How to Enhance Carboxylic Acid Protein Binding Properties?
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carboxylic Acid Binding Background and Objectives
Carboxylic acids play a crucial role in various biological processes, particularly in protein-ligand interactions. The binding properties of carboxylic acids to proteins have been a subject of extensive research due to their significance in drug discovery, enzyme catalysis, and molecular recognition. Over the years, scientists have made significant strides in understanding the mechanisms underlying these interactions, leading to the development of novel therapeutic agents and molecular tools.
The evolution of carboxylic acid-protein binding research can be traced back to the early 20th century when researchers first began to explore the chemical nature of enzyme-substrate interactions. As analytical techniques advanced, so did our understanding of the intricate molecular interactions involved in carboxylic acid binding. The advent of X-ray crystallography and nuclear magnetic resonance spectroscopy in the mid-20th century revolutionized the field, allowing researchers to visualize protein structures and binding sites with unprecedented detail.
In recent decades, the focus has shifted towards enhancing the binding properties of carboxylic acids to proteins. This pursuit is driven by the need for more effective drugs, improved molecular probes, and better understanding of biological processes. The primary objectives in this field include increasing binding affinity, improving selectivity, and optimizing pharmacokinetic properties of carboxylic acid-based ligands.
One of the key trends in this area is the development of structure-based design approaches. By leveraging high-resolution protein structures and computational modeling, researchers aim to rationally design carboxylic acid derivatives with enhanced binding properties. This approach has led to the discovery of novel compounds with improved potency and selectivity for specific protein targets.
Another significant trend is the exploration of non-covalent interactions beyond the traditional hydrogen bonding of the carboxyl group. Researchers are investigating the role of hydrophobic interactions, π-π stacking, and electrostatic complementarity in enhancing binding affinity and specificity. This holistic approach to ligand design has opened up new avenues for optimizing carboxylic acid-protein interactions.
The field is also witnessing a growing interest in the development of multivalent ligands. By incorporating multiple carboxylic acid moieties into a single molecule, researchers aim to exploit avidity effects and achieve stronger overall binding to protein targets. This strategy has shown promise in various applications, including the design of enzyme inhibitors and targeted drug delivery systems.
As we look to the future, the enhancement of carboxylic acid protein binding properties continues to be a dynamic and evolving field. The integration of advanced computational methods, high-throughput screening technologies, and novel synthetic approaches is expected to accelerate progress in this area. The ultimate goal remains the development of more effective and selective molecular tools for both basic research and therapeutic applications.
The evolution of carboxylic acid-protein binding research can be traced back to the early 20th century when researchers first began to explore the chemical nature of enzyme-substrate interactions. As analytical techniques advanced, so did our understanding of the intricate molecular interactions involved in carboxylic acid binding. The advent of X-ray crystallography and nuclear magnetic resonance spectroscopy in the mid-20th century revolutionized the field, allowing researchers to visualize protein structures and binding sites with unprecedented detail.
In recent decades, the focus has shifted towards enhancing the binding properties of carboxylic acids to proteins. This pursuit is driven by the need for more effective drugs, improved molecular probes, and better understanding of biological processes. The primary objectives in this field include increasing binding affinity, improving selectivity, and optimizing pharmacokinetic properties of carboxylic acid-based ligands.
One of the key trends in this area is the development of structure-based design approaches. By leveraging high-resolution protein structures and computational modeling, researchers aim to rationally design carboxylic acid derivatives with enhanced binding properties. This approach has led to the discovery of novel compounds with improved potency and selectivity for specific protein targets.
Another significant trend is the exploration of non-covalent interactions beyond the traditional hydrogen bonding of the carboxyl group. Researchers are investigating the role of hydrophobic interactions, π-π stacking, and electrostatic complementarity in enhancing binding affinity and specificity. This holistic approach to ligand design has opened up new avenues for optimizing carboxylic acid-protein interactions.
The field is also witnessing a growing interest in the development of multivalent ligands. By incorporating multiple carboxylic acid moieties into a single molecule, researchers aim to exploit avidity effects and achieve stronger overall binding to protein targets. This strategy has shown promise in various applications, including the design of enzyme inhibitors and targeted drug delivery systems.
As we look to the future, the enhancement of carboxylic acid protein binding properties continues to be a dynamic and evolving field. The integration of advanced computational methods, high-throughput screening technologies, and novel synthetic approaches is expected to accelerate progress in this area. The ultimate goal remains the development of more effective and selective molecular tools for both basic research and therapeutic applications.
Market Analysis for Enhanced Protein Binding
The market for enhanced protein binding, particularly in the context of carboxylic acid interactions, has shown significant growth potential in recent years. This trend is driven by the increasing demand for more effective drug delivery systems, improved biocatalysts, and advanced materials in various industries. The pharmaceutical sector, in particular, has been a major driver of this market, as enhanced protein binding properties can lead to more efficient drug formulations and improved therapeutic outcomes.
In the pharmaceutical industry, the global market for protein-based drugs is projected to reach substantial figures in the coming years, with a considerable portion of this market relying on technologies that enhance protein binding properties. The ability to improve carboxylic acid protein binding has direct implications for drug efficacy, bioavailability, and reduced side effects, making it a key focus area for pharmaceutical companies and research institutions.
Beyond pharmaceuticals, the biotechnology sector has also shown keen interest in enhanced protein binding technologies. Enzymes with improved binding properties are in high demand for industrial applications, including biofuel production, food processing, and environmental remediation. The global industrial enzymes market, which benefits from advancements in protein binding, has been experiencing steady growth.
The materials science industry is another significant player in this market. Enhanced protein binding properties are crucial for developing advanced biomaterials, such as tissue engineering scaffolds, biosensors, and smart materials for various applications. The growing interest in personalized medicine and regenerative therapies has further fueled the demand for materials with precisely controlled protein-binding characteristics.
In the agricultural sector, there is an emerging market for enhanced protein binding technologies in the development of more effective and environmentally friendly pesticides and fertilizers. By improving the binding properties of active ingredients to target proteins, these products can achieve better efficacy with lower environmental impact.
Geographically, North America and Europe currently lead the market for enhanced protein binding technologies, primarily due to their strong pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing investments in life sciences research and a rapidly expanding healthcare sector.
The market is characterized by a mix of large pharmaceutical and biotechnology companies, specialized research institutions, and innovative startups. Collaborations between academic institutions and industry players are becoming increasingly common, accelerating the pace of innovation in this field. As the demand for more sophisticated and targeted therapies continues to grow, the market for enhanced carboxylic acid protein binding properties is expected to expand further, offering significant opportunities for companies that can develop and commercialize effective solutions in this space.
In the pharmaceutical industry, the global market for protein-based drugs is projected to reach substantial figures in the coming years, with a considerable portion of this market relying on technologies that enhance protein binding properties. The ability to improve carboxylic acid protein binding has direct implications for drug efficacy, bioavailability, and reduced side effects, making it a key focus area for pharmaceutical companies and research institutions.
Beyond pharmaceuticals, the biotechnology sector has also shown keen interest in enhanced protein binding technologies. Enzymes with improved binding properties are in high demand for industrial applications, including biofuel production, food processing, and environmental remediation. The global industrial enzymes market, which benefits from advancements in protein binding, has been experiencing steady growth.
The materials science industry is another significant player in this market. Enhanced protein binding properties are crucial for developing advanced biomaterials, such as tissue engineering scaffolds, biosensors, and smart materials for various applications. The growing interest in personalized medicine and regenerative therapies has further fueled the demand for materials with precisely controlled protein-binding characteristics.
In the agricultural sector, there is an emerging market for enhanced protein binding technologies in the development of more effective and environmentally friendly pesticides and fertilizers. By improving the binding properties of active ingredients to target proteins, these products can achieve better efficacy with lower environmental impact.
Geographically, North America and Europe currently lead the market for enhanced protein binding technologies, primarily due to their strong pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing investments in life sciences research and a rapidly expanding healthcare sector.
The market is characterized by a mix of large pharmaceutical and biotechnology companies, specialized research institutions, and innovative startups. Collaborations between academic institutions and industry players are becoming increasingly common, accelerating the pace of innovation in this field. As the demand for more sophisticated and targeted therapies continues to grow, the market for enhanced carboxylic acid protein binding properties is expected to expand further, offering significant opportunities for companies that can develop and commercialize effective solutions in this space.
Current Challenges in Carboxylic Acid-Protein Interactions
The field of carboxylic acid-protein interactions faces several significant challenges that hinder the enhancement of binding properties. One of the primary obstacles is the inherent complexity of protein structures and their dynamic nature. Proteins are not static entities but constantly undergo conformational changes, making it difficult to predict and control their interactions with carboxylic acids accurately.
Another major challenge lies in the diverse chemical properties of carboxylic acids themselves. The varying chain lengths, degrees of saturation, and functional group substitutions result in a wide range of binding affinities and specificities. This diversity complicates the development of universal strategies to enhance binding properties across different carboxylic acid-protein systems.
The influence of environmental factors, such as pH and ionic strength, presents additional hurdles. These factors can significantly alter the ionization state of both carboxylic acids and protein residues, thereby affecting their electrostatic interactions and overall binding affinity. Researchers must carefully consider and control these parameters to achieve consistent and reproducible results.
Furthermore, the presence of competing molecules in biological systems poses a substantial challenge. In vivo, numerous other biomolecules can interfere with carboxylic acid-protein interactions, potentially reducing binding efficiency or leading to unintended side effects. Developing strategies to enhance specificity and overcome these competitive interactions remains a critical area of research.
The limited structural information available for many protein-ligand complexes involving carboxylic acids also impedes progress. While X-ray crystallography and NMR spectroscopy have provided valuable insights, obtaining high-resolution structures for all relevant complexes is time-consuming and often technically challenging. This lack of structural data hampers the rational design of improved binding strategies.
Additionally, the multifaceted nature of protein-ligand interactions, involving not only electrostatic forces but also hydrophobic effects, hydrogen bonding, and van der Waals interactions, complicates efforts to enhance binding properties. Balancing these various forces to achieve optimal binding while maintaining protein function and stability is a delicate task that requires sophisticated approaches.
Lastly, the challenge of translating in vitro findings to in vivo applications remains significant. Many strategies that show promise in controlled laboratory conditions may fail to perform adequately in the complex cellular environment. Overcoming this translational gap requires innovative approaches that can maintain enhanced binding properties under physiological conditions while ensuring biocompatibility and minimizing off-target effects.
Another major challenge lies in the diverse chemical properties of carboxylic acids themselves. The varying chain lengths, degrees of saturation, and functional group substitutions result in a wide range of binding affinities and specificities. This diversity complicates the development of universal strategies to enhance binding properties across different carboxylic acid-protein systems.
The influence of environmental factors, such as pH and ionic strength, presents additional hurdles. These factors can significantly alter the ionization state of both carboxylic acids and protein residues, thereby affecting their electrostatic interactions and overall binding affinity. Researchers must carefully consider and control these parameters to achieve consistent and reproducible results.
Furthermore, the presence of competing molecules in biological systems poses a substantial challenge. In vivo, numerous other biomolecules can interfere with carboxylic acid-protein interactions, potentially reducing binding efficiency or leading to unintended side effects. Developing strategies to enhance specificity and overcome these competitive interactions remains a critical area of research.
The limited structural information available for many protein-ligand complexes involving carboxylic acids also impedes progress. While X-ray crystallography and NMR spectroscopy have provided valuable insights, obtaining high-resolution structures for all relevant complexes is time-consuming and often technically challenging. This lack of structural data hampers the rational design of improved binding strategies.
Additionally, the multifaceted nature of protein-ligand interactions, involving not only electrostatic forces but also hydrophobic effects, hydrogen bonding, and van der Waals interactions, complicates efforts to enhance binding properties. Balancing these various forces to achieve optimal binding while maintaining protein function and stability is a delicate task that requires sophisticated approaches.
Lastly, the challenge of translating in vitro findings to in vivo applications remains significant. Many strategies that show promise in controlled laboratory conditions may fail to perform adequately in the complex cellular environment. Overcoming this translational gap requires innovative approaches that can maintain enhanced binding properties under physiological conditions while ensuring biocompatibility and minimizing off-target effects.
Existing Methods for Improving Carboxylic Acid Binding
01 Carboxylic acid interactions with protein binding sites
Carboxylic acids play a crucial role in protein binding by interacting with specific binding sites on proteins. These interactions can involve hydrogen bonding, electrostatic interactions, or other non-covalent forces. Understanding these interactions is essential for drug design and development, as many pharmaceuticals contain carboxylic acid groups that interact with target proteins.- Carboxylic acid interactions with protein binding sites: Carboxylic acids play a crucial role in protein binding by interacting with specific sites on proteins. These interactions can involve hydrogen bonding, electrostatic interactions, or van der Waals forces. The binding properties of carboxylic acids to proteins are influenced by factors such as pH, ionic strength, and the presence of other molecules in the environment.
- Structure-activity relationships of carboxylic acids in protein binding: The structural features of carboxylic acids, including chain length, branching, and functional group substitutions, significantly affect their protein binding properties. Understanding these structure-activity relationships helps in designing molecules with improved binding affinity and specificity for target proteins, which is crucial in drug discovery and development.
- Analytical methods for studying carboxylic acid-protein interactions: Various analytical techniques are employed to study the binding properties of carboxylic acids to proteins. These methods include spectroscopic techniques, calorimetry, surface plasmon resonance, and computational modeling. These approaches provide insights into binding kinetics, thermodynamics, and structural changes associated with carboxylic acid-protein interactions.
- Applications of carboxylic acid-protein binding in biotechnology: The understanding of carboxylic acid-protein binding properties has led to various applications in biotechnology. These include the development of affinity chromatography techniques, design of enzyme inhibitors, and creation of biosensors. The specific interactions between carboxylic acids and proteins are exploited to achieve selective separation, purification, or detection of target molecules.
- Role of carboxylic acids in protein function and regulation: Carboxylic acids play important roles in modulating protein function and regulation. They can act as allosteric regulators, cofactors, or post-translational modifications. The binding of carboxylic acids to proteins can induce conformational changes, alter enzymatic activity, or influence protein-protein interactions, thereby affecting various cellular processes and signaling pathways.
02 Influence of carboxylic acids on protein structure and function
Carboxylic acids can affect protein structure and function by altering the local environment around binding sites or inducing conformational changes. This influence can lead to changes in protein activity, stability, or interactions with other molecules. Studying these effects is important for understanding protein-ligand interactions and developing new therapeutic approaches.Expand Specific Solutions03 Analytical methods for studying carboxylic acid-protein interactions
Various analytical techniques are employed to study carboxylic acid-protein binding properties. These may include spectroscopic methods, calorimetry, surface plasmon resonance, and computational modeling. These techniques help researchers quantify binding affinities, determine binding kinetics, and elucidate the structural basis of carboxylic acid-protein interactions.Expand Specific Solutions04 Role of carboxylic acids in protein-based biosensors and diagnostics
Carboxylic acid-protein interactions are utilized in the development of biosensors and diagnostic tools. These interactions can be exploited to create highly specific and sensitive detection methods for various analytes, including biomarkers, drugs, and environmental contaminants. Understanding the binding properties of carboxylic acids with proteins is crucial for optimizing these diagnostic platforms.Expand Specific Solutions05 Carboxylic acid modifications for enhanced protein binding
Researchers explore various modifications of carboxylic acids to enhance their protein binding properties. These modifications may include the addition of functional groups, changes in chain length, or incorporation into larger molecular structures. Such modifications can lead to improved binding affinity, selectivity, or stability of carboxylic acid-protein complexes, with applications in drug discovery and materials science.Expand Specific Solutions
Key Players in Protein-Ligand Binding Research
The field of enhancing carboxylic acid protein binding properties is in a growth phase, with increasing market size and technological advancements. The global market for protein-based pharmaceuticals, which heavily relies on this technology, is projected to reach significant value in the coming years. Companies like Novo Nordisk, Amgen, and Roche Diagnostics are at the forefront, investing heavily in R&D to improve binding properties. The technology's maturity is advancing, with academic institutions such as Jiangnan University and the University of Bristol contributing to fundamental research. Collaborations between industry leaders and research institutions are driving innovation, pushing the boundaries of protein engineering and drug development.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a multifaceted approach to enhance carboxylic acid protein binding properties, focusing on both chemical modifications and innovative formulation techniques. Their strategy involves the development of novel carboxylic acid derivatives with enhanced lipophilicity and membrane permeability, while maintaining the essential acidic functionality[4]. DuPont's researchers have successfully synthesized a series of prodrugs that utilize enzymatic or pH-dependent cleavage to release the active carboxylic acid moiety at the target site, thereby improving bioavailability and target engagement[5]. Furthermore, they have explored the use of nanocarrier systems, such as lipid nanoparticles and polymeric micelles, to encapsulate and deliver carboxylic acid-containing compounds more effectively to their protein targets[6]. This approach has shown promise in overcoming solubility and stability issues often associated with carboxylic acids.
Strengths: Improved bioavailability and target engagement, versatile approach applicable to various compounds. Weaknesses: Potential complexity in manufacturing processes, regulatory challenges associated with novel formulations.
Roche Diagnostics GmbH
Technical Solution: Roche Diagnostics has developed an innovative platform for enhancing carboxylic acid protein binding properties, focusing on the application of fragment-based drug discovery (FBDD) and structure-guided design. Their approach involves the systematic screening of carboxylic acid-containing fragments against protein targets using high-throughput X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy[7]. By identifying key binding interactions and optimizing fragment combinations, Roche has successfully created lead compounds with improved affinity and specificity for target proteins. Additionally, they have implemented a novel "grow-link-merge" strategy, where carboxylic acid fragments are strategically expanded and linked to create larger molecules with enhanced binding properties[8]. This method has been particularly effective in developing potent inhibitors for challenging protein targets, such as protein-protein interactions.
Strengths: Highly rational and structure-guided approach, potential for discovering novel binding modes. Weaknesses: Resource-intensive process, may be limited by the availability of high-quality protein structures.
Innovative Approaches in Carboxylic Acid Modification
Novel plasma protein affinity tags
PatentWO2005028516A2
Innovation
- Development of novel plasma protein affinity tags, specifically carboxylic acid mimetics like α-acylsulfonamides, which can be covalently linked to therapeutic agents, enabling reversible binding to plasma proteins like albumin, thereby extending their half-life without compromising their biological activity.
Coupling method for peptide synthesis at elevated temperatures
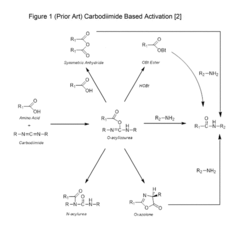
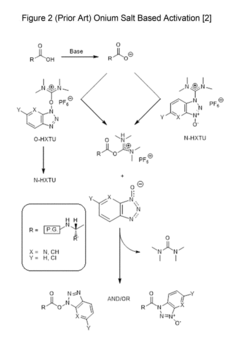
PatentActiveUS20170342104A1
Innovation
- A modified carbodiimide activation strategy that incorporates a strong base in an amount less than one equivalent compared to the amino acid, used during the activation and coupling process at temperatures greater than 30°C, to enhance coupling reaction speed and purity while minimizing side reactions.
Structure-Activity Relationship Studies
Structure-Activity Relationship (SAR) studies play a crucial role in enhancing the protein binding properties of carboxylic acids. These studies systematically investigate the relationship between chemical structure and biological activity, providing valuable insights for optimizing molecular design.
In the context of carboxylic acid protein binding, SAR studies typically focus on modifying various structural elements of the carboxylic acid molecule. These modifications may include altering the carbon chain length, introducing substituents, or changing the position of functional groups. By systematically varying these structural features, researchers can identify key factors that influence protein binding affinity and selectivity.
One important aspect of SAR studies is the exploration of different substituents on the carboxylic acid scaffold. Electron-withdrawing or electron-donating groups can significantly impact the electronic properties of the carboxyl group, thereby affecting its interaction with protein binding sites. For instance, the introduction of halogen atoms or nitro groups may enhance binding affinity through increased polarization or additional hydrogen bonding interactions.
The position of substituents relative to the carboxyl group is another critical factor in SAR studies. Researchers often investigate how the proximity of functional groups to the carboxyl moiety influences binding properties. This can lead to the identification of optimal spatial arrangements that maximize protein-ligand interactions.
Stereochemistry also plays a vital role in carboxylic acid protein binding. SAR studies frequently explore the impact of different stereoisomers on binding affinity and selectivity. This is particularly important when targeting specific protein binding sites that exhibit stereochemical preferences.
Furthermore, SAR studies often involve the synthesis and evaluation of carboxylic acid derivatives, such as esters or amides. These modifications can provide insights into the importance of the carboxyl group itself and help identify alternative functional groups that may enhance protein binding properties.
Computational methods, including molecular docking and quantitative structure-activity relationship (QSAR) analyses, are increasingly employed in SAR studies. These techniques allow researchers to predict binding affinities and explore a wider range of structural modifications in silico, complementing experimental approaches.
By systematically analyzing the results of SAR studies, researchers can identify trends and patterns in the relationship between chemical structure and protein binding properties. This knowledge can then be applied to design optimized carboxylic acid derivatives with enhanced binding characteristics, potentially leading to improved drug candidates or molecular probes for biological research.
In the context of carboxylic acid protein binding, SAR studies typically focus on modifying various structural elements of the carboxylic acid molecule. These modifications may include altering the carbon chain length, introducing substituents, or changing the position of functional groups. By systematically varying these structural features, researchers can identify key factors that influence protein binding affinity and selectivity.
One important aspect of SAR studies is the exploration of different substituents on the carboxylic acid scaffold. Electron-withdrawing or electron-donating groups can significantly impact the electronic properties of the carboxyl group, thereby affecting its interaction with protein binding sites. For instance, the introduction of halogen atoms or nitro groups may enhance binding affinity through increased polarization or additional hydrogen bonding interactions.
The position of substituents relative to the carboxyl group is another critical factor in SAR studies. Researchers often investigate how the proximity of functional groups to the carboxyl moiety influences binding properties. This can lead to the identification of optimal spatial arrangements that maximize protein-ligand interactions.
Stereochemistry also plays a vital role in carboxylic acid protein binding. SAR studies frequently explore the impact of different stereoisomers on binding affinity and selectivity. This is particularly important when targeting specific protein binding sites that exhibit stereochemical preferences.
Furthermore, SAR studies often involve the synthesis and evaluation of carboxylic acid derivatives, such as esters or amides. These modifications can provide insights into the importance of the carboxyl group itself and help identify alternative functional groups that may enhance protein binding properties.
Computational methods, including molecular docking and quantitative structure-activity relationship (QSAR) analyses, are increasingly employed in SAR studies. These techniques allow researchers to predict binding affinities and explore a wider range of structural modifications in silico, complementing experimental approaches.
By systematically analyzing the results of SAR studies, researchers can identify trends and patterns in the relationship between chemical structure and protein binding properties. This knowledge can then be applied to design optimized carboxylic acid derivatives with enhanced binding characteristics, potentially leading to improved drug candidates or molecular probes for biological research.
Computational Modeling for Binding Prediction
Computational modeling has emerged as a powerful tool for predicting and enhancing carboxylic acid protein binding properties. This approach leverages advanced algorithms and molecular simulation techniques to analyze and optimize the interactions between carboxylic acids and target proteins.
One of the primary methods employed in computational modeling for binding prediction is molecular docking. This technique simulates the binding process between a ligand (in this case, a carboxylic acid) and a protein receptor. By exploring various conformations and orientations, docking algorithms can identify potential binding sites and estimate binding affinities. These predictions help researchers prioritize promising carboxylic acid candidates for further experimental validation.
Molecular dynamics simulations offer another valuable approach for studying carboxylic acid-protein interactions. These simulations model the dynamic behavior of molecules over time, providing insights into the stability and flexibility of protein-ligand complexes. By analyzing trajectories from these simulations, researchers can identify key residues involved in binding and understand the energetics of the interaction.
Quantitative structure-activity relationship (QSAR) models are also widely used in computational binding prediction. These models correlate molecular descriptors of carboxylic acids with their experimentally determined binding affinities. By training machine learning algorithms on large datasets, QSAR models can predict the binding properties of novel carboxylic acid compounds, facilitating the design of improved ligands.
Fragment-based drug design (FBDD) is another computational strategy that has shown promise in enhancing carboxylic acid protein binding. This approach involves identifying small molecular fragments that bind to specific protein sites and then linking or growing these fragments to create more potent ligands. Computational tools can efficiently screen large fragment libraries and guide the optimization process.
Recent advancements in deep learning have led to the development of neural network-based models for predicting protein-ligand interactions. These models can learn complex patterns from large-scale structural and biochemical data, potentially offering more accurate predictions than traditional methods. However, the interpretability of these black-box models remains a challenge.
Integrating multiple computational approaches often yields more robust predictions. For example, combining molecular docking with molecular dynamics simulations can provide a more comprehensive understanding of binding mechanisms. Similarly, ensemble docking methods that consider multiple protein conformations can account for protein flexibility and improve prediction accuracy.
As computational power continues to increase and algorithms become more sophisticated, the role of computational modeling in enhancing carboxylic acid protein binding properties is expected to grow. These in silico approaches not only accelerate the drug discovery process but also provide valuable insights into the fundamental principles governing molecular recognition and binding.
One of the primary methods employed in computational modeling for binding prediction is molecular docking. This technique simulates the binding process between a ligand (in this case, a carboxylic acid) and a protein receptor. By exploring various conformations and orientations, docking algorithms can identify potential binding sites and estimate binding affinities. These predictions help researchers prioritize promising carboxylic acid candidates for further experimental validation.
Molecular dynamics simulations offer another valuable approach for studying carboxylic acid-protein interactions. These simulations model the dynamic behavior of molecules over time, providing insights into the stability and flexibility of protein-ligand complexes. By analyzing trajectories from these simulations, researchers can identify key residues involved in binding and understand the energetics of the interaction.
Quantitative structure-activity relationship (QSAR) models are also widely used in computational binding prediction. These models correlate molecular descriptors of carboxylic acids with their experimentally determined binding affinities. By training machine learning algorithms on large datasets, QSAR models can predict the binding properties of novel carboxylic acid compounds, facilitating the design of improved ligands.
Fragment-based drug design (FBDD) is another computational strategy that has shown promise in enhancing carboxylic acid protein binding. This approach involves identifying small molecular fragments that bind to specific protein sites and then linking or growing these fragments to create more potent ligands. Computational tools can efficiently screen large fragment libraries and guide the optimization process.
Recent advancements in deep learning have led to the development of neural network-based models for predicting protein-ligand interactions. These models can learn complex patterns from large-scale structural and biochemical data, potentially offering more accurate predictions than traditional methods. However, the interpretability of these black-box models remains a challenge.
Integrating multiple computational approaches often yields more robust predictions. For example, combining molecular docking with molecular dynamics simulations can provide a more comprehensive understanding of binding mechanisms. Similarly, ensemble docking methods that consider multiple protein conformations can account for protein flexibility and improve prediction accuracy.
As computational power continues to increase and algorithms become more sophisticated, the role of computational modeling in enhancing carboxylic acid protein binding properties is expected to grow. These in silico approaches not only accelerate the drug discovery process but also provide valuable insights into the fundamental principles governing molecular recognition and binding.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!