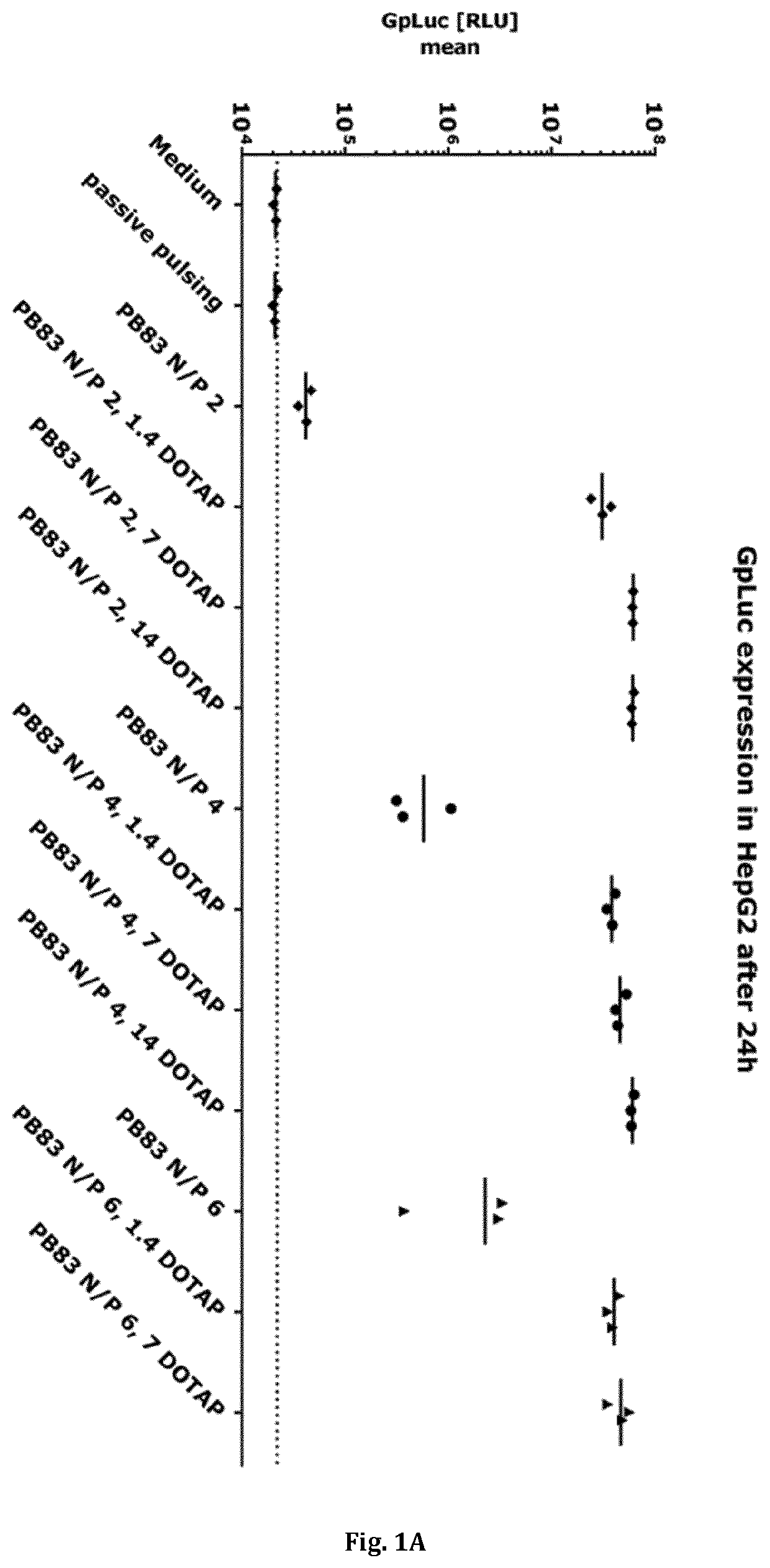
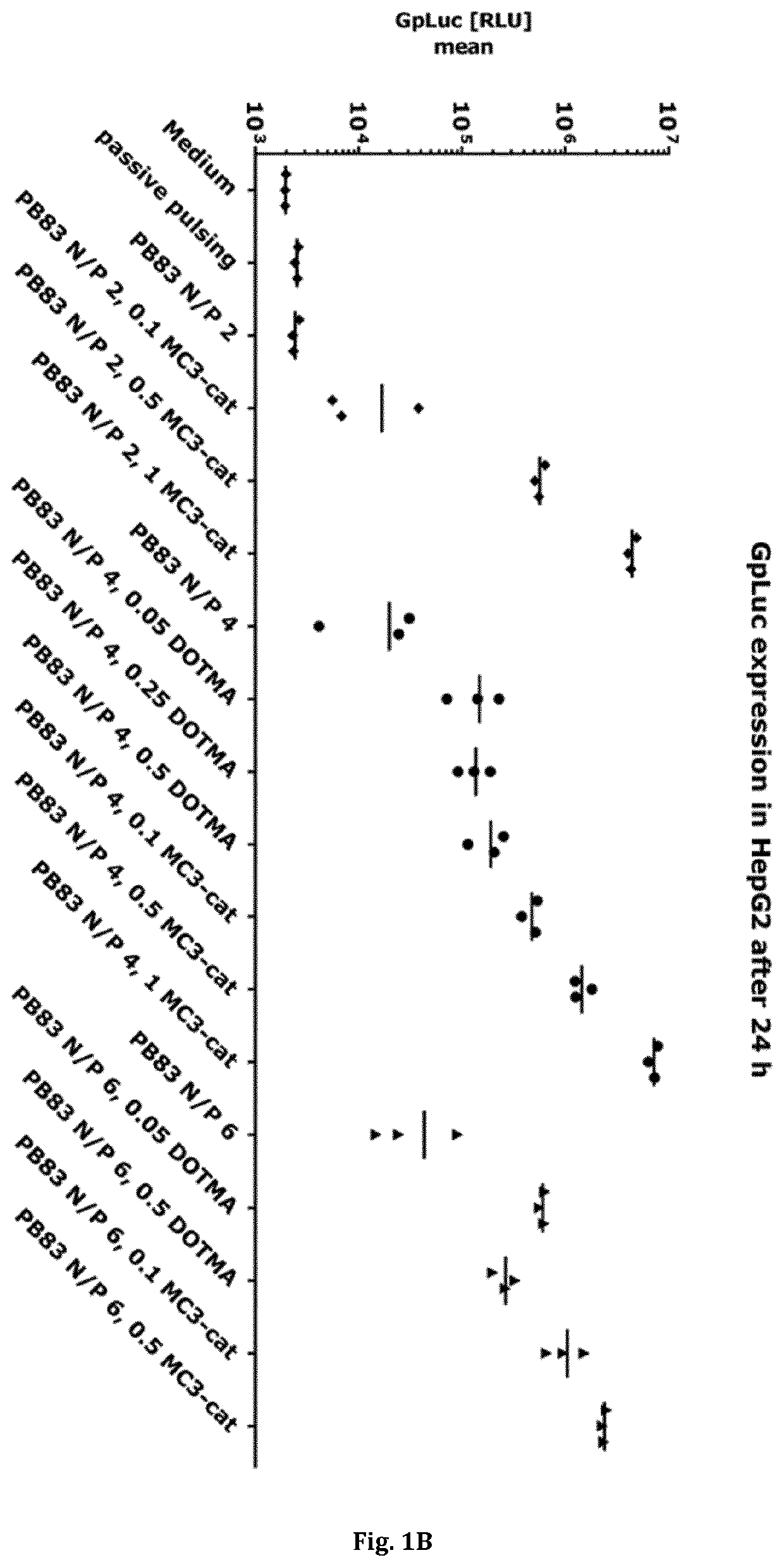
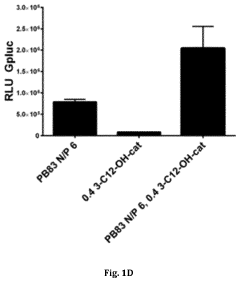
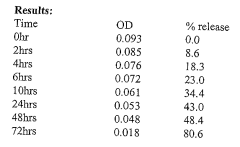
How to Implement Carboxylic Acid-Coated Nanoparticles in Medicine?
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle Coating Advancements and Objectives
The field of nanoparticle coating has witnessed significant advancements in recent years, particularly in the realm of carboxylic acid-coated nanoparticles for medical applications. This technology holds immense potential for revolutionizing drug delivery, diagnostic imaging, and therapeutic interventions. The primary objective of implementing carboxylic acid-coated nanoparticles in medicine is to enhance the efficacy and safety of various treatments while minimizing side effects.
One of the key goals is to improve the biocompatibility and stability of nanoparticles in biological environments. Carboxylic acid coatings provide a negatively charged surface that can reduce nonspecific protein adsorption and increase the circulation time of nanoparticles in the bloodstream. This extended circulation allows for more efficient targeting of diseased tissues and organs.
Another crucial objective is to develop precise targeting mechanisms. By functionalizing the carboxylic acid-coated nanoparticles with specific ligands or antibodies, researchers aim to create "smart" nanocarriers that can selectively bind to cancer cells or other pathological targets. This targeted approach has the potential to dramatically improve the therapeutic index of drugs and reduce off-target effects.
The controlled release of therapeutic agents is also a primary focus in this field. Researchers are exploring various stimuli-responsive mechanisms that can trigger the release of drugs from carboxylic acid-coated nanoparticles in response to specific environmental cues, such as pH changes, temperature fluctuations, or enzymatic activity. This controlled release strategy aims to optimize drug efficacy while minimizing systemic toxicity.
Furthermore, there is a growing interest in developing multifunctional nanoparticles that can combine diagnostic and therapeutic capabilities, known as theranostics. Carboxylic acid-coated nanoparticles serve as an excellent platform for integrating imaging agents and therapeutic compounds, enabling real-time monitoring of drug delivery and treatment response.
As the field progresses, researchers are also focusing on scaling up the production of carboxylic acid-coated nanoparticles to meet clinical demands. This involves optimizing synthesis methods, improving batch-to-batch consistency, and developing cost-effective manufacturing processes that comply with regulatory standards for pharmaceutical products.
Lastly, there is an ongoing effort to elucidate the long-term safety and biodistribution profiles of carboxylic acid-coated nanoparticles in vivo. Comprehensive toxicological studies and pharmacokinetic analyses are being conducted to ensure the safety and efficacy of these nanoparticles for various medical applications, paving the way for their successful translation from bench to bedside.
One of the key goals is to improve the biocompatibility and stability of nanoparticles in biological environments. Carboxylic acid coatings provide a negatively charged surface that can reduce nonspecific protein adsorption and increase the circulation time of nanoparticles in the bloodstream. This extended circulation allows for more efficient targeting of diseased tissues and organs.
Another crucial objective is to develop precise targeting mechanisms. By functionalizing the carboxylic acid-coated nanoparticles with specific ligands or antibodies, researchers aim to create "smart" nanocarriers that can selectively bind to cancer cells or other pathological targets. This targeted approach has the potential to dramatically improve the therapeutic index of drugs and reduce off-target effects.
The controlled release of therapeutic agents is also a primary focus in this field. Researchers are exploring various stimuli-responsive mechanisms that can trigger the release of drugs from carboxylic acid-coated nanoparticles in response to specific environmental cues, such as pH changes, temperature fluctuations, or enzymatic activity. This controlled release strategy aims to optimize drug efficacy while minimizing systemic toxicity.
Furthermore, there is a growing interest in developing multifunctional nanoparticles that can combine diagnostic and therapeutic capabilities, known as theranostics. Carboxylic acid-coated nanoparticles serve as an excellent platform for integrating imaging agents and therapeutic compounds, enabling real-time monitoring of drug delivery and treatment response.
As the field progresses, researchers are also focusing on scaling up the production of carboxylic acid-coated nanoparticles to meet clinical demands. This involves optimizing synthesis methods, improving batch-to-batch consistency, and developing cost-effective manufacturing processes that comply with regulatory standards for pharmaceutical products.
Lastly, there is an ongoing effort to elucidate the long-term safety and biodistribution profiles of carboxylic acid-coated nanoparticles in vivo. Comprehensive toxicological studies and pharmacokinetic analyses are being conducted to ensure the safety and efficacy of these nanoparticles for various medical applications, paving the way for their successful translation from bench to bedside.
Medical Applications and Market Demand
Carboxylic acid-coated nanoparticles have emerged as a promising technology in the field of medicine, with a wide range of potential applications and growing market demand. These nanoparticles offer unique properties that make them particularly suitable for drug delivery, diagnostic imaging, and therapeutic interventions.
In the realm of drug delivery, carboxylic acid-coated nanoparticles have shown significant potential for improving the efficacy and targeted delivery of various pharmaceutical compounds. The carboxylic acid coating provides a versatile surface for functionalization, allowing for the attachment of specific ligands or antibodies that can target diseased cells or tissues. This targeted approach not only enhances the therapeutic effect but also reduces side effects by minimizing drug exposure to healthy tissues.
The market for nanoparticle-based drug delivery systems is experiencing rapid growth, driven by the increasing prevalence of chronic diseases and the need for more effective treatment options. According to recent market research, the global nanomedicine market, which includes nanoparticle-based drug delivery systems, is expected to reach substantial value in the coming years, with a significant compound annual growth rate.
In the field of diagnostic imaging, carboxylic acid-coated nanoparticles have demonstrated promising results as contrast agents for various imaging modalities, including magnetic resonance imaging (MRI) and computed tomography (CT). These nanoparticles can be engineered to accumulate in specific tissues or organs, providing enhanced contrast and improved diagnostic accuracy. The market for advanced imaging agents is expanding, driven by the growing demand for early and accurate disease detection.
Therapeutic applications of carboxylic acid-coated nanoparticles extend beyond drug delivery to include areas such as photothermal therapy and gene therapy. In photothermal therapy, these nanoparticles can be designed to absorb near-infrared light and generate localized heat, effectively destroying cancer cells. The market for minimally invasive cancer treatments is growing rapidly, creating opportunities for nanoparticle-based therapies.
The increasing focus on personalized medicine and targeted therapies is expected to further drive the demand for carboxylic acid-coated nanoparticles in medical applications. These nanoparticles can be tailored to individual patient needs, offering the potential for more effective and personalized treatment strategies.
However, it is important to note that the market adoption of nanoparticle-based technologies faces challenges related to regulatory approval processes, safety concerns, and manufacturing scalability. Overcoming these hurdles will be crucial for realizing the full market potential of carboxylic acid-coated nanoparticles in medicine.
In the realm of drug delivery, carboxylic acid-coated nanoparticles have shown significant potential for improving the efficacy and targeted delivery of various pharmaceutical compounds. The carboxylic acid coating provides a versatile surface for functionalization, allowing for the attachment of specific ligands or antibodies that can target diseased cells or tissues. This targeted approach not only enhances the therapeutic effect but also reduces side effects by minimizing drug exposure to healthy tissues.
The market for nanoparticle-based drug delivery systems is experiencing rapid growth, driven by the increasing prevalence of chronic diseases and the need for more effective treatment options. According to recent market research, the global nanomedicine market, which includes nanoparticle-based drug delivery systems, is expected to reach substantial value in the coming years, with a significant compound annual growth rate.
In the field of diagnostic imaging, carboxylic acid-coated nanoparticles have demonstrated promising results as contrast agents for various imaging modalities, including magnetic resonance imaging (MRI) and computed tomography (CT). These nanoparticles can be engineered to accumulate in specific tissues or organs, providing enhanced contrast and improved diagnostic accuracy. The market for advanced imaging agents is expanding, driven by the growing demand for early and accurate disease detection.
Therapeutic applications of carboxylic acid-coated nanoparticles extend beyond drug delivery to include areas such as photothermal therapy and gene therapy. In photothermal therapy, these nanoparticles can be designed to absorb near-infrared light and generate localized heat, effectively destroying cancer cells. The market for minimally invasive cancer treatments is growing rapidly, creating opportunities for nanoparticle-based therapies.
The increasing focus on personalized medicine and targeted therapies is expected to further drive the demand for carboxylic acid-coated nanoparticles in medical applications. These nanoparticles can be tailored to individual patient needs, offering the potential for more effective and personalized treatment strategies.
However, it is important to note that the market adoption of nanoparticle-based technologies faces challenges related to regulatory approval processes, safety concerns, and manufacturing scalability. Overcoming these hurdles will be crucial for realizing the full market potential of carboxylic acid-coated nanoparticles in medicine.
Current Challenges in Carboxylic Acid Nanoparticle Synthesis
The synthesis of carboxylic acid-coated nanoparticles for medical applications faces several significant challenges that researchers and manufacturers must overcome. One of the primary obstacles is achieving consistent size control and uniformity of the nanoparticles. The size and shape of nanoparticles greatly influence their behavior in biological systems, affecting their biodistribution, cellular uptake, and therapeutic efficacy. Maintaining precise control over these parameters during large-scale production remains a complex task.
Another critical challenge lies in the stability of the carboxylic acid coating. The harsh physiological environment in the human body, including varying pH levels and the presence of enzymes, can potentially degrade or alter the carboxylic acid coating. This instability may lead to premature drug release or changes in the nanoparticle's surface properties, compromising its intended function and safety profile.
The biocompatibility and potential toxicity of carboxylic acid-coated nanoparticles present ongoing concerns. While carboxylic acid coatings are generally considered biocompatible, the long-term effects of these nanoparticles in the body are not fully understood. Researchers must conduct extensive in vitro and in vivo studies to assess potential cytotoxicity, immunogenicity, and genotoxicity.
Scalability and reproducibility in manufacturing processes pose significant hurdles. Transitioning from laboratory-scale synthesis to industrial production while maintaining consistent quality and properties of the nanoparticles is challenging. Variations in synthesis conditions can lead to batch-to-batch inconsistencies, affecting the overall efficacy and safety of the final product.
The functionalization of carboxylic acid-coated nanoparticles with targeting ligands or therapeutic agents introduces additional complexities. Achieving efficient and stable conjugation without compromising the integrity of the coating or the attached molecules requires careful optimization of reaction conditions and purification methods.
Regulatory compliance and characterization standards for nanoparticle-based medical products are still evolving. The lack of standardized protocols for characterizing and evaluating the safety and efficacy of these nanoparticles complicates the regulatory approval process. Manufacturers must navigate this uncertain regulatory landscape while developing their products.
Lastly, the cost-effectiveness of large-scale production remains a significant challenge. The complex synthesis processes, specialized equipment, and rigorous quality control measures required for producing medical-grade carboxylic acid-coated nanoparticles contribute to high production costs. Balancing these costs with the potential therapeutic benefits is crucial for the commercial viability of these nanoparticle-based medical applications.
Another critical challenge lies in the stability of the carboxylic acid coating. The harsh physiological environment in the human body, including varying pH levels and the presence of enzymes, can potentially degrade or alter the carboxylic acid coating. This instability may lead to premature drug release or changes in the nanoparticle's surface properties, compromising its intended function and safety profile.
The biocompatibility and potential toxicity of carboxylic acid-coated nanoparticles present ongoing concerns. While carboxylic acid coatings are generally considered biocompatible, the long-term effects of these nanoparticles in the body are not fully understood. Researchers must conduct extensive in vitro and in vivo studies to assess potential cytotoxicity, immunogenicity, and genotoxicity.
Scalability and reproducibility in manufacturing processes pose significant hurdles. Transitioning from laboratory-scale synthesis to industrial production while maintaining consistent quality and properties of the nanoparticles is challenging. Variations in synthesis conditions can lead to batch-to-batch inconsistencies, affecting the overall efficacy and safety of the final product.
The functionalization of carboxylic acid-coated nanoparticles with targeting ligands or therapeutic agents introduces additional complexities. Achieving efficient and stable conjugation without compromising the integrity of the coating or the attached molecules requires careful optimization of reaction conditions and purification methods.
Regulatory compliance and characterization standards for nanoparticle-based medical products are still evolving. The lack of standardized protocols for characterizing and evaluating the safety and efficacy of these nanoparticles complicates the regulatory approval process. Manufacturers must navigate this uncertain regulatory landscape while developing their products.
Lastly, the cost-effectiveness of large-scale production remains a significant challenge. The complex synthesis processes, specialized equipment, and rigorous quality control measures required for producing medical-grade carboxylic acid-coated nanoparticles contribute to high production costs. Balancing these costs with the potential therapeutic benefits is crucial for the commercial viability of these nanoparticle-based medical applications.
Existing Carboxylic Acid Coating Methodologies
01 Synthesis of carboxylic acid-coated nanoparticles
Methods for synthesizing nanoparticles coated with carboxylic acids, including techniques for controlling particle size, shape, and surface properties. These processes often involve the use of specific precursors and reaction conditions to achieve desired nanoparticle characteristics.- Synthesis and functionalization of carboxylic acid-coated nanoparticles: Methods for synthesizing and functionalizing nanoparticles with carboxylic acid coatings. These processes involve chemical reactions to attach carboxylic acid groups to the surface of various types of nanoparticles, enhancing their stability and functionality for diverse applications.
- Applications in energy storage and conversion: Carboxylic acid-coated nanoparticles are utilized in energy-related applications, such as battery electrodes, fuel cells, and solar cells. The coating improves the nanoparticles' conductivity, stability, and integration with other materials in these energy storage and conversion devices.
- Biomedical applications and drug delivery: The use of carboxylic acid-coated nanoparticles in biomedical fields, particularly for drug delivery systems. The coating enhances biocompatibility, allows for functionalization with therapeutic agents, and improves targeting capabilities in various medical treatments.
- Environmental remediation and water treatment: Application of carboxylic acid-coated nanoparticles in environmental cleanup and water purification processes. These nanoparticles can adsorb pollutants, heavy metals, and other contaminants, making them effective for water treatment and soil remediation.
- Surface modification for improved dispersion and stability: Techniques for modifying the surface of nanoparticles with carboxylic acid coatings to enhance their dispersion in various media and improve their colloidal stability. This modification is crucial for preventing aggregation and maintaining the unique properties of nanoparticles in different applications.
02 Applications in energy storage and conversion
Carboxylic acid-coated nanoparticles are utilized in various energy-related applications, such as battery electrodes, fuel cells, and solar cells. The surface modification enhances their performance and stability in these devices, improving overall energy efficiency and storage capacity.Expand Specific Solutions03 Biomedical applications and drug delivery
These nanoparticles find extensive use in biomedical fields, particularly in drug delivery systems. The carboxylic acid coating can be functionalized to target specific cells or tissues, allowing for controlled release of therapeutic agents and improved biocompatibility.Expand Specific Solutions04 Environmental remediation and catalysis
Carboxylic acid-coated nanoparticles are employed in environmental applications, such as water purification and pollutant removal. They also serve as effective catalysts in various chemical reactions, owing to their high surface area and tunable surface properties.Expand Specific Solutions05 Characterization and analysis techniques
Advanced methods for characterizing carboxylic acid-coated nanoparticles, including spectroscopic, microscopic, and chromatographic techniques. These analytical approaches help in understanding the surface chemistry, particle size distribution, and other crucial properties of the nanoparticles.Expand Specific Solutions
Key Players in Nanoparticle-Based Drug Delivery
The implementation of carboxylic acid-coated nanoparticles in medicine is in a nascent stage of development, with the market still emerging and showing significant growth potential. The technology's maturity is progressing, as evidenced by research efforts from institutions like Massachusetts Institute of Technology, University of Connecticut, and Shanghai Jiao Tong University. Companies such as Midatech Ltd. and NanoDel Technologies GmbH are at the forefront of commercializing this technology. The competitive landscape is characterized by a mix of academic research, start-ups, and established pharmaceutical companies, indicating a diverse and dynamic field with opportunities for innovation and market entry.
Midatech Ltd.
Technical Solution: Midatech Ltd. has developed a proprietary platform for carboxylic acid-coated gold nanoparticles in medicine. Their technology involves creating ultra-small gold nanoparticles (2-5 nm) coated with carboxylic acid ligands, which provide stability and allow for further functionalization[3]. These nanoparticles are designed for targeted drug delivery, particularly in oncology and rare diseases. Midatech's approach includes the ability to attach multiple drug molecules and targeting peptides to a single nanoparticle, enhancing therapeutic efficacy[4]. The company has demonstrated improved drug solubility, stability, and cellular uptake using their carboxylic acid-coated nanoparticle platform in various preclinical studies.
Strengths: Versatile platform technology, ultra-small nanoparticle size for enhanced tissue penetration, potential for combination therapies. Weaknesses: Gold nanoparticles may face regulatory hurdles, potential long-term accumulation concerns in the body.
Massachusetts Institute of Technology
Technical Solution: MIT has developed carboxylic acid-coated nanoparticles for targeted drug delivery in cancer treatment. Their approach involves synthesizing iron oxide nanoparticles with a carboxylic acid coating, which enhances stability and biocompatibility. These nanoparticles are designed to accumulate in tumor tissues through the enhanced permeability and retention (EPR) effect[1]. The carboxylic acid coating allows for further functionalization with targeting ligands or therapeutic agents. MIT researchers have demonstrated improved drug efficacy and reduced side effects in preclinical studies using this nanoparticle system[2].
Strengths: Advanced synthesis techniques, proven efficacy in preclinical studies, potential for multifunctional nanoparticles. Weaknesses: Potential challenges in scaling up production, need for extensive clinical trials before widespread medical use.
Innovations in Carboxylic Acid-Nanoparticle Conjugation
Cationic carriers for nucleic acid delivery
PatentInactiveUS20190336608A1
Innovation
- A composition comprising a cationisable or permanently cationic lipid or lipidoid non-covalently associated with a nucleic acid compound, formulated to form nanoparticles that can efficiently cross cell membranes and avoid immune recognition, allowing for targeted delivery and release of nucleic acids.
Smart polymeric nanoparticles which overcome multidrug resistance to cancer chemotherapeutics and treatment-related systemic toxicity
PatentWO2012078831A2
Innovation
- Development of cross-linked polymeric nanoparticles with curcumin and chemotherapeutic agents like doxorubicin, where curcumin is encapsulated in the hydrophobic core and doxorubicin is conjugated to the surface, overcoming multidrug resistance and reducing systemic toxicity.
Regulatory Framework for Nanoparticle-Based Medicines
The regulatory framework for nanoparticle-based medicines is a complex and evolving landscape that plays a crucial role in the implementation of carboxylic acid-coated nanoparticles in medicine. As these novel therapeutic agents continue to advance, regulatory bodies worldwide are adapting their guidelines to ensure the safety and efficacy of nanomedicines while promoting innovation.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory approaches for nanoparticle-based medicines. The FDA's Nanotechnology Task Force, established in 2006, has been instrumental in addressing the unique challenges posed by nanomedicines. The agency has issued several guidance documents specific to nanotechnology-based products, including those related to safety assessments, manufacturing processes, and characterization methods.
The European Medicines Agency (EMA) has also been proactive in developing regulatory frameworks for nanomedicines. The EMA's approach emphasizes the need for case-by-case evaluation of nanoparticle-based products, recognizing the diverse nature of these materials. The agency has published reflection papers and guidelines on various aspects of nanomedicines, including quality, non-clinical, and clinical considerations.
Internationally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working towards harmonizing regulatory standards for nanomedicines across different regions. This effort aims to streamline the development and approval processes for nanoparticle-based medicines on a global scale.
One of the key challenges in regulating nanomedicines is the need for standardized characterization methods. Regulatory agencies are collaborating with research institutions and industry partners to develop and validate analytical techniques specific to nanoparticles. These efforts focus on establishing reliable methods for assessing particle size, surface properties, and stability, which are critical for ensuring the consistency and quality of carboxylic acid-coated nanoparticles.
Safety assessment is another crucial aspect of the regulatory framework for nanoparticle-based medicines. Regulatory bodies are developing guidelines for evaluating the potential toxicity and long-term effects of nanomedicines, taking into account their unique properties and biodistribution patterns. This includes considerations for nanoparticle accumulation in specific organs and potential interactions with biological systems.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to adapt accordingly. Ongoing research and collaboration between regulatory agencies, academia, and industry will be essential in addressing emerging challenges and refining guidelines for the development and approval of carboxylic acid-coated nanoparticles and other nanomedicines.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory approaches for nanoparticle-based medicines. The FDA's Nanotechnology Task Force, established in 2006, has been instrumental in addressing the unique challenges posed by nanomedicines. The agency has issued several guidance documents specific to nanotechnology-based products, including those related to safety assessments, manufacturing processes, and characterization methods.
The European Medicines Agency (EMA) has also been proactive in developing regulatory frameworks for nanomedicines. The EMA's approach emphasizes the need for case-by-case evaluation of nanoparticle-based products, recognizing the diverse nature of these materials. The agency has published reflection papers and guidelines on various aspects of nanomedicines, including quality, non-clinical, and clinical considerations.
Internationally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working towards harmonizing regulatory standards for nanomedicines across different regions. This effort aims to streamline the development and approval processes for nanoparticle-based medicines on a global scale.
One of the key challenges in regulating nanomedicines is the need for standardized characterization methods. Regulatory agencies are collaborating with research institutions and industry partners to develop and validate analytical techniques specific to nanoparticles. These efforts focus on establishing reliable methods for assessing particle size, surface properties, and stability, which are critical for ensuring the consistency and quality of carboxylic acid-coated nanoparticles.
Safety assessment is another crucial aspect of the regulatory framework for nanoparticle-based medicines. Regulatory bodies are developing guidelines for evaluating the potential toxicity and long-term effects of nanomedicines, taking into account their unique properties and biodistribution patterns. This includes considerations for nanoparticle accumulation in specific organs and potential interactions with biological systems.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to adapt accordingly. Ongoing research and collaboration between regulatory agencies, academia, and industry will be essential in addressing emerging challenges and refining guidelines for the development and approval of carboxylic acid-coated nanoparticles and other nanomedicines.
Biocompatibility and Toxicity Considerations
The implementation of carboxylic acid-coated nanoparticles in medicine necessitates a thorough evaluation of their biocompatibility and potential toxicity. These considerations are crucial for ensuring the safety and efficacy of nanoparticle-based medical applications.
Biocompatibility is a primary concern when introducing any foreign material into the human body. Carboxylic acid-coated nanoparticles must demonstrate minimal adverse reactions with biological tissues and fluids. The surface chemistry of these nanoparticles plays a significant role in their interaction with cellular components and proteins. Studies have shown that the carboxylic acid coating can enhance biocompatibility by reducing non-specific protein adsorption and improving colloidal stability in physiological environments.
However, the potential for immune system activation remains a critical factor to assess. The size, shape, and surface properties of nanoparticles can influence their recognition by immune cells. Careful design and optimization of carboxylic acid-coated nanoparticles are essential to minimize unwanted immune responses while maintaining their intended therapeutic functions.
Toxicity considerations encompass both acute and long-term effects of nanoparticle exposure. Acute toxicity may manifest as immediate cellular damage or inflammatory responses, while chronic toxicity could lead to accumulation in organs and potential long-term health consequences. Extensive in vitro and in vivo studies are necessary to evaluate the toxicological profile of carboxylic acid-coated nanoparticles across various cell types and organ systems.
The biodistribution and clearance of these nanoparticles are critical aspects of their safety profile. Understanding how they are processed and eliminated by the body is essential for predicting potential toxicity and optimizing dosing regimens. Factors such as particle size, surface charge, and coating stability can significantly influence their pharmacokinetics and biodistribution patterns.
Genotoxicity and carcinogenicity assessments are also vital components of the safety evaluation process. While carboxylic acid coatings are generally considered biocompatible, the potential for nanoparticles to interact with genetic material or induce oxidative stress must be thoroughly investigated to ensure long-term safety.
Regulatory compliance is another crucial aspect of implementing carboxylic acid-coated nanoparticles in medicine. Adhering to guidelines set by regulatory bodies such as the FDA and EMA is essential for the successful translation of these nanoparticles from bench to bedside. This includes conducting rigorous preclinical and clinical studies to demonstrate safety and efficacy.
In conclusion, while carboxylic acid-coated nanoparticles show promise in medical applications, their implementation requires a comprehensive assessment of biocompatibility and toxicity. Balancing the therapeutic benefits with potential risks is crucial for developing safe and effective nanoparticle-based medical interventions.
Biocompatibility is a primary concern when introducing any foreign material into the human body. Carboxylic acid-coated nanoparticles must demonstrate minimal adverse reactions with biological tissues and fluids. The surface chemistry of these nanoparticles plays a significant role in their interaction with cellular components and proteins. Studies have shown that the carboxylic acid coating can enhance biocompatibility by reducing non-specific protein adsorption and improving colloidal stability in physiological environments.
However, the potential for immune system activation remains a critical factor to assess. The size, shape, and surface properties of nanoparticles can influence their recognition by immune cells. Careful design and optimization of carboxylic acid-coated nanoparticles are essential to minimize unwanted immune responses while maintaining their intended therapeutic functions.
Toxicity considerations encompass both acute and long-term effects of nanoparticle exposure. Acute toxicity may manifest as immediate cellular damage or inflammatory responses, while chronic toxicity could lead to accumulation in organs and potential long-term health consequences. Extensive in vitro and in vivo studies are necessary to evaluate the toxicological profile of carboxylic acid-coated nanoparticles across various cell types and organ systems.
The biodistribution and clearance of these nanoparticles are critical aspects of their safety profile. Understanding how they are processed and eliminated by the body is essential for predicting potential toxicity and optimizing dosing regimens. Factors such as particle size, surface charge, and coating stability can significantly influence their pharmacokinetics and biodistribution patterns.
Genotoxicity and carcinogenicity assessments are also vital components of the safety evaluation process. While carboxylic acid coatings are generally considered biocompatible, the potential for nanoparticles to interact with genetic material or induce oxidative stress must be thoroughly investigated to ensure long-term safety.
Regulatory compliance is another crucial aspect of implementing carboxylic acid-coated nanoparticles in medicine. Adhering to guidelines set by regulatory bodies such as the FDA and EMA is essential for the successful translation of these nanoparticles from bench to bedside. This includes conducting rigorous preclinical and clinical studies to demonstrate safety and efficacy.
In conclusion, while carboxylic acid-coated nanoparticles show promise in medical applications, their implementation requires a comprehensive assessment of biocompatibility and toxicity. Balancing the therapeutic benefits with potential risks is crucial for developing safe and effective nanoparticle-based medical interventions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!