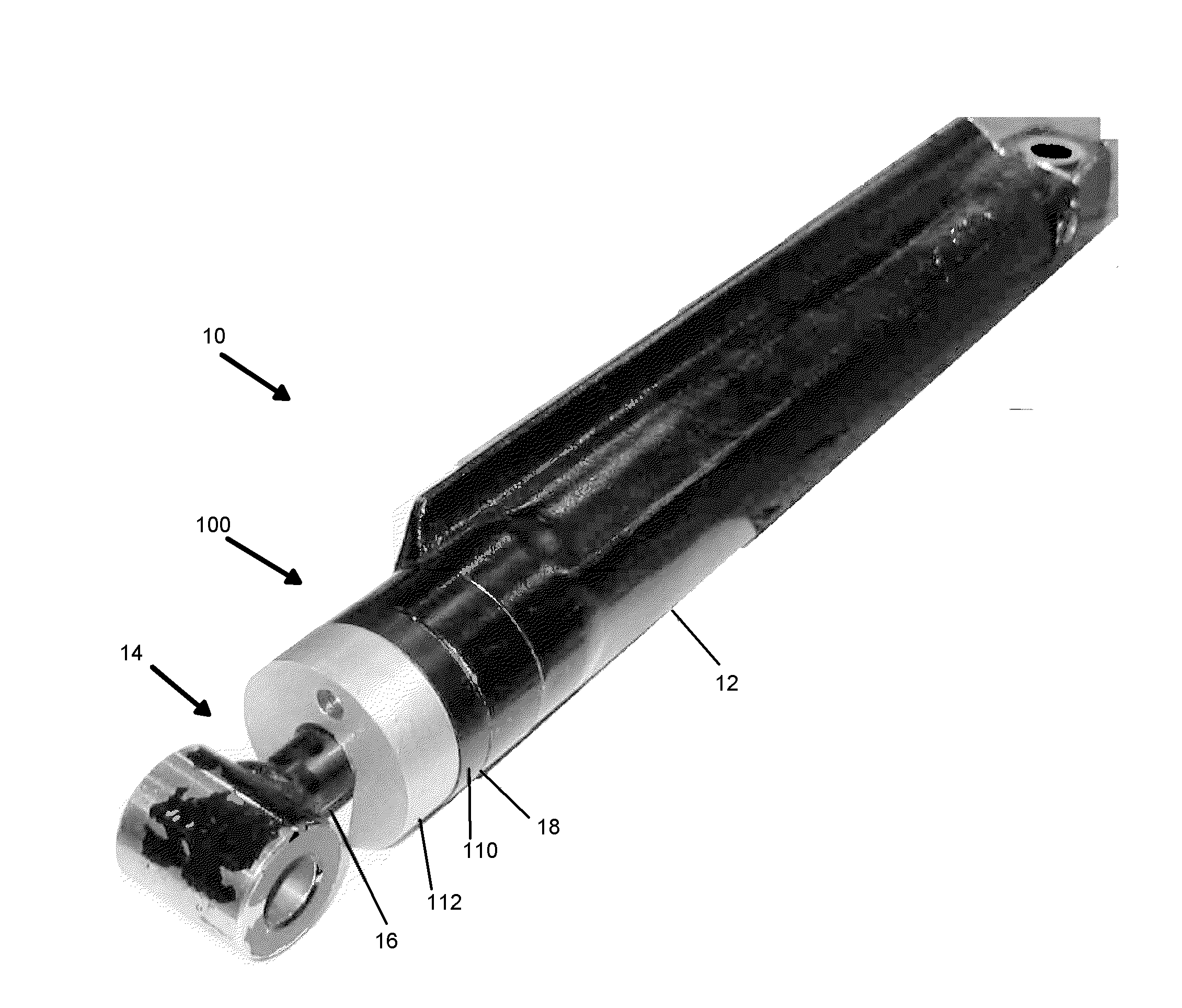
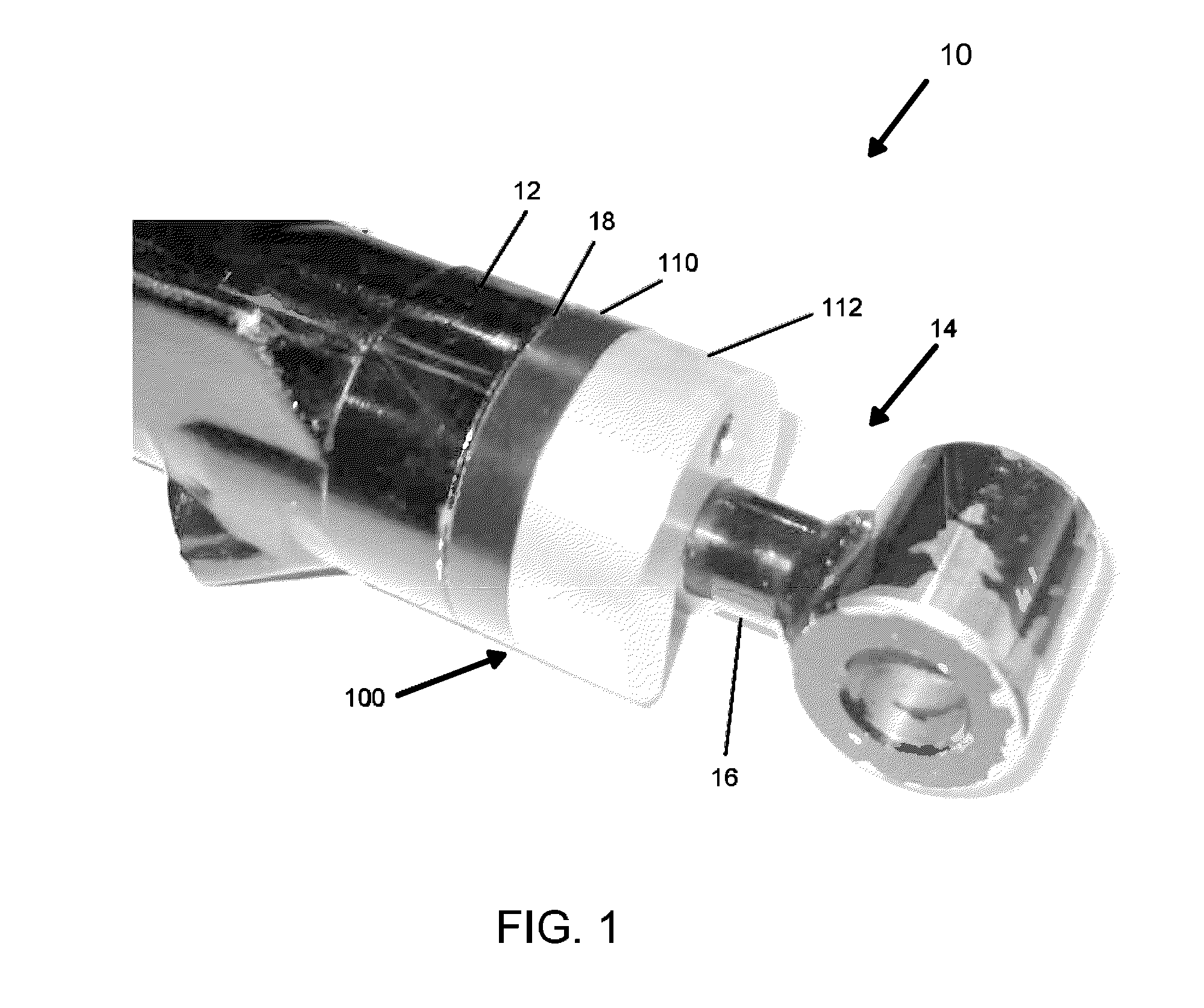
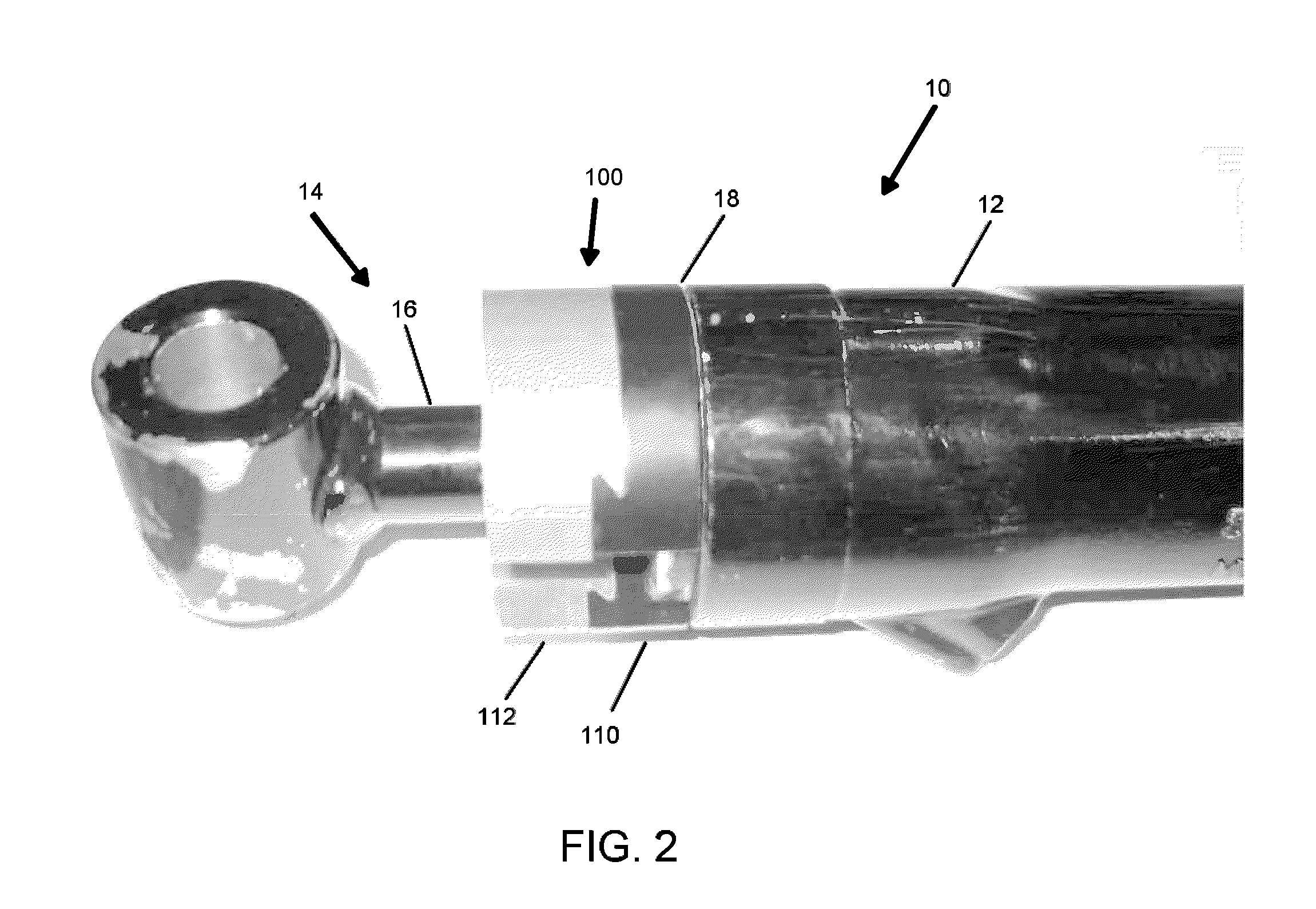
How Sacrificial Anodes Maintain Performance With Mixed Metals And Dissimilar Joints?
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sacrificial Anode Technology Background and Objectives
Sacrificial anode technology has evolved significantly since its inception in the early 19th century when Sir Humphry Davy first demonstrated the electrochemical protection of copper-clad ships using zinc and iron anodes. This galvanic corrosion protection method has since become fundamental across numerous industries including marine, oil and gas, underground pipelines, and structural engineering. The technology leverages the principle of galvanic series, where less noble metals preferentially corrode to protect more noble metals in the same electrolytic environment.
The evolution of sacrificial anode technology has been marked by significant advancements in material science, particularly in the development of specialized alloys. Early systems primarily utilized zinc, aluminum, and magnesium, but modern applications now incorporate precisely engineered alloys with controlled impurity levels and activation elements to enhance performance characteristics such as current capacity, operating life, and dissolution patterns.
In mixed-metal environments, the complexity of electrochemical interactions increases exponentially. When dissimilar metals are joined or placed in proximity within the same electrolytic medium, they create potential differences that accelerate corrosion processes. This phenomenon presents unique challenges for protective systems, particularly in modern industrial applications where material diversity is increasingly common due to performance and cost optimization requirements.
The technical objective of contemporary sacrificial anode systems in mixed-metal environments is to maintain consistent protective potential across all components despite varying electrochemical properties. This requires precise calculation of anode mass, distribution, and composition to ensure adequate current output throughout the designed service life while accommodating the different current demands of various metals in the system.
Recent research has focused on developing advanced modeling techniques to predict the behavior of sacrificial anodes in complex mixed-metal systems. These models incorporate factors such as electrolyte resistivity variations, temperature fluctuations, coating degradation patterns, and the influence of dissimilar metal joints on current distribution. The goal is to optimize anode placement and composition for maximum protection efficiency while minimizing material consumption.
The industry is moving toward more sophisticated approaches that consider the entire system's electrochemical behavior rather than treating individual components in isolation. This holistic perspective has led to innovations in anode design, including gradient composition anodes that can provide varying levels of protection potential across different sections of a structure, addressing the specific needs of dissimilar metal interfaces.
As industrial applications continue to incorporate increasingly diverse material combinations, the development of next-generation sacrificial anode technologies specifically engineered for mixed-metal environments represents a critical research direction with significant implications for infrastructure longevity and maintenance cost reduction.
The evolution of sacrificial anode technology has been marked by significant advancements in material science, particularly in the development of specialized alloys. Early systems primarily utilized zinc, aluminum, and magnesium, but modern applications now incorporate precisely engineered alloys with controlled impurity levels and activation elements to enhance performance characteristics such as current capacity, operating life, and dissolution patterns.
In mixed-metal environments, the complexity of electrochemical interactions increases exponentially. When dissimilar metals are joined or placed in proximity within the same electrolytic medium, they create potential differences that accelerate corrosion processes. This phenomenon presents unique challenges for protective systems, particularly in modern industrial applications where material diversity is increasingly common due to performance and cost optimization requirements.
The technical objective of contemporary sacrificial anode systems in mixed-metal environments is to maintain consistent protective potential across all components despite varying electrochemical properties. This requires precise calculation of anode mass, distribution, and composition to ensure adequate current output throughout the designed service life while accommodating the different current demands of various metals in the system.
Recent research has focused on developing advanced modeling techniques to predict the behavior of sacrificial anodes in complex mixed-metal systems. These models incorporate factors such as electrolyte resistivity variations, temperature fluctuations, coating degradation patterns, and the influence of dissimilar metal joints on current distribution. The goal is to optimize anode placement and composition for maximum protection efficiency while minimizing material consumption.
The industry is moving toward more sophisticated approaches that consider the entire system's electrochemical behavior rather than treating individual components in isolation. This holistic perspective has led to innovations in anode design, including gradient composition anodes that can provide varying levels of protection potential across different sections of a structure, addressing the specific needs of dissimilar metal interfaces.
As industrial applications continue to incorporate increasingly diverse material combinations, the development of next-generation sacrificial anode technologies specifically engineered for mixed-metal environments represents a critical research direction with significant implications for infrastructure longevity and maintenance cost reduction.
Market Demand Analysis for Galvanic Corrosion Protection
The global market for galvanic corrosion protection solutions has been experiencing steady growth, driven primarily by increasing infrastructure development and the expanding use of mixed metal systems across various industries. The market value for sacrificial anodes specifically reached approximately $2.8 billion in 2022, with projections indicating a compound annual growth rate of 5.7% through 2030.
Maritime and offshore industries represent the largest market segment, accounting for roughly 38% of total demand. This is attributed to the harsh saltwater environments that accelerate galvanic corrosion processes and the critical nature of maintaining structural integrity in these applications. The oil and gas sector follows closely, comprising about 27% of market demand, particularly for pipeline protection and offshore platform maintenance.
Recent market research indicates a significant shift toward advanced sacrificial anode materials that offer improved performance in complex mixed-metal environments. Aluminum-zinc-indium alloys have gained particular traction, showing a market growth rate nearly double that of traditional zinc anodes due to their superior performance characteristics when protecting dissimilar metal joints.
Geographically, Asia-Pacific dominates the market with approximately 42% share, driven by massive infrastructure development and maritime activities in China, Japan, and South Korea. North America and Europe collectively account for 45% of the market, with particularly strong demand in specialized industrial applications involving mixed metal systems.
Industry surveys reveal that end-users increasingly prioritize long-term performance over initial cost, with 73% of procurement specialists citing "extended service life in mixed metal environments" as a critical purchasing factor. This represents a significant shift from previous market dynamics where initial cost was the primary consideration.
Regulatory factors are also shaping market demand, with environmental regulations in Europe and North America driving the development of more environmentally friendly anode materials. The transition away from certain heavy metal components has created new market opportunities for innovative alloy formulations specifically designed for mixed metal protection.
The construction sector represents the fastest-growing market segment, with 8.3% annual growth, driven by increasing use of dissimilar metal joints in modern building designs. This trend is particularly evident in high-rise construction and infrastructure projects where galvanic compatibility issues must be addressed to ensure structural longevity.
Market analysis indicates that technological innovation in this space is primarily focused on developing sacrificial anodes with more predictable degradation rates when protecting multiple metal types simultaneously, addressing a key pain point identified by 67% of industrial users in recent industry surveys.
Maritime and offshore industries represent the largest market segment, accounting for roughly 38% of total demand. This is attributed to the harsh saltwater environments that accelerate galvanic corrosion processes and the critical nature of maintaining structural integrity in these applications. The oil and gas sector follows closely, comprising about 27% of market demand, particularly for pipeline protection and offshore platform maintenance.
Recent market research indicates a significant shift toward advanced sacrificial anode materials that offer improved performance in complex mixed-metal environments. Aluminum-zinc-indium alloys have gained particular traction, showing a market growth rate nearly double that of traditional zinc anodes due to their superior performance characteristics when protecting dissimilar metal joints.
Geographically, Asia-Pacific dominates the market with approximately 42% share, driven by massive infrastructure development and maritime activities in China, Japan, and South Korea. North America and Europe collectively account for 45% of the market, with particularly strong demand in specialized industrial applications involving mixed metal systems.
Industry surveys reveal that end-users increasingly prioritize long-term performance over initial cost, with 73% of procurement specialists citing "extended service life in mixed metal environments" as a critical purchasing factor. This represents a significant shift from previous market dynamics where initial cost was the primary consideration.
Regulatory factors are also shaping market demand, with environmental regulations in Europe and North America driving the development of more environmentally friendly anode materials. The transition away from certain heavy metal components has created new market opportunities for innovative alloy formulations specifically designed for mixed metal protection.
The construction sector represents the fastest-growing market segment, with 8.3% annual growth, driven by increasing use of dissimilar metal joints in modern building designs. This trend is particularly evident in high-rise construction and infrastructure projects where galvanic compatibility issues must be addressed to ensure structural longevity.
Market analysis indicates that technological innovation in this space is primarily focused on developing sacrificial anodes with more predictable degradation rates when protecting multiple metal types simultaneously, addressing a key pain point identified by 67% of industrial users in recent industry surveys.
Current Challenges in Mixed Metal Protection Systems
Despite significant advancements in cathodic protection systems, several critical challenges persist in protecting mixed metal assemblies. The fundamental issue stems from galvanic compatibility disparities between dissimilar metals, creating complex electrochemical interactions that conventional sacrificial anode systems struggle to address uniformly. When metals with substantially different electrode potentials are joined, the resulting galvanic cells can accelerate corrosion rates beyond the protective capacity of standard anode configurations.
A primary technical obstacle involves achieving balanced protection across all metal surfaces simultaneously. Current sacrificial anode systems often provide either excessive protection to one metal while leaving others vulnerable, or deliver insufficient protection across the entire assembly. This protection imbalance is particularly problematic in marine environments where chloride ions accelerate localized corrosion at dissimilar metal interfaces.
The effective distribution of protective current represents another significant challenge. In complex structures with intricate geometries and multiple metal types, ensuring uniform current distribution becomes increasingly difficult. Shadow effects and current attenuation in recessed areas create protection dead zones where corrosion can initiate and propagate undetected.
Environmental variability further complicates protection strategies. Fluctuating salinity, temperature, and oxygen levels in operational environments create dynamic corrosion conditions that static protection systems cannot adequately address. These variations can dramatically alter the performance parameters of sacrificial anodes, reducing their effectiveness and operational lifespan.
Material selection constraints also present significant hurdles. The limited range of commercially available sacrificial anode materials (primarily zinc, aluminum, and magnesium alloys) restricts optimization options for mixed metal systems. Each anode material offers specific protection characteristics that may not be ideal for all metals in a complex assembly.
Monitoring and maintenance challenges compound these technical issues. Traditional inspection methods often fail to detect early-stage corrosion at dissimilar metal interfaces, particularly in submerged or inaccessible locations. The lack of reliable real-time monitoring solutions for mixed metal systems prevents adaptive protection strategies that could address changing environmental conditions.
Cost considerations further restrict implementation of comprehensive solutions. While multi-anode systems with sophisticated control mechanisms could theoretically provide optimized protection, their complexity and expense limit widespread adoption, particularly in price-sensitive industries where corrosion is viewed as an inevitable operational cost rather than a preventable failure mode.
A primary technical obstacle involves achieving balanced protection across all metal surfaces simultaneously. Current sacrificial anode systems often provide either excessive protection to one metal while leaving others vulnerable, or deliver insufficient protection across the entire assembly. This protection imbalance is particularly problematic in marine environments where chloride ions accelerate localized corrosion at dissimilar metal interfaces.
The effective distribution of protective current represents another significant challenge. In complex structures with intricate geometries and multiple metal types, ensuring uniform current distribution becomes increasingly difficult. Shadow effects and current attenuation in recessed areas create protection dead zones where corrosion can initiate and propagate undetected.
Environmental variability further complicates protection strategies. Fluctuating salinity, temperature, and oxygen levels in operational environments create dynamic corrosion conditions that static protection systems cannot adequately address. These variations can dramatically alter the performance parameters of sacrificial anodes, reducing their effectiveness and operational lifespan.
Material selection constraints also present significant hurdles. The limited range of commercially available sacrificial anode materials (primarily zinc, aluminum, and magnesium alloys) restricts optimization options for mixed metal systems. Each anode material offers specific protection characteristics that may not be ideal for all metals in a complex assembly.
Monitoring and maintenance challenges compound these technical issues. Traditional inspection methods often fail to detect early-stage corrosion at dissimilar metal interfaces, particularly in submerged or inaccessible locations. The lack of reliable real-time monitoring solutions for mixed metal systems prevents adaptive protection strategies that could address changing environmental conditions.
Cost considerations further restrict implementation of comprehensive solutions. While multi-anode systems with sophisticated control mechanisms could theoretically provide optimized protection, their complexity and expense limit widespread adoption, particularly in price-sensitive industries where corrosion is viewed as an inevitable operational cost rather than a preventable failure mode.
Current Technical Solutions for Dissimilar Metal Joints
01 Material selection for sacrificial anodes
The choice of material for sacrificial anodes significantly impacts their performance and longevity. Commonly used materials include zinc, aluminum, and magnesium alloys, each with specific electrochemical properties suitable for different environments. The composition and purity of these materials affect the anode's efficiency, with higher purity materials generally providing better protection. Alloying elements can be added to enhance performance characteristics such as current capacity and dissolution behavior, ensuring optimal cathodic protection.- Material selection for sacrificial anodes: The choice of material for sacrificial anodes significantly impacts their performance and longevity. Commonly used materials include zinc, aluminum, and magnesium alloys, each with specific electrochemical properties suitable for different environments. The composition and purity of these materials directly affect the anode's efficiency, with higher purity materials generally providing better protection. Proper material selection ensures optimal galvanic protection while maximizing the service life of the sacrificial anode system.
- Monitoring and inspection techniques: Regular monitoring and inspection of sacrificial anode systems are essential for maintaining their effectiveness. This includes visual inspections, electrical potential measurements, and corrosion rate assessments. Advanced monitoring techniques may involve remote sensing technologies and automated data collection systems that provide real-time information about the anode's condition and performance. Implementing a systematic inspection schedule helps identify deterioration early and ensures the continued protection of the cathodically protected structure.
- Environmental factors affecting performance: Environmental conditions significantly impact sacrificial anode performance. Factors such as water temperature, salinity, pH, oxygen content, and flow rate can alter the electrochemical behavior of anodes. In marine environments, seasonal variations in water chemistry can affect dissolution rates, while in soil applications, moisture content and resistivity play crucial roles. Understanding these environmental influences allows for better design and maintenance strategies to optimize anode performance across varying conditions.
- Anode attachment and positioning systems: The method of attaching sacrificial anodes to the protected structure and their strategic positioning significantly affects protection efficiency. Proper electrical connection between the anode and the structure is critical for electron flow. Various attachment methods include welding, bolting, clamping, or using specialized brackets. Optimal positioning ensures uniform current distribution across the protected surface, preventing localized corrosion. Advanced designs incorporate adjustable mounting systems that allow for repositioning as conditions change or as anodes partially deplete.
- Replacement and maintenance protocols: Establishing systematic replacement and maintenance protocols is essential for continuous cathodic protection. This includes determining optimal replacement intervals based on anode consumption rates, developing procedures for safely removing depleted anodes, and installing new ones without disrupting protection. Maintenance activities may involve cleaning marine growth or scale from anodes, repairing damaged connections, and adjusting the system based on performance data. Implementing these protocols extends the overall system lifespan and ensures uninterrupted protection of valuable assets.
02 Monitoring and inspection systems
Regular monitoring and inspection of sacrificial anodes are essential for maintaining their effectiveness. Advanced monitoring systems can track anode consumption rates, remaining life, and protection levels in real-time. These systems often include sensors that measure electrical potential, current output, and environmental parameters. Periodic visual inspections and electrical measurements help identify issues before they compromise protection. Implementing scheduled inspection protocols ensures timely replacement of depleted anodes and maintains continuous protection of the cathodic structure.Expand Specific Solutions03 Installation techniques and positioning
Proper installation and strategic positioning of sacrificial anodes are crucial for optimal performance. The distance between anodes, their orientation relative to the protected structure, and attachment methods all affect protection efficiency. Techniques such as welding, bolting, or using specialized brackets ensure good electrical contact. For large structures, distributed placement of anodes provides uniform protection coverage. In complex systems, computational modeling can help determine optimal anode placement to maximize protection while minimizing the number of anodes required.Expand Specific Solutions04 Environmental adaptation and performance enhancement
Sacrificial anodes must be adapted to specific environmental conditions to maintain performance. In varying water salinity, temperature, or pH levels, different anode formulations may be required. Performance can be enhanced through surface treatments, protective coatings that control dissolution rates, or design modifications that increase current output. For harsh environments, specialized anode designs with improved resistance to passivation or increased current capacity can be employed. Some systems incorporate environmental sensors to adjust protection parameters based on changing conditions.Expand Specific Solutions05 Maintenance procedures and replacement strategies
Effective maintenance procedures and replacement strategies extend the service life of cathodic protection systems. This includes cleaning anodes to remove calcareous deposits or marine growth that may impede performance, implementing scheduled replacement programs based on calculated anode life, and documenting performance history to optimize future installations. Replacement strategies may involve phased approaches to ensure continuous protection or complete system overhauls during scheduled maintenance periods. Training personnel in proper maintenance techniques ensures consistent protection and maximizes the cost-effectiveness of sacrificial anode systems.Expand Specific Solutions
Key Industry Players in Sacrificial Anode Manufacturing
The sacrificial anode technology market is currently in a growth phase, with increasing applications across marine, oil and gas, and infrastructure sectors. The global cathodic protection market, which includes sacrificial anodes, is estimated at approximately $6.5 billion, with projected annual growth of 5-7%. Leading companies like BAC Corrosion Control, Vector Corrosion Technologies, and Magnesium Elektron are driving innovation in mixed-metal applications, while research institutions such as Naval Research Laboratory and Texas A&M University contribute to advancing technical solutions. PetroChina, China Petroleum & Chemical Corp, and State Grid Corp are major end-users implementing these technologies at scale. The market is characterized by increasing technical sophistication in addressing galvanic corrosion challenges in complex environments, with specialized solutions emerging for marine, industrial, and infrastructure applications.
BAC Corrosion Control Ltd
Technical Solution: BAC Corrosion Control has developed advanced sacrificial anode systems specifically engineered for mixed metal environments. Their technology utilizes high-potential aluminum-zinc-indium alloy anodes that provide effective cathodic protection across dissimilar metal joints. The company's proprietary anode composition features carefully calibrated percentages of zinc (4-6%) and indium (0.01-0.02%) to optimize current output and longevity when protecting multiple metal types simultaneously. BAC's systems incorporate specialized mounting configurations that ensure optimal current distribution across dissimilar joints, with strategic placement protocols that account for the varying electrochemical properties of different metals. Their anodes are designed with activation enhancers that reduce initial polarization time by up to 60%, allowing for faster protection of vulnerable dissimilar metal interfaces.
Strengths: Superior current distribution across multiple metal types; extended anode lifespan in complex environments; rapid polarization capabilities. Weakness: Higher initial cost compared to standard zinc or magnesium anodes; requires more precise installation to achieve optimal protection across dissimilar joints.
Magnesium Elektron Ltd.
Technical Solution: Magnesium Elektron has pioneered high-performance magnesium-based sacrificial anode systems specifically formulated for mixed metal environments. Their proprietary AZ63 alloy incorporates precisely controlled amounts of aluminum (6%) and zinc (3%) with trace elements that enhance performance when protecting dissimilar metals. The company's technology features a unique microstructure that promotes uniform dissolution patterns, preventing preferential corrosion paths that typically occur when protecting mixed metals. Their anodes maintain a consistent driving potential of -1.5V to -1.6V (vs. SCE) throughout their service life, ensuring continuous protection even as the anode mass decreases. Magnesium Elektron's systems include specialized connection hardware designed to minimize transition resistance between the anode and various protected metals, addressing one of the key challenges in mixed metal protection.
Strengths: High driving voltage suitable for high-resistivity environments; excellent performance in freshwater and soil applications; good protection range for multiple metal types. Weakness: Faster consumption rate than aluminum or zinc anodes; potential for hydrogen evolution at cathode surfaces; less efficient in high-chloride environments.
Core Innovations in Sacrificial Anode Materials
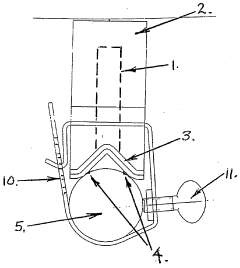
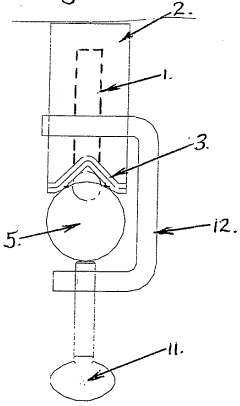
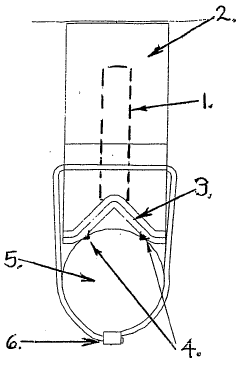
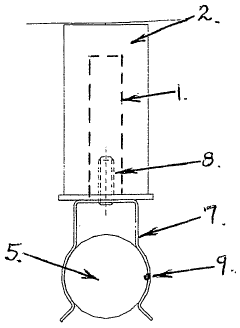
Anode mount assembly
PatentActiveUS20110017589A1
Innovation
- An anode mount assembly comprising a mount component made from non-corroding materials like stainless steel or aluminum, integrated or separately formed, with a sacrificial anode component such as zinc or magnesium, featuring a cooperating protrusion and recess pair and a single fastening mechanism for quick and secure mounting/dismounting.
Anode assembly and means of attachment
PatentWO2005080637A1
Innovation
- The use of deliquescent or hygroscopic chemicals like lithium nitrate and lithium bromide to maintain anodes in an active state, combined with a clamp system that provides a reliable low-resistance electrical connection to the reinforcing steel using metallic or polymeric clamps with spring action, knife-edges, or screw devices for secure attachment.
Environmental Impact of Sacrificial Anode Systems
The environmental impact of sacrificial anode systems represents a significant consideration in corrosion protection strategies, particularly when applied to mixed metal systems and dissimilar joints. These systems, while effective for corrosion prevention, introduce various environmental concerns that merit careful examination.
The primary environmental issue stems from the deliberate dissolution of sacrificial anodes, which releases metal ions into surrounding environments. In marine applications, zinc, aluminum, and magnesium anodes gradually disperse their constituent metals into seawater, potentially affecting local aquatic ecosystems. Studies have shown that zinc and aluminum ions can accumulate in sediments near heavily protected structures, with potential bioaccumulation in marine organisms occurring in high-concentration areas.
Water quality impacts vary significantly depending on water flow rates and the scale of anode implementation. In closed or semi-closed water systems, such as harbors or lakes, the concentration of dissolved metals may reach levels of environmental concern more rapidly than in open ocean environments where dilution factors are substantially higher. Regulatory frameworks in many jurisdictions now include specific provisions limiting the metal content in sacrificial anodes to mitigate these effects.
The manufacturing process of sacrificial anodes also carries an environmental footprint. Energy consumption during production, particularly for high-purity anodes required for mixed metal protection, contributes to carbon emissions. Additionally, the mining and processing of anode materials—especially zinc and aluminum—involve substantial resource extraction impacts and potential habitat disruption.
Recent technological developments have focused on creating more environmentally sustainable sacrificial anode systems. These include formulations with reduced heavy metal content, improved efficiency that requires less anode mass for equivalent protection periods, and enhanced activation properties that minimize unnecessary dissolution when protection is not required. For mixed metal systems specifically, targeted anode placement strategies help optimize protection while minimizing overall material consumption.
End-of-life considerations present another environmental dimension. Spent anodes often contain residual metal content that, if improperly disposed of, may contribute to landfill contamination. Recycling programs for sacrificial anode remnants have emerged as an important mitigation strategy, allowing for the recovery and reuse of valuable metals while reducing waste stream impacts.
The environmental trade-off calculation must also consider the alternative—unprotected systems experiencing accelerated corrosion may require more frequent replacement of entire structures, potentially resulting in greater overall environmental impact than that caused by controlled sacrificial anode systems.
The primary environmental issue stems from the deliberate dissolution of sacrificial anodes, which releases metal ions into surrounding environments. In marine applications, zinc, aluminum, and magnesium anodes gradually disperse their constituent metals into seawater, potentially affecting local aquatic ecosystems. Studies have shown that zinc and aluminum ions can accumulate in sediments near heavily protected structures, with potential bioaccumulation in marine organisms occurring in high-concentration areas.
Water quality impacts vary significantly depending on water flow rates and the scale of anode implementation. In closed or semi-closed water systems, such as harbors or lakes, the concentration of dissolved metals may reach levels of environmental concern more rapidly than in open ocean environments where dilution factors are substantially higher. Regulatory frameworks in many jurisdictions now include specific provisions limiting the metal content in sacrificial anodes to mitigate these effects.
The manufacturing process of sacrificial anodes also carries an environmental footprint. Energy consumption during production, particularly for high-purity anodes required for mixed metal protection, contributes to carbon emissions. Additionally, the mining and processing of anode materials—especially zinc and aluminum—involve substantial resource extraction impacts and potential habitat disruption.
Recent technological developments have focused on creating more environmentally sustainable sacrificial anode systems. These include formulations with reduced heavy metal content, improved efficiency that requires less anode mass for equivalent protection periods, and enhanced activation properties that minimize unnecessary dissolution when protection is not required. For mixed metal systems specifically, targeted anode placement strategies help optimize protection while minimizing overall material consumption.
End-of-life considerations present another environmental dimension. Spent anodes often contain residual metal content that, if improperly disposed of, may contribute to landfill contamination. Recycling programs for sacrificial anode remnants have emerged as an important mitigation strategy, allowing for the recovery and reuse of valuable metals while reducing waste stream impacts.
The environmental trade-off calculation must also consider the alternative—unprotected systems experiencing accelerated corrosion may require more frequent replacement of entire structures, potentially resulting in greater overall environmental impact than that caused by controlled sacrificial anode systems.
Standards and Testing Protocols for Mixed Metal Applications
The standardization of sacrificial anode systems for mixed metal applications represents a critical aspect of corrosion protection engineering. ASTM International has established several key standards that govern the testing and implementation of sacrificial anodes in mixed metal environments, including ASTM G71 for galvanic corrosion testing and ASTM B843 for quality requirements of zinc anodes. These standards provide essential frameworks for ensuring consistent performance across various industrial applications.
Testing protocols for sacrificial anodes in mixed metal systems typically involve accelerated corrosion testing under controlled conditions. Salt spray testing (ASTM B117) remains one of the most widely utilized methods, allowing engineers to evaluate anode performance in simulated aggressive environments. Electrochemical impedance spectroscopy (EIS) offers more sophisticated analysis by measuring the electrical response of the protected system across a range of frequencies, providing insights into the protective mechanisms at dissimilar metal interfaces.
Field exposure testing represents another crucial evaluation method, particularly for marine and offshore applications where mixed metal systems face extreme environmental challenges. These tests often follow standards such as NACE TM0169/G31 for laboratory immersion testing or ISO 9223 for atmospheric corrosion classification, with exposure periods ranging from months to years to capture long-term performance data.
The electrical potential measurement protocol, standardized under ASTM C876, serves as a non-destructive evaluation technique for monitoring sacrificial anode systems in real-time. This approach enables maintenance engineers to assess protection levels without system disruption, particularly valuable for complex mixed metal assemblies in critical infrastructure.
Quality assurance standards for sacrificial anodes have evolved significantly, with ISO 15589-2 specifically addressing cathodic protection of offshore structures and DNV-RP-B401 focusing on cathodic protection design. These standards establish minimum requirements for chemical composition, microstructure, and electrochemical properties of anodes used in mixed metal applications.
Performance validation protocols typically include current output testing and consumption rate analysis. The former measures the electrical current generated by the anode when connected to the protected metals, while the latter quantifies material loss over time. Both metrics must meet industry-specific thresholds established by organizations such as NACE International and the American Bureau of Shipping.
Recent advancements in testing methodologies have incorporated digital monitoring systems that enable continuous data collection on anode performance in mixed metal environments. These systems, while not yet fully standardized, represent the cutting edge of quality control for sacrificial anode applications, allowing for predictive maintenance and optimization of protection systems across diverse industrial settings.
Testing protocols for sacrificial anodes in mixed metal systems typically involve accelerated corrosion testing under controlled conditions. Salt spray testing (ASTM B117) remains one of the most widely utilized methods, allowing engineers to evaluate anode performance in simulated aggressive environments. Electrochemical impedance spectroscopy (EIS) offers more sophisticated analysis by measuring the electrical response of the protected system across a range of frequencies, providing insights into the protective mechanisms at dissimilar metal interfaces.
Field exposure testing represents another crucial evaluation method, particularly for marine and offshore applications where mixed metal systems face extreme environmental challenges. These tests often follow standards such as NACE TM0169/G31 for laboratory immersion testing or ISO 9223 for atmospheric corrosion classification, with exposure periods ranging from months to years to capture long-term performance data.
The electrical potential measurement protocol, standardized under ASTM C876, serves as a non-destructive evaluation technique for monitoring sacrificial anode systems in real-time. This approach enables maintenance engineers to assess protection levels without system disruption, particularly valuable for complex mixed metal assemblies in critical infrastructure.
Quality assurance standards for sacrificial anodes have evolved significantly, with ISO 15589-2 specifically addressing cathodic protection of offshore structures and DNV-RP-B401 focusing on cathodic protection design. These standards establish minimum requirements for chemical composition, microstructure, and electrochemical properties of anodes used in mixed metal applications.
Performance validation protocols typically include current output testing and consumption rate analysis. The former measures the electrical current generated by the anode when connected to the protected metals, while the latter quantifies material loss over time. Both metrics must meet industry-specific thresholds established by organizations such as NACE International and the American Bureau of Shipping.
Recent advancements in testing methodologies have incorporated digital monitoring systems that enable continuous data collection on anode performance in mixed metal environments. These systems, while not yet fully standardized, represent the cutting edge of quality control for sacrificial anode applications, allowing for predictive maintenance and optimization of protection systems across diverse industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!