiveUS20100147703A1
Innovation
- A sacrificial anode assembly with a cell and cathode in ionic contact but not electronic contact, connected to a metal section via an electron conductor, and a sacrificial anode in series with a power supply to enhance voltage and current flow, allowing for increased spacing and reduced anode count, and embedding the anode in concrete for strong attachment.
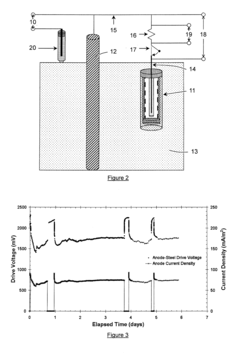
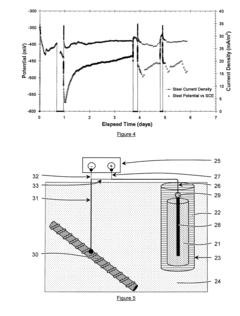
Sacrificial anode and treatment of concrete
PatentActiveUS20120261270A1
Innovation
- A sacrificial anode assembly with an anode and cathode in ionic contact but not electronic contact, connected to a power source via an electron conducting connector, allowing increased voltage and current flow by combining external power supply voltage with galvanic voltage, and embedding the anode in a concrete cavity for strong attachment and reduced resistance.
Environmental Impact Assessment of Sacrificial Anode Materials
The environmental impact of sacrificial anode materials used in concrete structures represents a critical consideration in sustainable construction practices. These anodes, typically composed of zinc, aluminum, or magnesium alloys, are designed to corrode preferentially to protect steel reinforcement. However, their implementation introduces various environmental concerns throughout their lifecycle.
Primary environmental impacts occur during the extraction and processing of raw materials for sacrificial anodes. Mining operations for zinc, aluminum, and magnesium contribute to habitat destruction, soil erosion, and water contamination. The energy-intensive refining processes generate significant carbon emissions, with aluminum production being particularly demanding, requiring approximately 14 kWh of electricity per kilogram produced.
During installation and service life, sacrificial anodes release metal ions into the surrounding environment as they corrode. In chloride-rich environments, these ions may combine with chlorides to form potentially harmful compounds. Research indicates that zinc anodes in high-alkalinity concrete can form zincate ions (Zn(OH)4²⁻), which may affect local microbial communities when leached into soil or groundwater systems.
The long-term environmental persistence of these materials warrants attention. While the corrosion products generally remain bound within the concrete matrix, deteriorating structures may release these compounds during demolition or natural degradation. Studies have shown that under certain pH conditions, particularly in acidic environments, the mobility of these metal ions increases significantly, potentially impacting aquatic ecosystems.
Comparative lifecycle assessments reveal that despite these concerns, sacrificial anodes often present a more environmentally favorable option than alternative protection methods or premature structure replacement. The extended service life of protected concrete structures reduces the need for resource-intensive reconstruction, resulting in net environmental benefits when properly implemented.
Recent innovations focus on developing more environmentally compatible sacrificial anode materials. These include zinc alloys with reduced heavy metal content and composite anodes incorporating recycled materials. Additionally, research into biodegradable sacrificial systems shows promise for applications where temporary protection is sufficient.
Regulatory frameworks increasingly address the environmental aspects of sacrificial anode use, with particular emphasis on leaching characteristics and end-of-life management. The European Union's Construction Products Regulation and the United States EPA guidelines now include specific provisions for monitoring and limiting the environmental impact of cathodic protection systems in infrastructure applications.
Primary environmental impacts occur during the extraction and processing of raw materials for sacrificial anodes. Mining operations for zinc, aluminum, and magnesium contribute to habitat destruction, soil erosion, and water contamination. The energy-intensive refining processes generate significant carbon emissions, with aluminum production being particularly demanding, requiring approximately 14 kWh of electricity per kilogram produced.
During installation and service life, sacrificial anodes release metal ions into the surrounding environment as they corrode. In chloride-rich environments, these ions may combine with chlorides to form potentially harmful compounds. Research indicates that zinc anodes in high-alkalinity concrete can form zincate ions (Zn(OH)4²⁻), which may affect local microbial communities when leached into soil or groundwater systems.
The long-term environmental persistence of these materials warrants attention. While the corrosion products generally remain bound within the concrete matrix, deteriorating structures may release these compounds during demolition or natural degradation. Studies have shown that under certain pH conditions, particularly in acidic environments, the mobility of these metal ions increases significantly, potentially impacting aquatic ecosystems.
Comparative lifecycle assessments reveal that despite these concerns, sacrificial anodes often present a more environmentally favorable option than alternative protection methods or premature structure replacement. The extended service life of protected concrete structures reduces the need for resource-intensive reconstruction, resulting in net environmental benefits when properly implemented.
Recent innovations focus on developing more environmentally compatible sacrificial anode materials. These include zinc alloys with reduced heavy metal content and composite anodes incorporating recycled materials. Additionally, research into biodegradable sacrificial systems shows promise for applications where temporary protection is sufficient.
Regulatory frameworks increasingly address the environmental aspects of sacrificial anode use, with particular emphasis on leaching characteristics and end-of-life management. The European Union's Construction Products Regulation and the United States EPA guidelines now include specific provisions for monitoring and limiting the environmental impact of cathodic protection systems in infrastructure applications.
Durability Testing Standards and Performance Metrics
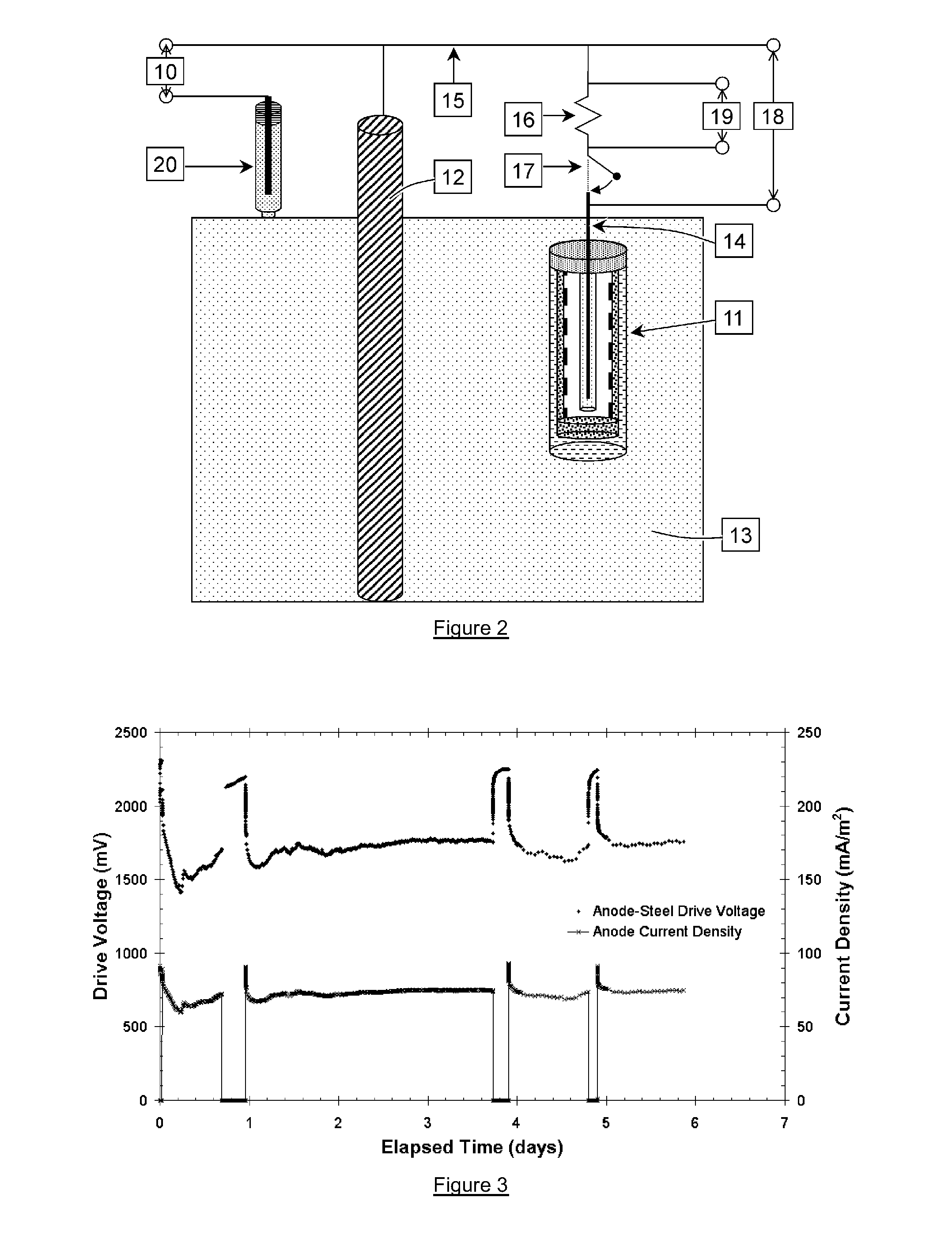
The durability testing standards for sacrificial anodes in concrete structures are critical for ensuring long-term performance in chloride-rich environments. ASTM G116 provides standardized methods for evaluating galvanic anode performance, measuring current output and protective potential shifts when connected to reinforcing steel. This standard establishes baseline criteria for comparing different anode systems under controlled laboratory conditions.
ISO 12696 offers comprehensive guidelines specifically for cathodic protection of steel in concrete, detailing performance metrics such as protective current density requirements (typically 2-20 mA/m²) and depolarization criteria. The standard stipulates that a minimum 100mV depolarization over a 24-hour period indicates effective protection against corrosion processes.
Field performance evaluation follows NACE SP0290, which establishes protocols for monitoring sacrificial anode systems in concrete structures exposed to marine environments. This standard requires periodic measurement of steel-to-concrete potentials, current output monitoring, and visual inspection of concrete condition to assess ongoing protection efficacy.
Accelerated testing methodologies have been developed to predict long-term performance within reasonable timeframes. ASTM B117 salt spray testing, modified for concrete applications, subjects anode systems to concentrated chloride exposure. Additionally, cyclic wetting/drying tests simulate coastal environmental conditions, with performance measured by weight loss, current output stability, and protective potential maintenance over time.
Key performance metrics for sacrificial anodes include service life expectancy (typically 10-25 years depending on exposure conditions), current capacity (measured in amp-hours/kg), and driving voltage (the potential difference between the anode and steel). The efficiency ratio—comparing theoretical to actual current capacity—serves as a critical quality indicator, with high-performance systems achieving 80-95% efficiency.
Environmental factors significantly impact testing protocols, with temperature cycling (-20°C to +50°C) and varying humidity conditions (30-95% RH) incorporated into comprehensive evaluation regimes. Modern standards increasingly include alkalinity resistance testing, as concrete pH environments (12.5-13.5) can significantly affect anode dissolution mechanisms and long-term performance.
Recent developments in testing standards have introduced electrochemical impedance spectroscopy (EIS) as a non-destructive evaluation method, allowing for more precise characterization of anode-concrete interfaces and corrosion protection mechanisms without destructive sampling.
ISO 12696 offers comprehensive guidelines specifically for cathodic protection of steel in concrete, detailing performance metrics such as protective current density requirements (typically 2-20 mA/m²) and depolarization criteria. The standard stipulates that a minimum 100mV depolarization over a 24-hour period indicates effective protection against corrosion processes.
Field performance evaluation follows NACE SP0290, which establishes protocols for monitoring sacrificial anode systems in concrete structures exposed to marine environments. This standard requires periodic measurement of steel-to-concrete potentials, current output monitoring, and visual inspection of concrete condition to assess ongoing protection efficacy.
Accelerated testing methodologies have been developed to predict long-term performance within reasonable timeframes. ASTM B117 salt spray testing, modified for concrete applications, subjects anode systems to concentrated chloride exposure. Additionally, cyclic wetting/drying tests simulate coastal environmental conditions, with performance measured by weight loss, current output stability, and protective potential maintenance over time.
Key performance metrics for sacrificial anodes include service life expectancy (typically 10-25 years depending on exposure conditions), current capacity (measured in amp-hours/kg), and driving voltage (the potential difference between the anode and steel). The efficiency ratio—comparing theoretical to actual current capacity—serves as a critical quality indicator, with high-performance systems achieving 80-95% efficiency.
Environmental factors significantly impact testing protocols, with temperature cycling (-20°C to +50°C) and varying humidity conditions (30-95% RH) incorporated into comprehensive evaluation regimes. Modern standards increasingly include alkalinity resistance testing, as concrete pH environments (12.5-13.5) can significantly affect anode dissolution mechanisms and long-term performance.
Recent developments in testing standards have introduced electrochemical impedance spectroscopy (EIS) as a non-destructive evaluation method, allowing for more precise characterization of anode-concrete interfaces and corrosion protection mechanisms without destructive sampling.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!