How to Enhance Carboxylic Acid Stability in Chemical Reactions?
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carboxylic Acid Stability Background and Objectives
Carboxylic acids are fundamental organic compounds with widespread applications in various industries, including pharmaceuticals, food production, and materials science. The stability of these compounds during chemical reactions is crucial for maintaining their efficacy and ensuring the quality of end products. Over the past decades, researchers have been exploring methods to enhance the stability of carboxylic acids, recognizing the significant impact this could have on numerous chemical processes.
The evolution of carboxylic acid stability techniques has been driven by the increasing demand for more efficient and sustainable chemical reactions. Early approaches focused primarily on controlling reaction conditions, such as temperature and pH, to minimize degradation. However, as our understanding of molecular interactions and reaction mechanisms has advanced, more sophisticated strategies have emerged.
Recent technological advancements have opened up new possibilities for enhancing carboxylic acid stability. These include the development of novel protective groups, the use of enzyme-catalyzed reactions, and the application of microfluidic systems for precise reaction control. Additionally, computational chemistry has played a crucial role in predicting and optimizing reaction conditions, leading to more targeted approaches in stability enhancement.
The primary objective in this field is to develop robust methods that can maintain the structural integrity of carboxylic acids across a wide range of reaction conditions. This includes protecting the acid group from unwanted side reactions, preventing decarboxylation, and ensuring the stability of the carbon-oxygen bond. Researchers aim to achieve these goals while minimizing the use of harsh reagents or extreme conditions, aligning with the principles of green chemistry.
Another key objective is to enhance the stability of carboxylic acids in complex reaction environments, such as those found in biological systems or multi-step synthetic processes. This requires a deep understanding of the interplay between carboxylic acids and various other functional groups, as well as the development of selective protection strategies that do not interfere with desired reactions.
Looking ahead, the field is moving towards more integrated approaches that combine multiple stability-enhancing techniques. This includes the development of smart reaction systems that can adapt to changing conditions in real-time, ensuring optimal carboxylic acid stability throughout the reaction process. Furthermore, there is a growing interest in leveraging nanotechnology and advanced materials to create novel stabilization methods, such as encapsulation or surface modification techniques.
The evolution of carboxylic acid stability techniques has been driven by the increasing demand for more efficient and sustainable chemical reactions. Early approaches focused primarily on controlling reaction conditions, such as temperature and pH, to minimize degradation. However, as our understanding of molecular interactions and reaction mechanisms has advanced, more sophisticated strategies have emerged.
Recent technological advancements have opened up new possibilities for enhancing carboxylic acid stability. These include the development of novel protective groups, the use of enzyme-catalyzed reactions, and the application of microfluidic systems for precise reaction control. Additionally, computational chemistry has played a crucial role in predicting and optimizing reaction conditions, leading to more targeted approaches in stability enhancement.
The primary objective in this field is to develop robust methods that can maintain the structural integrity of carboxylic acids across a wide range of reaction conditions. This includes protecting the acid group from unwanted side reactions, preventing decarboxylation, and ensuring the stability of the carbon-oxygen bond. Researchers aim to achieve these goals while minimizing the use of harsh reagents or extreme conditions, aligning with the principles of green chemistry.
Another key objective is to enhance the stability of carboxylic acids in complex reaction environments, such as those found in biological systems or multi-step synthetic processes. This requires a deep understanding of the interplay between carboxylic acids and various other functional groups, as well as the development of selective protection strategies that do not interfere with desired reactions.
Looking ahead, the field is moving towards more integrated approaches that combine multiple stability-enhancing techniques. This includes the development of smart reaction systems that can adapt to changing conditions in real-time, ensuring optimal carboxylic acid stability throughout the reaction process. Furthermore, there is a growing interest in leveraging nanotechnology and advanced materials to create novel stabilization methods, such as encapsulation or surface modification techniques.
Industrial Applications and Market Demand
Carboxylic acids play a crucial role in various industrial applications, driving significant market demand across multiple sectors. The stability enhancement of carboxylic acids in chemical reactions is of paramount importance in industries such as pharmaceuticals, food and beverages, agriculture, and materials science.
In the pharmaceutical industry, carboxylic acids serve as key intermediates and building blocks for drug synthesis. Improving their stability during reactions can lead to higher yields, reduced production costs, and enhanced drug efficacy. This is particularly relevant in the production of nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, and other therapeutic compounds. The global pharmaceutical market, which heavily relies on carboxylic acid chemistry, is projected to reach substantial growth in the coming years, underscoring the need for advanced stability enhancement techniques.
The food and beverage industry also heavily utilizes carboxylic acids as preservatives, flavor enhancers, and pH regulators. Enhancing their stability can extend product shelf life, improve taste consistency, and ensure food safety. This is especially critical in the production of processed foods, beverages, and dairy products. As consumer demand for natural and clean-label products grows, developing stable carboxylic acid derivatives from natural sources becomes increasingly important.
In agriculture, carboxylic acids are employed in the formulation of herbicides, pesticides, and plant growth regulators. Improving their stability can lead to more effective and longer-lasting agricultural products, reducing the frequency of application and minimizing environmental impact. This aligns with the global trend towards sustainable agriculture and precision farming practices.
The materials science sector also benefits from enhanced carboxylic acid stability. These compounds are used in the production of polymers, resins, and coatings. Increased stability can result in improved material properties, such as durability, weather resistance, and longevity. This is particularly relevant in the automotive, construction, and packaging industries, where high-performance materials are in constant demand.
Furthermore, the growing emphasis on green chemistry and sustainable production processes has created a new market segment for bio-based carboxylic acids. Enhancing the stability of these environmentally friendly alternatives can accelerate their adoption across industries, aligning with global sustainability goals and regulatory requirements.
As industries continue to innovate and seek more efficient processes, the demand for advanced carboxylic acid stability enhancement technologies is expected to rise. This presents opportunities for research and development in areas such as novel catalysts, reaction conditions optimization, and innovative stabilization techniques. Companies that can develop and commercialize these solutions stand to gain a significant competitive advantage in the global market.
In the pharmaceutical industry, carboxylic acids serve as key intermediates and building blocks for drug synthesis. Improving their stability during reactions can lead to higher yields, reduced production costs, and enhanced drug efficacy. This is particularly relevant in the production of nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, and other therapeutic compounds. The global pharmaceutical market, which heavily relies on carboxylic acid chemistry, is projected to reach substantial growth in the coming years, underscoring the need for advanced stability enhancement techniques.
The food and beverage industry also heavily utilizes carboxylic acids as preservatives, flavor enhancers, and pH regulators. Enhancing their stability can extend product shelf life, improve taste consistency, and ensure food safety. This is especially critical in the production of processed foods, beverages, and dairy products. As consumer demand for natural and clean-label products grows, developing stable carboxylic acid derivatives from natural sources becomes increasingly important.
In agriculture, carboxylic acids are employed in the formulation of herbicides, pesticides, and plant growth regulators. Improving their stability can lead to more effective and longer-lasting agricultural products, reducing the frequency of application and minimizing environmental impact. This aligns with the global trend towards sustainable agriculture and precision farming practices.
The materials science sector also benefits from enhanced carboxylic acid stability. These compounds are used in the production of polymers, resins, and coatings. Increased stability can result in improved material properties, such as durability, weather resistance, and longevity. This is particularly relevant in the automotive, construction, and packaging industries, where high-performance materials are in constant demand.
Furthermore, the growing emphasis on green chemistry and sustainable production processes has created a new market segment for bio-based carboxylic acids. Enhancing the stability of these environmentally friendly alternatives can accelerate their adoption across industries, aligning with global sustainability goals and regulatory requirements.
As industries continue to innovate and seek more efficient processes, the demand for advanced carboxylic acid stability enhancement technologies is expected to rise. This presents opportunities for research and development in areas such as novel catalysts, reaction conditions optimization, and innovative stabilization techniques. Companies that can develop and commercialize these solutions stand to gain a significant competitive advantage in the global market.
Current Challenges in Carboxylic Acid Stabilization
The stabilization of carboxylic acids in chemical reactions presents several significant challenges that researchers and industry professionals continue to grapple with. One of the primary issues is the inherent reactivity of the carboxyl group, which can lead to undesired side reactions and degradation under various conditions. This reactivity is particularly problematic in the presence of nucleophiles, where the carbonyl carbon is susceptible to nucleophilic attack, potentially resulting in esterification or amidation reactions that alter the desired product.
Another major challenge is the acid-base sensitivity of carboxylic acids. In strongly basic environments, deprotonation can occur, leading to the formation of carboxylate anions. While this may be desirable in some cases, it can also lead to increased solubility in aqueous media and potential loss of the compound during extraction or purification steps. Conversely, in highly acidic conditions, protonation of the carboxyl group can occur, potentially catalyzing unwanted reactions or altering the compound's properties.
The thermal instability of certain carboxylic acids poses additional difficulties, especially in high-temperature reactions or during prolonged storage. Some carboxylic acids can undergo decarboxylation, losing carbon dioxide and forming less complex molecules. This is particularly problematic for β-keto acids and other structurally similar compounds, where the proximity of certain functional groups facilitates this decomposition pathway.
Oxidative and reductive environments also present challenges for carboxylic acid stability. In the presence of strong oxidizing agents, carboxylic acids can be further oxidized to form peroxy acids or undergo other oxidative transformations. Conversely, in reducing environments, carboxylic acids may be reduced to aldehydes or alcohols, altering the desired product and potentially complicating purification processes.
The presence of metal ions in reaction mixtures can also impact carboxylic acid stability. Many transition metals can form complexes with carboxylate groups, potentially catalyzing unwanted reactions or altering the reactivity of the carboxylic acid. This complexation can lead to unexpected side products or affect the overall yield of the desired reaction.
Furthermore, the stability of carboxylic acids can be influenced by the surrounding chemical environment, including solvent effects and pH fluctuations. Certain solvents may promote esterification or other transformations, while pH changes can affect the protonation state and reactivity of the carboxyl group. Balancing these factors to maintain carboxylic acid stability throughout a reaction sequence or during product storage remains a significant challenge in many chemical processes.
Another major challenge is the acid-base sensitivity of carboxylic acids. In strongly basic environments, deprotonation can occur, leading to the formation of carboxylate anions. While this may be desirable in some cases, it can also lead to increased solubility in aqueous media and potential loss of the compound during extraction or purification steps. Conversely, in highly acidic conditions, protonation of the carboxyl group can occur, potentially catalyzing unwanted reactions or altering the compound's properties.
The thermal instability of certain carboxylic acids poses additional difficulties, especially in high-temperature reactions or during prolonged storage. Some carboxylic acids can undergo decarboxylation, losing carbon dioxide and forming less complex molecules. This is particularly problematic for β-keto acids and other structurally similar compounds, where the proximity of certain functional groups facilitates this decomposition pathway.
Oxidative and reductive environments also present challenges for carboxylic acid stability. In the presence of strong oxidizing agents, carboxylic acids can be further oxidized to form peroxy acids or undergo other oxidative transformations. Conversely, in reducing environments, carboxylic acids may be reduced to aldehydes or alcohols, altering the desired product and potentially complicating purification processes.
The presence of metal ions in reaction mixtures can also impact carboxylic acid stability. Many transition metals can form complexes with carboxylate groups, potentially catalyzing unwanted reactions or altering the reactivity of the carboxylic acid. This complexation can lead to unexpected side products or affect the overall yield of the desired reaction.
Furthermore, the stability of carboxylic acids can be influenced by the surrounding chemical environment, including solvent effects and pH fluctuations. Certain solvents may promote esterification or other transformations, while pH changes can affect the protonation state and reactivity of the carboxyl group. Balancing these factors to maintain carboxylic acid stability throughout a reaction sequence or during product storage remains a significant challenge in many chemical processes.
Existing Stabilization Methods and Approaches
01 Stabilization through structural modifications
Carboxylic acid stability can be enhanced through structural modifications such as introducing substituents or altering the carbon chain length. These modifications can affect the acid's reactivity, solubility, and resistance to degradation, thereby improving its overall stability in various environments and applications.- Stabilization through structural modifications: Carboxylic acid stability can be enhanced through structural modifications. This includes introducing substituents or altering the molecular structure to reduce reactivity and increase resistance to degradation. Such modifications can improve the acid's thermal stability, resistance to oxidation, and overall chemical stability.
- Use of stabilizing additives: Incorporating stabilizing additives can significantly improve the stability of carboxylic acids. These additives may include antioxidants, pH buffers, or other compounds that prevent degradation or unwanted reactions. The choice of stabilizer depends on the specific carboxylic acid and its intended application.
- Environmental control for stability: Controlling environmental factors such as temperature, light exposure, and humidity can enhance carboxylic acid stability. Proper storage conditions, including the use of appropriate containers and controlled atmospheres, can prevent degradation and extend the shelf life of carboxylic acids.
- Formulation techniques for improved stability: Specific formulation techniques can be employed to improve carboxylic acid stability in various products. This may involve creating emulsions, microencapsulation, or using specific solvent systems that protect the acid from degradation. These techniques are particularly useful in pharmaceutical and cosmetic applications.
- Stabilization through salt formation: Converting carboxylic acids into their salt forms can often improve stability. Salt formation can alter the physical properties of the compound, making it less susceptible to degradation. This approach is commonly used in pharmaceutical formulations to enhance the stability and bioavailability of active ingredients.
02 Use of stabilizing additives
Incorporating stabilizing additives into formulations containing carboxylic acids can significantly improve their stability. These additives may include antioxidants, pH buffers, or chelating agents that prevent degradation, maintain optimal pH, or inhibit metal-catalyzed reactions, respectively, thus extending the shelf life and efficacy of carboxylic acid-containing products.Expand Specific Solutions03 Environmental control for stability
Controlling environmental factors such as temperature, light exposure, and humidity can greatly enhance the stability of carboxylic acids. Proper storage conditions, including the use of appropriate packaging materials and controlled atmosphere, can prevent degradation and maintain the acid's chemical integrity over extended periods.Expand Specific Solutions04 Formulation techniques for improved stability
Advanced formulation techniques can be employed to improve carboxylic acid stability in various products. These may include microencapsulation, emulsion technology, or the use of specialized delivery systems that protect the acid from degradation and control its release, thereby enhancing its stability and effectiveness in the final product.Expand Specific Solutions05 Stability enhancement through salt formation
Converting carboxylic acids into their salt forms can significantly improve their stability. Salt formation can alter the acid's solubility, melting point, and reactivity, often resulting in enhanced stability against thermal degradation, oxidation, and hydrolysis. This approach is particularly useful in pharmaceutical and industrial applications.Expand Specific Solutions
Key Players in Carboxylic Acid Research
The competition landscape for enhancing carboxylic acid stability in chemical reactions is characterized by a mature market with significant potential for growth. Major players like DuPont, Bayer AG, and Ecolab USA are investing heavily in research and development to improve existing technologies. The market is driven by increasing demand in pharmaceuticals, agrochemicals, and industrial applications. Companies such as Astellas Pharma and Eli Lilly are focusing on innovative approaches to stabilize carboxylic acids in drug formulations. Emerging technologies from research institutions like the Agency for Science, Technology & Research are expected to further advance the field. The market is highly competitive, with both established chemical companies and specialized firms like LyondellBasell Acetyls LLC vying for market share.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed a proprietary technology called "Acid-Stable Emulsion" (ASE) to enhance carboxylic acid stability in various chemical reactions and formulations. This technology involves creating a microemulsion system where carboxylic acid molecules are dispersed in nano-sized droplets surrounded by a carefully engineered surfactant layer[2]. The surfactant layer acts as a protective barrier, preventing acid degradation and unwanted side reactions. Henkel has also incorporated antioxidants and chelating agents into their ASE formulations to further improve stability against oxidation and metal-catalyzed decomposition[4]. The company has successfully applied this technology in personal care products, adhesives, and industrial cleaners, demonstrating significant improvements in product shelf-life and performance[6].
Strengths: Broad applicability in consumer and industrial products, improved product efficacy, and extended shelf-life. Weaknesses: May require reformulation of existing products and potential regulatory challenges in some applications.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed an innovative approach to enhance carboxylic acid stability in chemical reactions, particularly focusing on industrial and institutional cleaning applications. Their technology, known as "Stabilized Acid Technology" (SAT), involves the use of synergistic stabilizer blends that work in conjunction with carboxylic acids to maintain their efficacy in challenging environments[1]. The SAT system incorporates pH buffers, metal chelators, and proprietary organic stabilizers that form protective complexes around carboxylic acid molecules[3]. This multi-faceted approach helps prevent acid dissociation, reduces unwanted side reactions, and maintains the desired pH range for optimal performance. Ecolab has also developed specialized delivery systems that minimize acid exposure to destabilizing factors during storage and application[5].
Strengths: Highly effective in harsh industrial environments, improved safety profile, and reduced chemical consumption. Weaknesses: May be more expensive than traditional acid formulations and potentially limited to specific application areas.
Innovative Stabilization Technologies
Stabilization of peroxycarboxylic acids using amine acid salts
PatentInactiveUS20140120179A1
Innovation
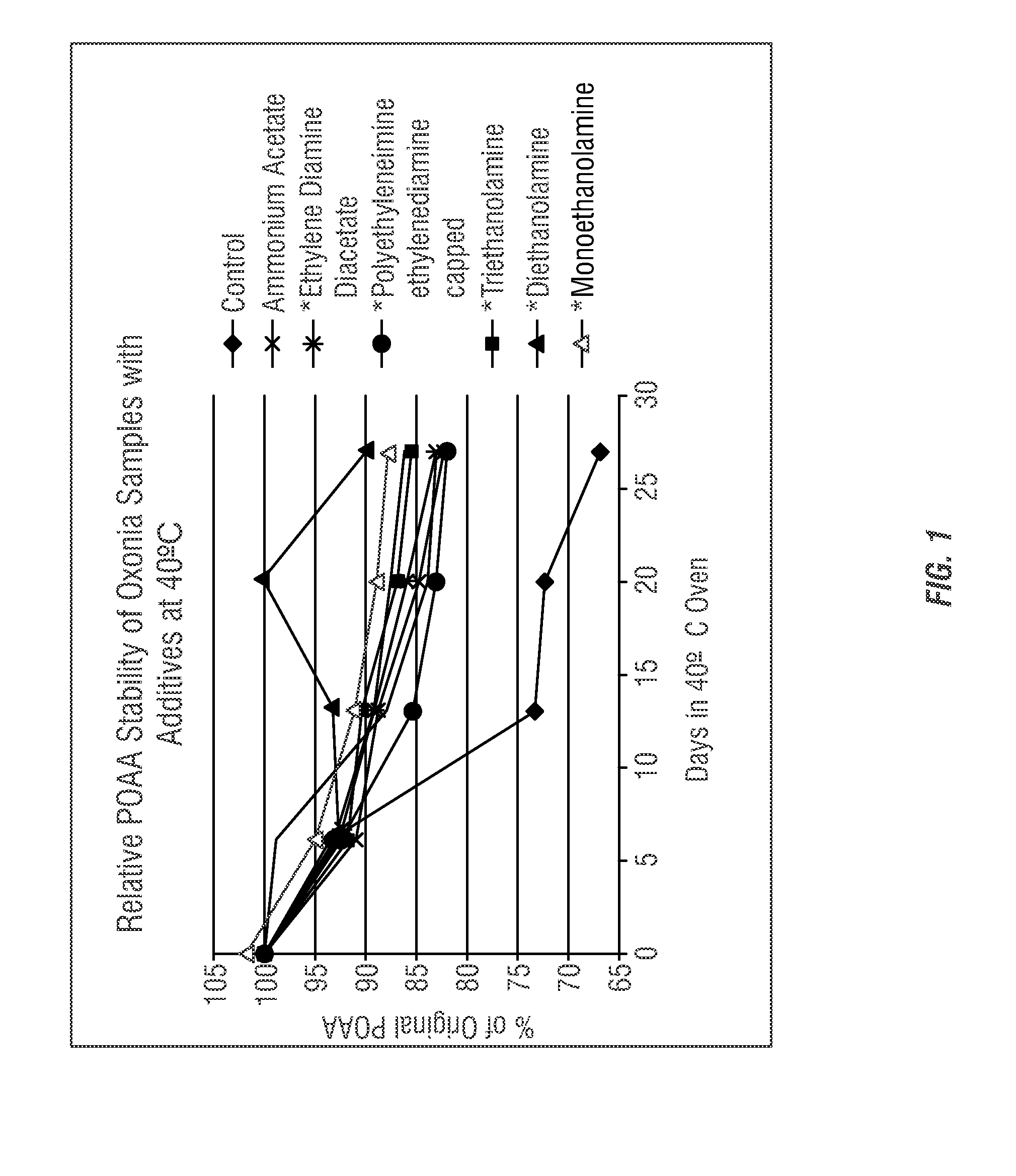
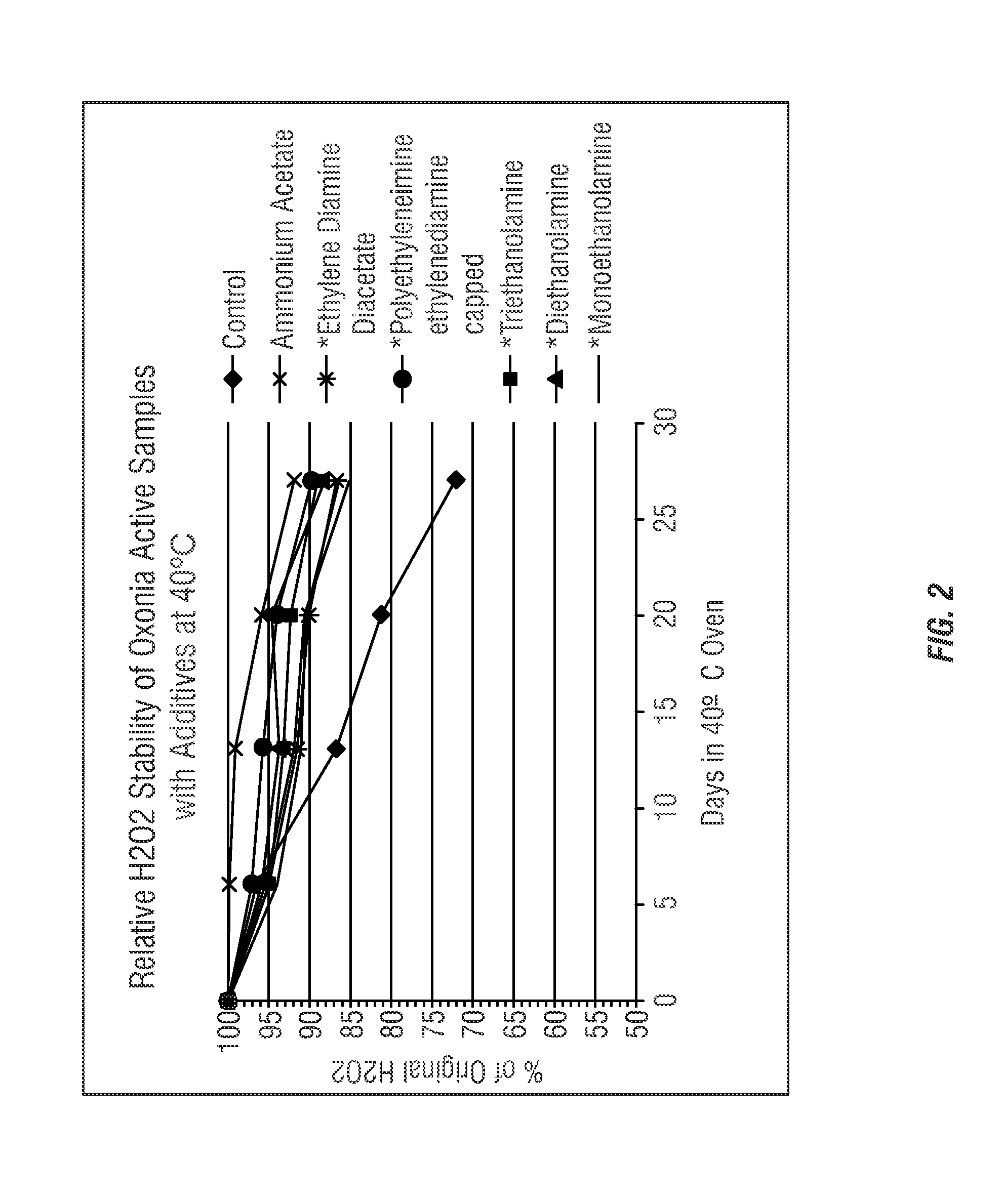
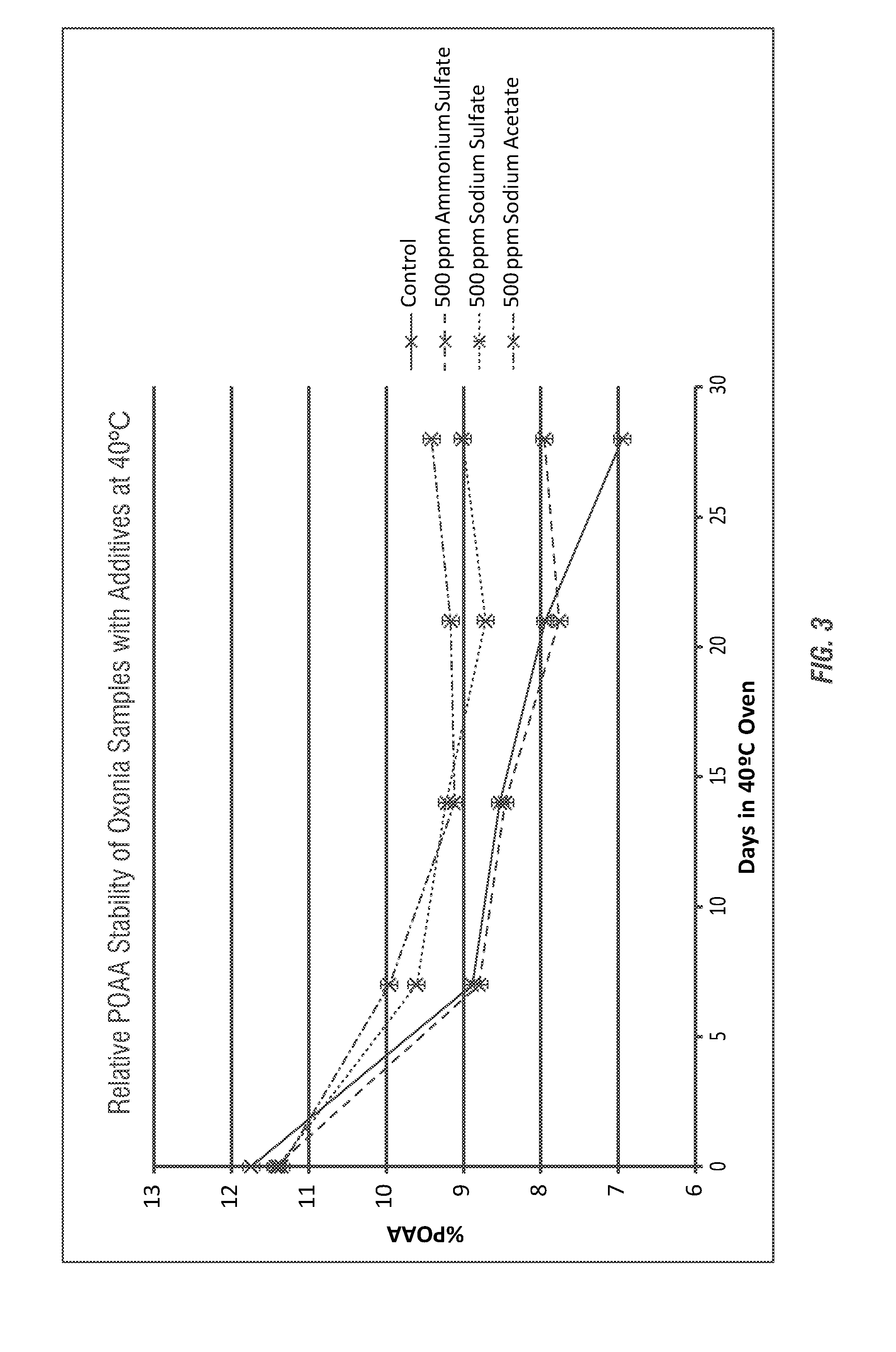
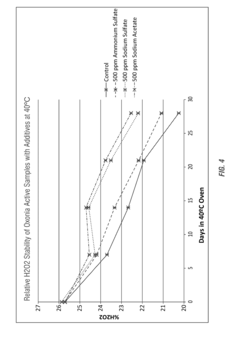
- The use of ammonium salts, polymeric amines, amine salts, and sodium salts of acetic acid and sulfuric acid as stabilizing agents in peroxycarboxylic acid compositions to enhance stability, reducing decomposition and odor, thereby improving shelf-life and antimicrobial efficacy.
Method for manufacturing thermoplastic poly(vinyl alcohol) derivative in a melt state reaction and products thereof
PatentActiveUS11897974B2
Innovation
- A method involving a melt state condensation reaction between poly(vinyl alcohol) and organic acid anhydride to produce a thermoplastic poly(vinyl alcohol) derivative with ester bonded pendant chains, which forms a primer layer that enhances the anchorage of silicone compounds without using solvents, reducing the risk of cross-linker migration and improving reaction efficiency.
Environmental Impact of Stabilization Processes
The stabilization processes used to enhance carboxylic acid stability in chemical reactions can have significant environmental implications. These processes often involve the use of various chemicals and energy-intensive methods, which may contribute to environmental pollution and resource depletion if not managed properly.
One of the primary environmental concerns is the potential release of harmful substances during stabilization processes. Some stabilizers or additives used to improve carboxylic acid stability may be toxic or persistent in the environment. If these chemicals are not fully consumed in the reaction or are released as byproducts, they can contaminate soil, water, and air, potentially harming ecosystems and human health.
Energy consumption is another critical factor to consider. Many stabilization techniques require elevated temperatures or pressures, leading to increased energy usage and associated greenhouse gas emissions. This contributes to the overall carbon footprint of the chemical industry and exacerbates climate change concerns.
Water usage and wastewater generation are also important environmental aspects of stabilization processes. Some methods may require large volumes of water for cooling or as reaction media. The resulting wastewater can contain residual chemicals, acids, or other contaminants that require treatment before discharge, placing additional strain on water resources and treatment facilities.
The production and disposal of stabilizers themselves can have environmental impacts. Manufacturing these compounds often involves complex chemical processes that may generate hazardous waste or emissions. Additionally, the disposal of spent stabilizers or reaction byproducts can pose challenges for waste management systems and potentially lead to environmental contamination if not handled properly.
However, it is important to note that enhancing carboxylic acid stability can also have positive environmental effects. Improved stability can lead to more efficient reactions, reducing the need for excess reagents and minimizing waste generation. This can result in overall resource conservation and a reduction in the environmental footprint of chemical processes.
To mitigate the environmental impact of stabilization processes, researchers and industry professionals are exploring greener alternatives. These include the development of bio-based stabilizers, the use of catalytic systems that operate under milder conditions, and the implementation of closed-loop systems that minimize waste and maximize resource recovery. Additionally, advancements in process intensification and continuous flow chemistry are helping to reduce energy consumption and improve overall efficiency in carboxylic acid stabilization processes.
One of the primary environmental concerns is the potential release of harmful substances during stabilization processes. Some stabilizers or additives used to improve carboxylic acid stability may be toxic or persistent in the environment. If these chemicals are not fully consumed in the reaction or are released as byproducts, they can contaminate soil, water, and air, potentially harming ecosystems and human health.
Energy consumption is another critical factor to consider. Many stabilization techniques require elevated temperatures or pressures, leading to increased energy usage and associated greenhouse gas emissions. This contributes to the overall carbon footprint of the chemical industry and exacerbates climate change concerns.
Water usage and wastewater generation are also important environmental aspects of stabilization processes. Some methods may require large volumes of water for cooling or as reaction media. The resulting wastewater can contain residual chemicals, acids, or other contaminants that require treatment before discharge, placing additional strain on water resources and treatment facilities.
The production and disposal of stabilizers themselves can have environmental impacts. Manufacturing these compounds often involves complex chemical processes that may generate hazardous waste or emissions. Additionally, the disposal of spent stabilizers or reaction byproducts can pose challenges for waste management systems and potentially lead to environmental contamination if not handled properly.
However, it is important to note that enhancing carboxylic acid stability can also have positive environmental effects. Improved stability can lead to more efficient reactions, reducing the need for excess reagents and minimizing waste generation. This can result in overall resource conservation and a reduction in the environmental footprint of chemical processes.
To mitigate the environmental impact of stabilization processes, researchers and industry professionals are exploring greener alternatives. These include the development of bio-based stabilizers, the use of catalytic systems that operate under milder conditions, and the implementation of closed-loop systems that minimize waste and maximize resource recovery. Additionally, advancements in process intensification and continuous flow chemistry are helping to reduce energy consumption and improve overall efficiency in carboxylic acid stabilization processes.
Regulatory Considerations for Chemical Stabilization
Regulatory considerations play a crucial role in the development and implementation of chemical stabilization techniques for carboxylic acids. These considerations are designed to ensure the safety, efficacy, and environmental sustainability of chemical processes involving carboxylic acids.
One of the primary regulatory concerns is the potential environmental impact of stabilization methods. Regulatory bodies such as the Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) in the European Union have established strict guidelines for the use and disposal of chemicals used in stabilization processes. These regulations aim to minimize the release of harmful substances into the environment and protect ecosystems from potential damage.
Worker safety is another critical aspect of regulatory compliance in chemical stabilization. Occupational Safety and Health Administration (OSHA) regulations in the US, and similar bodies in other countries, mandate specific safety protocols for handling and working with carboxylic acids and their stabilizers. This includes requirements for personal protective equipment, proper ventilation systems, and emergency response procedures.
The pharmaceutical industry faces particularly stringent regulations when it comes to carboxylic acid stabilization. The Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in the EU have established Good Manufacturing Practice (GMP) guidelines that dictate the quality control and assurance measures required for pharmaceutical production. These guidelines extend to the stabilization of carboxylic acids used in drug formulations, ensuring that the final products meet safety and efficacy standards.
Regulatory bodies also focus on the purity and quality of stabilized carboxylic acids, especially when used in food and cosmetic applications. The FDA's Food Additive Regulations and the EU's Regulation on Food Additives set specific limits on impurities and require thorough documentation of stabilization processes. Similarly, cosmetic regulations such as the EU Cosmetics Regulation and the FDA's Federal Food, Drug, and Cosmetic Act impose strict requirements on the use of stabilized carboxylic acids in personal care products.
As sustainability becomes an increasingly important consideration, regulatory frameworks are evolving to encourage the development of green chemistry approaches to carboxylic acid stabilization. This includes incentives for using bio-based stabilizers, implementing energy-efficient processes, and reducing waste generation. Companies that can demonstrate compliance with these emerging sustainability regulations may gain a competitive advantage in the market.
Compliance with these diverse regulatory requirements necessitates a comprehensive approach to chemical stabilization. Companies must invest in robust quality management systems, conduct thorough risk assessments, and maintain detailed documentation of their stabilization processes. Regular audits and inspections by regulatory authorities ensure ongoing compliance and may lead to updates in stabilization protocols as new regulations emerge.
One of the primary regulatory concerns is the potential environmental impact of stabilization methods. Regulatory bodies such as the Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) in the European Union have established strict guidelines for the use and disposal of chemicals used in stabilization processes. These regulations aim to minimize the release of harmful substances into the environment and protect ecosystems from potential damage.
Worker safety is another critical aspect of regulatory compliance in chemical stabilization. Occupational Safety and Health Administration (OSHA) regulations in the US, and similar bodies in other countries, mandate specific safety protocols for handling and working with carboxylic acids and their stabilizers. This includes requirements for personal protective equipment, proper ventilation systems, and emergency response procedures.
The pharmaceutical industry faces particularly stringent regulations when it comes to carboxylic acid stabilization. The Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in the EU have established Good Manufacturing Practice (GMP) guidelines that dictate the quality control and assurance measures required for pharmaceutical production. These guidelines extend to the stabilization of carboxylic acids used in drug formulations, ensuring that the final products meet safety and efficacy standards.
Regulatory bodies also focus on the purity and quality of stabilized carboxylic acids, especially when used in food and cosmetic applications. The FDA's Food Additive Regulations and the EU's Regulation on Food Additives set specific limits on impurities and require thorough documentation of stabilization processes. Similarly, cosmetic regulations such as the EU Cosmetics Regulation and the FDA's Federal Food, Drug, and Cosmetic Act impose strict requirements on the use of stabilized carboxylic acids in personal care products.
As sustainability becomes an increasingly important consideration, regulatory frameworks are evolving to encourage the development of green chemistry approaches to carboxylic acid stabilization. This includes incentives for using bio-based stabilizers, implementing energy-efficient processes, and reducing waste generation. Companies that can demonstrate compliance with these emerging sustainability regulations may gain a competitive advantage in the market.
Compliance with these diverse regulatory requirements necessitates a comprehensive approach to chemical stabilization. Companies must invest in robust quality management systems, conduct thorough risk assessments, and maintain detailed documentation of their stabilization processes. Regular audits and inspections by regulatory authorities ensure ongoing compliance and may lead to updates in stabilization protocols as new regulations emerge.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!