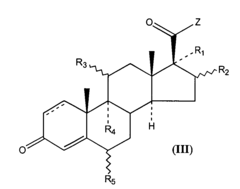
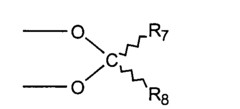
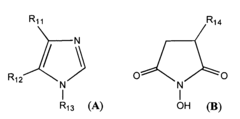
How Carboxylic Acid Optimizes Biochemical Reaction Rates?
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carboxylic Acid Catalysis Background and Objectives
Carboxylic acids have played a pivotal role in biochemical reactions since the dawn of life on Earth. These organic compounds, characterized by their carboxyl group (-COOH), are ubiquitous in nature and serve as key players in numerous metabolic processes. The study of carboxylic acid catalysis has evolved significantly over the past century, with researchers unraveling its complex mechanisms and diverse applications in biological systems.
The primary objective of investigating carboxylic acid catalysis in biochemical reactions is to understand how these molecules optimize reaction rates. This knowledge is crucial for advancing our comprehension of cellular metabolism, enzyme function, and the development of novel therapeutic strategies. By elucidating the intricate ways in which carboxylic acids influence reaction kinetics, scientists aim to harness this understanding for biotechnological applications and drug design.
Historically, the exploration of carboxylic acid catalysis began with the pioneering work of chemists in the early 20th century. As analytical techniques improved, researchers gained deeper insights into the structural properties and reactive nature of carboxylic acids. The discovery of their role in enzyme-catalyzed reactions marked a significant milestone, leading to a surge in research focused on their catalytic mechanisms.
The field has witnessed several paradigm shifts, from the initial focus on simple acid-base catalysis to the recognition of more complex roles in biological systems. These include their involvement in proton shuttling, stabilization of transition states, and modulation of substrate binding affinities. Each breakthrough has contributed to a more nuanced understanding of how carboxylic acids optimize biochemical reaction rates.
Recent technological advancements, particularly in spectroscopic and computational methods, have revolutionized the study of carboxylic acid catalysis. High-resolution structural analyses, coupled with sophisticated molecular modeling, now allow researchers to probe the atomic-level interactions between carboxylic acids and their substrates. This has led to a more precise understanding of catalytic mechanisms and the factors influencing reaction rates.
The current research landscape is characterized by a multidisciplinary approach, combining insights from organic chemistry, biochemistry, and biophysics. Scientists are exploring the role of carboxylic acids in diverse biological contexts, from cellular signaling pathways to the regulation of enzymatic activity. This holistic perspective is essential for unraveling the full potential of carboxylic acid catalysis in optimizing biochemical reactions.
As we look to the future, the field of carboxylic acid catalysis holds immense promise for addressing global challenges in healthcare, agriculture, and environmental sustainability. By deepening our understanding of how these molecules fine-tune reaction rates, we pave the way for innovative solutions in drug delivery, biofuel production, and the development of eco-friendly catalysts.
The primary objective of investigating carboxylic acid catalysis in biochemical reactions is to understand how these molecules optimize reaction rates. This knowledge is crucial for advancing our comprehension of cellular metabolism, enzyme function, and the development of novel therapeutic strategies. By elucidating the intricate ways in which carboxylic acids influence reaction kinetics, scientists aim to harness this understanding for biotechnological applications and drug design.
Historically, the exploration of carboxylic acid catalysis began with the pioneering work of chemists in the early 20th century. As analytical techniques improved, researchers gained deeper insights into the structural properties and reactive nature of carboxylic acids. The discovery of their role in enzyme-catalyzed reactions marked a significant milestone, leading to a surge in research focused on their catalytic mechanisms.
The field has witnessed several paradigm shifts, from the initial focus on simple acid-base catalysis to the recognition of more complex roles in biological systems. These include their involvement in proton shuttling, stabilization of transition states, and modulation of substrate binding affinities. Each breakthrough has contributed to a more nuanced understanding of how carboxylic acids optimize biochemical reaction rates.
Recent technological advancements, particularly in spectroscopic and computational methods, have revolutionized the study of carboxylic acid catalysis. High-resolution structural analyses, coupled with sophisticated molecular modeling, now allow researchers to probe the atomic-level interactions between carboxylic acids and their substrates. This has led to a more precise understanding of catalytic mechanisms and the factors influencing reaction rates.
The current research landscape is characterized by a multidisciplinary approach, combining insights from organic chemistry, biochemistry, and biophysics. Scientists are exploring the role of carboxylic acids in diverse biological contexts, from cellular signaling pathways to the regulation of enzymatic activity. This holistic perspective is essential for unraveling the full potential of carboxylic acid catalysis in optimizing biochemical reactions.
As we look to the future, the field of carboxylic acid catalysis holds immense promise for addressing global challenges in healthcare, agriculture, and environmental sustainability. By deepening our understanding of how these molecules fine-tune reaction rates, we pave the way for innovative solutions in drug delivery, biofuel production, and the development of eco-friendly catalysts.
Biochemical Industry Demand for Reaction Rate Optimization
The biochemical industry has witnessed a growing demand for reaction rate optimization, driven by the need for increased efficiency, productivity, and sustainability in various processes. Carboxylic acids play a crucial role in this optimization, offering significant potential to enhance biochemical reaction rates across multiple applications.
In the pharmaceutical sector, the optimization of reaction rates is paramount for drug discovery and development. Carboxylic acids are extensively used as catalysts or reactants in the synthesis of active pharmaceutical ingredients (APIs), enabling faster and more efficient production of life-saving medications. The ability to accelerate these reactions translates directly into reduced time-to-market for new drugs and improved cost-effectiveness in manufacturing processes.
The food and beverage industry also benefits greatly from optimized reaction rates involving carboxylic acids. Fermentation processes, which are fundamental to the production of various food products and beverages, rely heavily on precise control of reaction kinetics. By optimizing these reactions, manufacturers can achieve better flavor profiles, increased product consistency, and improved shelf life, all while reducing production times and energy consumption.
In the field of biofuels and renewable energy, reaction rate optimization is critical for enhancing the efficiency of biomass conversion processes. Carboxylic acids play a vital role in the pretreatment and hydrolysis of lignocellulosic materials, key steps in the production of bioethanol and other biofuels. Faster reaction rates in these processes can significantly increase the yield of fermentable sugars, thereby improving the overall economics of biofuel production.
The agrochemical industry also demands optimized reaction rates for the synthesis of pesticides, herbicides, and fertilizers. Carboxylic acids are often used as building blocks or intermediates in these processes. By enhancing reaction rates, manufacturers can reduce production costs, minimize waste generation, and develop more environmentally friendly agricultural products.
In the realm of industrial biotechnology, optimized biochemical reaction rates are essential for the production of bio-based chemicals and materials. Carboxylic acids are frequently employed in the synthesis of biodegradable plastics, specialty chemicals, and other sustainable alternatives to petroleum-based products. Faster and more efficient reactions in these processes can significantly contribute to the growth of the bio-based economy and the transition towards more sustainable industrial practices.
The demand for reaction rate optimization extends to environmental applications as well. In wastewater treatment and bioremediation processes, carboxylic acids play a crucial role in accelerating the breakdown of organic pollutants. Optimized reaction rates in these applications lead to more effective and rapid purification of water and soil, addressing pressing environmental challenges.
In the pharmaceutical sector, the optimization of reaction rates is paramount for drug discovery and development. Carboxylic acids are extensively used as catalysts or reactants in the synthesis of active pharmaceutical ingredients (APIs), enabling faster and more efficient production of life-saving medications. The ability to accelerate these reactions translates directly into reduced time-to-market for new drugs and improved cost-effectiveness in manufacturing processes.
The food and beverage industry also benefits greatly from optimized reaction rates involving carboxylic acids. Fermentation processes, which are fundamental to the production of various food products and beverages, rely heavily on precise control of reaction kinetics. By optimizing these reactions, manufacturers can achieve better flavor profiles, increased product consistency, and improved shelf life, all while reducing production times and energy consumption.
In the field of biofuels and renewable energy, reaction rate optimization is critical for enhancing the efficiency of biomass conversion processes. Carboxylic acids play a vital role in the pretreatment and hydrolysis of lignocellulosic materials, key steps in the production of bioethanol and other biofuels. Faster reaction rates in these processes can significantly increase the yield of fermentable sugars, thereby improving the overall economics of biofuel production.
The agrochemical industry also demands optimized reaction rates for the synthesis of pesticides, herbicides, and fertilizers. Carboxylic acids are often used as building blocks or intermediates in these processes. By enhancing reaction rates, manufacturers can reduce production costs, minimize waste generation, and develop more environmentally friendly agricultural products.
In the realm of industrial biotechnology, optimized biochemical reaction rates are essential for the production of bio-based chemicals and materials. Carboxylic acids are frequently employed in the synthesis of biodegradable plastics, specialty chemicals, and other sustainable alternatives to petroleum-based products. Faster and more efficient reactions in these processes can significantly contribute to the growth of the bio-based economy and the transition towards more sustainable industrial practices.
The demand for reaction rate optimization extends to environmental applications as well. In wastewater treatment and bioremediation processes, carboxylic acids play a crucial role in accelerating the breakdown of organic pollutants. Optimized reaction rates in these applications lead to more effective and rapid purification of water and soil, addressing pressing environmental challenges.
Current Challenges in Carboxylic Acid Catalysis
Despite the widespread use of carboxylic acids in catalyzing biochemical reactions, several challenges persist in optimizing their catalytic efficiency. One of the primary obstacles is the precise control of reaction rates in complex biological systems. Carboxylic acids often exhibit varying degrees of effectiveness depending on the specific biochemical environment, making it difficult to predict and fine-tune their catalytic performance across different scenarios.
Another significant challenge lies in maintaining the stability of carboxylic acid catalysts under diverse physiological conditions. pH fluctuations, temperature variations, and the presence of competing molecules can all impact the catalytic activity of carboxylic acids. Researchers are grappling with the task of developing robust catalytic systems that can withstand these environmental changes while maintaining optimal reaction rates.
The selectivity of carboxylic acid catalysts also presents a considerable hurdle. In many biochemical processes, multiple reaction pathways are possible, and achieving high selectivity towards the desired product is crucial. Enhancing the specificity of carboxylic acid catalysts to target particular substrates or promote specific reaction mechanisms remains an ongoing challenge in the field.
Furthermore, the scalability of carboxylic acid-catalyzed reactions poses difficulties in industrial applications. While these catalysts may perform efficiently at laboratory scales, translating their effectiveness to large-scale production processes often encounters unforeseen complications. Issues such as mass transfer limitations, catalyst recovery, and product separation become more pronounced at industrial scales.
The development of sustainable and environmentally friendly catalytic systems based on carboxylic acids is another area of concern. As the chemical industry moves towards greener processes, there is a growing need for catalysts that are not only efficient but also derived from renewable sources and biodegradable. Balancing these sustainability requirements with high catalytic performance remains a significant challenge.
Additionally, the mechanistic understanding of carboxylic acid catalysis in complex biochemical systems is still incomplete. While general principles are well-established, the intricate interplay between carboxylic acids and various biomolecules in cellular environments is not fully elucidated. This knowledge gap hinders the rational design of more effective catalytic systems and limits our ability to predict and optimize reaction outcomes in diverse biological contexts.
Lastly, the integration of carboxylic acid catalysts with emerging technologies, such as microfluidics and artificial intelligence, presents both opportunities and challenges. Harnessing these advanced tools to enhance catalyst design, reaction monitoring, and process optimization is a frontier area that requires interdisciplinary expertise and innovative approaches.
Another significant challenge lies in maintaining the stability of carboxylic acid catalysts under diverse physiological conditions. pH fluctuations, temperature variations, and the presence of competing molecules can all impact the catalytic activity of carboxylic acids. Researchers are grappling with the task of developing robust catalytic systems that can withstand these environmental changes while maintaining optimal reaction rates.
The selectivity of carboxylic acid catalysts also presents a considerable hurdle. In many biochemical processes, multiple reaction pathways are possible, and achieving high selectivity towards the desired product is crucial. Enhancing the specificity of carboxylic acid catalysts to target particular substrates or promote specific reaction mechanisms remains an ongoing challenge in the field.
Furthermore, the scalability of carboxylic acid-catalyzed reactions poses difficulties in industrial applications. While these catalysts may perform efficiently at laboratory scales, translating their effectiveness to large-scale production processes often encounters unforeseen complications. Issues such as mass transfer limitations, catalyst recovery, and product separation become more pronounced at industrial scales.
The development of sustainable and environmentally friendly catalytic systems based on carboxylic acids is another area of concern. As the chemical industry moves towards greener processes, there is a growing need for catalysts that are not only efficient but also derived from renewable sources and biodegradable. Balancing these sustainability requirements with high catalytic performance remains a significant challenge.
Additionally, the mechanistic understanding of carboxylic acid catalysis in complex biochemical systems is still incomplete. While general principles are well-established, the intricate interplay between carboxylic acids and various biomolecules in cellular environments is not fully elucidated. This knowledge gap hinders the rational design of more effective catalytic systems and limits our ability to predict and optimize reaction outcomes in diverse biological contexts.
Lastly, the integration of carboxylic acid catalysts with emerging technologies, such as microfluidics and artificial intelligence, presents both opportunities and challenges. Harnessing these advanced tools to enhance catalyst design, reaction monitoring, and process optimization is a frontier area that requires interdisciplinary expertise and innovative approaches.
Existing Carboxylic Acid Optimization Techniques
01 Factors affecting carboxylic acid reaction rates
Various factors influence the reaction rates of carboxylic acids, including temperature, concentration, catalysts, and the structure of the acid. Understanding these factors is crucial for optimizing reaction conditions and improving yields in industrial processes involving carboxylic acids.- Factors affecting carboxylic acid reaction rates: Various factors influence the reaction rates of carboxylic acids, including temperature, concentration, catalysts, and the structure of the acid. Understanding these factors is crucial for optimizing reaction conditions and improving yields in industrial processes involving carboxylic acids.
- Catalysts for enhancing carboxylic acid reactions: The use of specific catalysts can significantly increase the reaction rates of carboxylic acids. These catalysts may include metal complexes, enzymes, or other compounds that lower the activation energy of the reaction, allowing it to proceed more quickly and efficiently.
- Reaction kinetics of carboxylic acid derivatives: The reaction rates of carboxylic acid derivatives, such as esters, amides, and anhydrides, can differ from those of the parent acids. Understanding these differences is important for predicting and controlling the behavior of these compounds in various chemical processes and applications.
- Effect of substituents on carboxylic acid reactivity: The presence and nature of substituents on the carbon chain or aromatic ring of carboxylic acids can significantly impact their reaction rates. Electron-withdrawing or electron-donating groups can alter the acidity and reactivity of the carboxylic acid group, leading to changes in reaction kinetics.
- Measurement and analysis of carboxylic acid reaction rates: Various techniques and methods are employed to measure and analyze the reaction rates of carboxylic acids. These may include spectroscopic methods, chromatography, and kinetic studies. Accurate measurement and analysis are essential for understanding reaction mechanisms and optimizing processes involving carboxylic acids.
02 Catalytic processes for carboxylic acid reactions
Catalysts play a significant role in enhancing the reaction rates of carboxylic acids. Different types of catalysts, such as metal complexes, enzymes, and heterogeneous catalysts, can be employed to accelerate specific reactions involving carboxylic acids, leading to improved efficiency and selectivity.Expand Specific Solutions03 Kinetic studies of carboxylic acid reactions
Kinetic studies are essential for understanding the reaction mechanisms and rates of carboxylic acid reactions. These studies involve measuring reaction rates under various conditions, determining rate laws, and identifying rate-determining steps. Such information is valuable for process optimization and reaction control.Expand Specific Solutions04 Structural effects on carboxylic acid reactivity
The structure of carboxylic acids significantly influences their reaction rates. Factors such as chain length, branching, and substituents can affect the reactivity of the carboxyl group. Understanding these structure-reactivity relationships is crucial for predicting and controlling reaction outcomes in organic synthesis and industrial applications.Expand Specific Solutions05 Novel methods for accelerating carboxylic acid reactions
Researchers are continuously developing new methods to enhance the reaction rates of carboxylic acids. These include the use of microwave irradiation, ultrasound, and flow chemistry techniques. Such innovative approaches can lead to faster reactions, improved yields, and more environmentally friendly processes in the synthesis and modification of carboxylic acid derivatives.Expand Specific Solutions
Key Players in Carboxylic Acid Catalysis Research
The carboxylic acid optimization of biochemical reaction rates is in a mature stage of industry development, with a substantial market size and high technical maturity. Key players like DuPont, Dow, and BASF have established strong positions in this field, leveraging their extensive R&D capabilities and industrial scale. The market is characterized by ongoing innovation in enzyme engineering and process optimization, with a focus on improving efficiency and sustainability. Emerging players, such as Auspex Pharmaceuticals and Ceramatec, are contributing to the competitive landscape by developing specialized applications and novel catalytic systems, further driving the sector's growth and technological advancement.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed innovative catalysts and process technologies to optimize carboxylic acid production and utilization in biochemical reactions. Their approach involves using metal-organic frameworks (MOFs) as heterogeneous catalysts, which enhance reaction rates and selectivity[1]. These MOFs provide high surface areas and tunable pore structures, allowing for efficient mass transfer and increased catalytic activity[3]. DuPont has also implemented continuous flow reactors for carboxylic acid synthesis, enabling precise control over reaction conditions and improving overall process efficiency[5]. Additionally, they have explored the use of biocatalysts, such as engineered enzymes, to catalyze carboxylic acid-mediated reactions under mild conditions, reducing energy consumption and improving product yields[7].
Strengths: Advanced catalyst design, process intensification, and sustainable chemistry approaches. Weaknesses: Potential scalability challenges and high initial investment costs for new technologies.
Solvay SA
Technical Solution: Solvay has focused on developing green chemistry solutions for carboxylic acid-based reactions. They have pioneered the use of supercritical CO2 as a solvent and reactant in carboxylic acid synthesis, which significantly enhances reaction rates and reduces environmental impact[2]. Solvay's researchers have also developed novel ionic liquid systems that act as both solvents and catalysts for carboxylic acid reactions, improving selectivity and yield[4]. Furthermore, they have implemented advanced process analytical technologies (PAT) to monitor and control carboxylic acid reactions in real-time, optimizing reaction conditions and ensuring consistent product quality[6]. Solvay has also explored the use of microreactor technology for rapid screening and optimization of carboxylic acid-mediated reactions, accelerating the development of new processes[8].
Strengths: Sustainable chemistry focus, innovative solvent systems, and advanced process control. Weaknesses: Potential challenges in scaling up novel technologies and higher production costs compared to traditional methods.
Innovative Approaches in Carboxylic Acid Catalysis
Methods, reagents and cells for biosynthesizing compounds
PatentActiveUS20150361467A1
Innovation
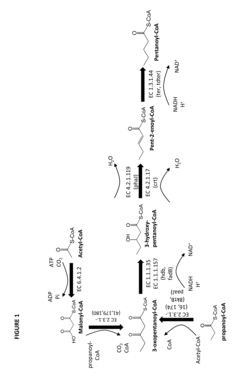
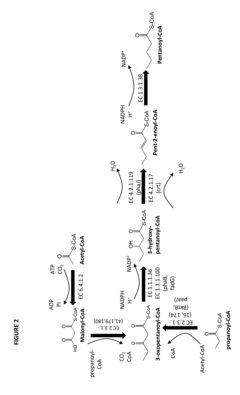
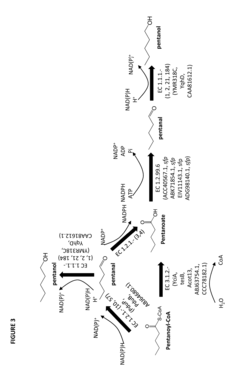
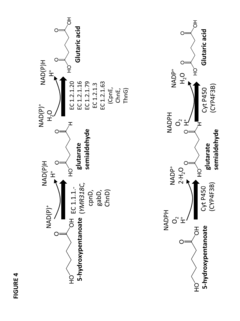
- Construction of biochemical pathways using isolated enzymes like fatty acid O-methyltransferase, monooxygenase, and esterase, along with recombinant host cells, to convert pentanoate and pentanoic acid into these C5 building blocks through multiple enzymatic steps, including CoA-dependent carbon chain elongation and functional group formation.
Process for the preparation of steroidal 17 beta-carbothioates
PatentInactiveEP1466920A1
Innovation
- The use of novel in situ generated 17β-carboxy imidazolyl- or succinimidyl esters as intermediates allows for a continuous one-pot synthesis, reducing hydrolysis and eliminating the need for anhydrous conditions, thereby increasing the efficiency and yield of the steroidal carbothioate production.
Environmental Impact of Carboxylic Acid Catalysis
The environmental impact of carboxylic acid catalysis in biochemical reactions is a critical consideration in the broader context of sustainable chemistry and industrial processes. Carboxylic acids, while effective in optimizing reaction rates, can have both positive and negative effects on the environment depending on their application and management.
One of the primary environmental benefits of carboxylic acid catalysis is its potential to reduce energy consumption in biochemical processes. By enhancing reaction rates, these catalysts can lower the overall energy requirements for industrial-scale reactions, contributing to reduced carbon emissions and improved energy efficiency. This aligns with global efforts to mitigate climate change and promote sustainable industrial practices.
However, the production and use of carboxylic acids can also pose environmental challenges. The synthesis of these compounds often involves petrochemical feedstocks, which are derived from non-renewable resources. This reliance on fossil fuels contributes to carbon emissions and resource depletion, highlighting the need for more sustainable production methods.
Water pollution is another significant concern associated with carboxylic acid catalysis. If not properly managed, these acids can enter aquatic ecosystems, altering pH levels and potentially harming aquatic life. Industrial wastewater containing carboxylic acids requires careful treatment to prevent environmental contamination and maintain ecological balance.
On the other hand, certain carboxylic acids, particularly those derived from renewable sources, can offer environmentally friendly alternatives to traditional catalysts. For instance, citric acid and lactic acid, which can be produced through fermentation processes, present more sustainable options for catalysis in various biochemical applications.
The biodegradability of carboxylic acids is another important environmental factor. Many of these compounds can be naturally broken down by microorganisms, reducing their long-term environmental persistence. However, the rate of biodegradation can vary significantly depending on the specific acid and environmental conditions, necessitating careful consideration in their application and disposal.
In terms of air quality, the volatile nature of some carboxylic acids can lead to atmospheric emissions. While these emissions are generally less harmful than those of many other industrial chemicals, they still contribute to air pollution and can potentially form secondary pollutants through atmospheric reactions.
To mitigate these environmental impacts, ongoing research focuses on developing greener synthesis methods for carboxylic acids, improving catalytic efficiency to reduce waste, and exploring bio-based alternatives. Additionally, advancements in process engineering aim to enhance containment and recycling of these compounds in industrial settings, minimizing their release into the environment.
One of the primary environmental benefits of carboxylic acid catalysis is its potential to reduce energy consumption in biochemical processes. By enhancing reaction rates, these catalysts can lower the overall energy requirements for industrial-scale reactions, contributing to reduced carbon emissions and improved energy efficiency. This aligns with global efforts to mitigate climate change and promote sustainable industrial practices.
However, the production and use of carboxylic acids can also pose environmental challenges. The synthesis of these compounds often involves petrochemical feedstocks, which are derived from non-renewable resources. This reliance on fossil fuels contributes to carbon emissions and resource depletion, highlighting the need for more sustainable production methods.
Water pollution is another significant concern associated with carboxylic acid catalysis. If not properly managed, these acids can enter aquatic ecosystems, altering pH levels and potentially harming aquatic life. Industrial wastewater containing carboxylic acids requires careful treatment to prevent environmental contamination and maintain ecological balance.
On the other hand, certain carboxylic acids, particularly those derived from renewable sources, can offer environmentally friendly alternatives to traditional catalysts. For instance, citric acid and lactic acid, which can be produced through fermentation processes, present more sustainable options for catalysis in various biochemical applications.
The biodegradability of carboxylic acids is another important environmental factor. Many of these compounds can be naturally broken down by microorganisms, reducing their long-term environmental persistence. However, the rate of biodegradation can vary significantly depending on the specific acid and environmental conditions, necessitating careful consideration in their application and disposal.
In terms of air quality, the volatile nature of some carboxylic acids can lead to atmospheric emissions. While these emissions are generally less harmful than those of many other industrial chemicals, they still contribute to air pollution and can potentially form secondary pollutants through atmospheric reactions.
To mitigate these environmental impacts, ongoing research focuses on developing greener synthesis methods for carboxylic acids, improving catalytic efficiency to reduce waste, and exploring bio-based alternatives. Additionally, advancements in process engineering aim to enhance containment and recycling of these compounds in industrial settings, minimizing their release into the environment.
Scalability and Industrial Applications
The scalability and industrial applications of carboxylic acid in optimizing biochemical reaction rates present significant opportunities for various sectors. The ability to scale up the use of carboxylic acids in industrial processes has far-reaching implications for manufacturing efficiency and product quality.
In the pharmaceutical industry, carboxylic acids play a crucial role in drug synthesis and formulation. Large-scale production of pharmaceuticals often involves complex biochemical reactions that can be optimized using carboxylic acids. By fine-tuning reaction conditions and incorporating carboxylic acids as catalysts or reactants, manufacturers can achieve higher yields, improved purity, and reduced production costs. This scalability is particularly important for the mass production of essential medicines and novel therapeutics.
The food and beverage industry also benefits from the scalable application of carboxylic acids in biochemical processes. Fermentation, a key process in food production, can be enhanced by carefully controlling pH levels using carboxylic acids. This optimization leads to more consistent product quality and increased production efficiency. Additionally, carboxylic acids serve as preservatives and flavor enhancers, allowing for large-scale production of shelf-stable food products without compromising taste or safety.
In the field of biofuels and renewable energy, carboxylic acids play a vital role in optimizing enzymatic reactions for the conversion of biomass into usable energy sources. As the demand for sustainable energy solutions grows, the ability to scale up these processes becomes increasingly important. Carboxylic acids can enhance the efficiency of biocatalysts, leading to higher yields of biofuels and reduced production costs. This scalability is crucial for making biofuels economically viable alternatives to fossil fuels.
The textile industry utilizes carboxylic acids in various processes, including dyeing and finishing. By optimizing these reactions on an industrial scale, manufacturers can achieve more uniform coloration, improved fabric properties, and reduced environmental impact. The ability to scale up these processes while maintaining precise control over reaction rates is essential for meeting the demands of global textile production.
In the realm of materials science, carboxylic acids are employed in the synthesis of polymers and advanced materials. The scalability of these processes allows for the production of high-performance materials with tailored properties for applications in aerospace, automotive, and electronics industries. By optimizing reaction rates using carboxylic acids, manufacturers can achieve better control over molecular weight distribution and material properties, leading to more consistent and higher-quality products.
As industries continue to seek more sustainable and efficient production methods, the role of carboxylic acids in optimizing biochemical reaction rates will likely expand. Future developments may include the integration of advanced process control systems and artificial intelligence to further enhance the scalability and efficiency of carboxylic acid-mediated reactions in industrial settings.
In the pharmaceutical industry, carboxylic acids play a crucial role in drug synthesis and formulation. Large-scale production of pharmaceuticals often involves complex biochemical reactions that can be optimized using carboxylic acids. By fine-tuning reaction conditions and incorporating carboxylic acids as catalysts or reactants, manufacturers can achieve higher yields, improved purity, and reduced production costs. This scalability is particularly important for the mass production of essential medicines and novel therapeutics.
The food and beverage industry also benefits from the scalable application of carboxylic acids in biochemical processes. Fermentation, a key process in food production, can be enhanced by carefully controlling pH levels using carboxylic acids. This optimization leads to more consistent product quality and increased production efficiency. Additionally, carboxylic acids serve as preservatives and flavor enhancers, allowing for large-scale production of shelf-stable food products without compromising taste or safety.
In the field of biofuels and renewable energy, carboxylic acids play a vital role in optimizing enzymatic reactions for the conversion of biomass into usable energy sources. As the demand for sustainable energy solutions grows, the ability to scale up these processes becomes increasingly important. Carboxylic acids can enhance the efficiency of biocatalysts, leading to higher yields of biofuels and reduced production costs. This scalability is crucial for making biofuels economically viable alternatives to fossil fuels.
The textile industry utilizes carboxylic acids in various processes, including dyeing and finishing. By optimizing these reactions on an industrial scale, manufacturers can achieve more uniform coloration, improved fabric properties, and reduced environmental impact. The ability to scale up these processes while maintaining precise control over reaction rates is essential for meeting the demands of global textile production.
In the realm of materials science, carboxylic acids are employed in the synthesis of polymers and advanced materials. The scalability of these processes allows for the production of high-performance materials with tailored properties for applications in aerospace, automotive, and electronics industries. By optimizing reaction rates using carboxylic acids, manufacturers can achieve better control over molecular weight distribution and material properties, leading to more consistent and higher-quality products.
As industries continue to seek more sustainable and efficient production methods, the role of carboxylic acids in optimizing biochemical reaction rates will likely expand. Future developments may include the integration of advanced process control systems and artificial intelligence to further enhance the scalability and efficiency of carboxylic acid-mediated reactions in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!